Abstract
Objective:
To assess cognitive abilities of healthy first-degree relatives of Ashkenazi patients with Parkinson disease (PD), carriers of the G2019S mutation in the LRRK2 gene.
Methods:
In this observational study, 60 consecutive healthy first-degree relatives (aged 50.9 ± 6.2 years; 48% male; 30 G2019S carriers) were assessed using a computerized cognitive program, the Montreal Cognitive Assessment questionnaire, the Unified Parkinson's Disease Rating Scale Part III, and the Geriatric Depression Scale.
Results:
G2019S carriers scored significantly lower on the computerized executive function index (p = 0.04) and on specific executive function tasks (Stroop test, p = 0.007).
Conclusion:
Carrying the LRRK2 G2019S mutation was associated with lower executive performance in a population at risk for PD.
The G2019S mutation in the LRRK2 gene is one of the most common genetic causes of Parkinson disease (PD).1 Although the clinical motor signs of PD in carriers of the G2019S mutation are largely typical, an earlier age at onset of motor symptoms has been reported in some studies.2,3 The exact penetrance among carriers of the G2019S mutation is currently unknown, with rates ranging between 17% at age 50 and 85% at age 70.4,5 Therefore, asymptomatic healthy carriers of the G2019S mutation in the LRRK2 gene are an at-risk population for future development of PD.6
Cognitive impairment is a well-recognized nonmotor feature of PD, affecting most patients if tested with sensitive tools. One of the main features of cognitive decline associated with PD is represented by impairment of executive functions (EFs), which can already be demonstrated shortly after motor symptoms appear.7,8 Computerized cognitive assessment tools have been used extensively over the past decade to assess different cognitive domains. They have been used and validated in PD9,10 but never in asymptomatic mutation carriers. The purpose of this study was to assess the cognitive performance of healthy asymptomatic carriers and noncarriers of the G2019S mutation in the LRRK2 gene. We hypothesized that the computerized assessment battery would identify subtle cognitive differences between nonmanifesting carriers of the G2019S LRRK2 mutation and their first-degree noncarrier relatives.
METHODS
Subjects.
A convenience sample of healthy, consecutive, asymptomatic, first-degree relatives of Ashkenazi Jewish (AJ) patients with PD who carry the G2019S mutation in the LRRK2 gene were invited to participate in a comprehensive study that assessed cognitive capabilities. The study population of the AJ PD cohort included 920 patients treated in the Movement Disorders Unit at the Tel Aviv Sourasky Medical Center. All patients had a diagnosis of clinically definite PD made by a movement disorder specialist, according to the Parkinson's UK Brain Bank criteria.11 All patients underwent a detailed interview to disclose family history of PD or other movement disorders, age at onset of motor symptoms and at diagnosis, and environmental and occupational risk factors. Ancestry and country of origin of both parents were reported by each participant, and only those with 2 AJ parents were included in the cohort. A total of 138 patients with PD were found to be carriers of the G2019S LRRK2 mutation. These patients were approached, and, after receiving their consent to contact their first-degree relatives, recruitment for this study commenced.
Subjects were included in the study only if they reported no overt symptoms of PD, depression, or history of significant head trauma. Cognitive impairment was not an exclusion criterion in this study; however, none of the participants complained of functional significant cognitive decline. All first-degree relatives included in the study were assessed by a neurologist to guarantee that they did not fulfill the criteria for diagnosis of PD.
Subjects were recruited on a rolling basis and were only subsequently genotyped. After 45 recruits, a study coordinator examined the groups to assess matching. There were more mutation carriers than nonmutation carriers; therefore, a paired sampling was performed to ensure equality in group numbers. Subjects and researchers were blinded to mutation status throughout the study until the time of data analysis.
This study was performed in the Tel Aviv Sourasky Medical Center as part of a larger effort to understand the significance of the G2019S mutation in the LRRK2 gene among AJ individuals by a consortium created and supported by the Michael J. Fox Foundation, which also includes Beth Israel Medical Center and Columbia Presbyterian Medical Center in New York, New York.
Standard protocol approvals, registrations, and patient consents.
Before the beginning of the study, all subjects signed an informed consent form approved by Tel Aviv Sourasky Medical Center institutional review board. Basic demographic data, medical history, and medications were collected for all participants. Motor signs were quantified using the motor portion (Part III) of the Unified Parkinson's Disease Rating Scale (UPDRS).12 Cognitive screening was performed using the Montreal Cognitive Assessment test (MoCA).13 Depression was assessed using the Geriatric Depression Scale (GDS).14 All participants were Hebrew speakers. Subjects completed a computerized cognitive test battery (MindStreams; NeuroTrax Corp., NY)15 designed to evaluate multiple cognitive domains including attention, memory, EF, visuospatial, and motor skills. The tests did not require prior knowledge and included subset scores of different tasks including Go-No-Go, verbal memory, Stroop, nonverbal memory, finger tapping, catch game, visuospatial processing, and verbal function. All tests were run in the same fixed order on a desktop computer using a mouse and a keyboard.
Subjects were familiarized with the test procedure before the beginning of the test. The program provides both raw scores such as accuracy rates, reaction times (RTs), response selection, response inhibition, and speed of processing on each domain, as well as an index score relating to the domain tested. Indices were normalized to age and years of education and are presented similarly to an IQ-like scale (mean ± SD 100 ± 15).15
Genetic testing was performed subsequently to the clinical and cognitive assessment. Genomic DNA was isolated from peripheral blood using standard protocols or from saliva according to the manufacturer's instructions (Oragene, Ottawa, Canada). To detect the 6055G_A (G2019S) mutation (rs34637584) in LRRK2 exon 41, we amplified a 171-bp fragment with the following primers: forward 5′ CCTGTGCATTTTCTGGCAGATA 3′ and reverse 5′ CCTCTGATGTTTTTATCCCCATTC 3′.2 PCR fragments were sequenced using the BigDye Terminator Chemistry (Applied Biosystems, Foster City, CA) and analyzed using an automated ABI Prism 3130xl Genetic Analyzer (Applied Biosystems). In addition, G2019S LRRK2 mutation was also detected using TaqMan assay C_63498123_10 in the StepOnePlus Real-Time PCR System (Applied Biosystems).
Statistical analysis.
Means and SDs were calculated for all dependent variables. Histograms and frequency distributions were constructed to evaluate the normality and homogeneity of the dependent variables. The relationship between the presence of the G2019S mutation and different cognitive indices was examined using the Student's t test or χ2 for continuous and dichotomous variables, respectively.
The subtests of each of the cognitive domains that were significantly different between the groups were further examined. Four subjects, not otherwise atypical in any of the cognitive or clinical tests (1 noncarrier and 3 carriers with MoCA scores between 23 and 27), had extreme poorer scores on the Stroop test (>3 SD above the mean). Their data were removed from the analysis to avoid disproportionate leverage on the statistical models. Generalized estimating equations were used to assess any cluster effect based on familial data. Analysis was adjusted for multiple comparisons. p values reported are based on two-tailed comparisons, with significance levels set at 0.05. Statistical analysis was performed with SPSS version 17 (SPSS Inc., Chicago, IL).
RESULTS
Sixty asymptomatic subjects (mean age 50.9 ± 6.2 years; 30 carriers of G2019S mutation) participated in this study. Of the subjects, 44 were children of patients, 15 were siblings, and 1 was a parent of a patient with PD. Subjects' characteristics are presented in the table. Groups were well-matched with regard to age, gender, and years of education. MoCA scores were similar between groups (noncarriers 26.4 ± 2.2 vs carriers 26 ± 2.4, p = 0.54). None of the subjects had motor signs suggesting PD based on the motor UPDRS (noncarriers 3.1 ± 2.4 vs carriers 2.7 ± 2.9, p = 0.60) or were deemed to be depressed based on the GDS (noncarriers 2.5 ± 3.2 vs carriers 1.8 ± 1.7, p = 0.29).
Table.
Characteristics of the study population
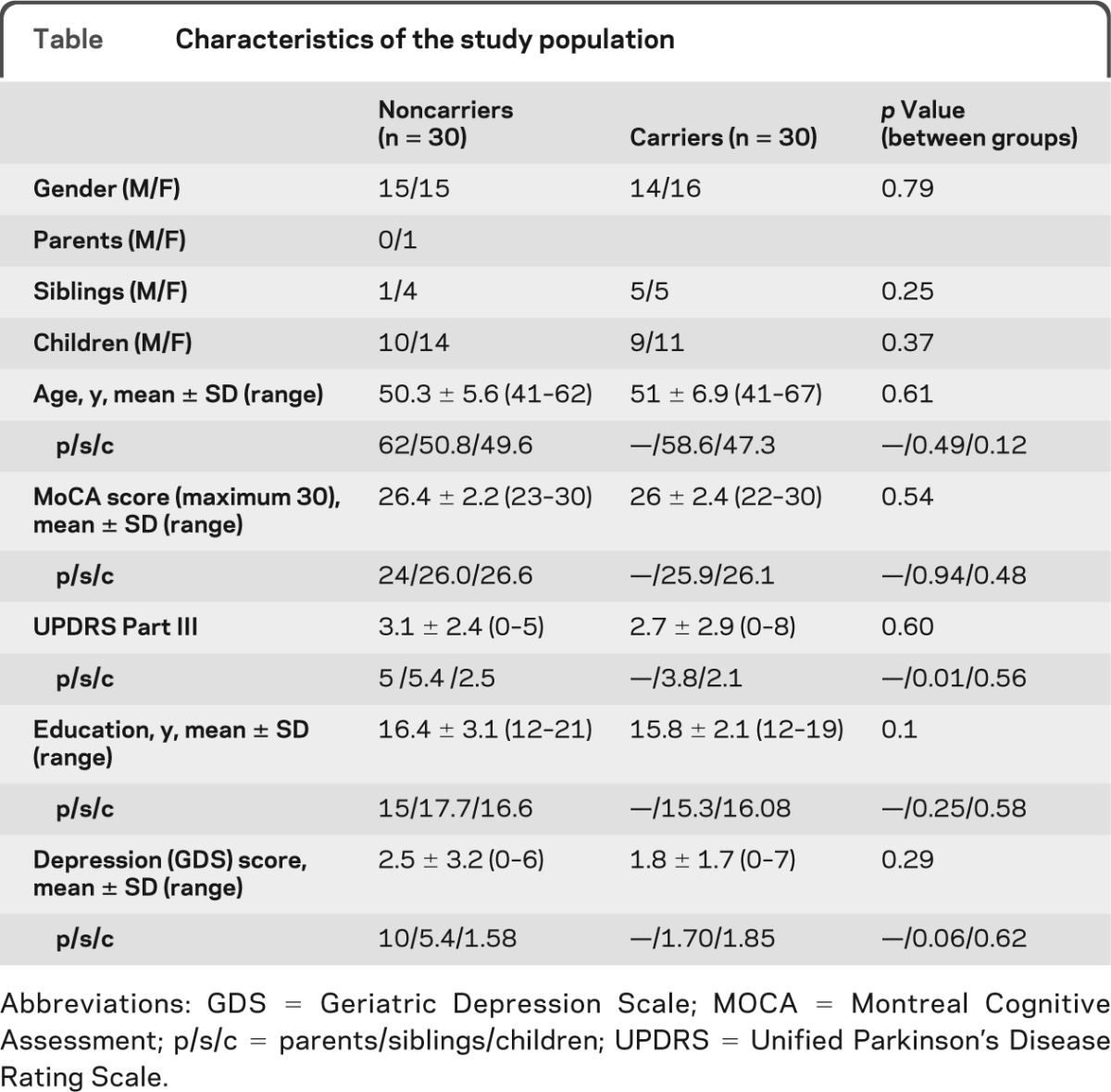
Abbreviations: GDS = Geriatric Depression Scale; MOCA = Montreal Cognitive Assessment; p/s/c = parents/siblings/children; UPDRS = Unified Parkinson's Disease Rating Scale.
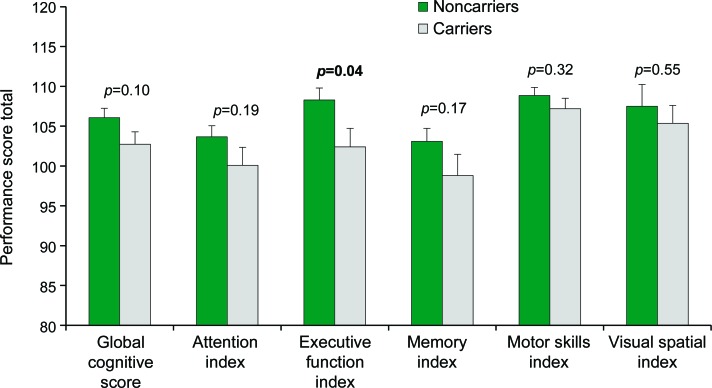
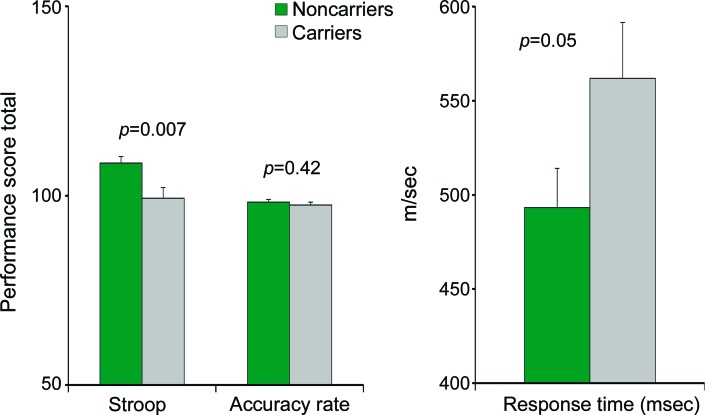
Average total scores on the MindStreams battery were within the normal range (figure 1) and did not reflect cognitive impairment. Significant between-group differences were observed in the EF index score (p = 0.04) with better performance by the noncarriers. In addition, noncarriers performed significantly better on the Stroop interference task (p = 0.007), with a significant difference in RT of performance (p = 0.05) (figure 2).
Figure 1. Comparison between carriers and noncarriers of the G2019S mutation in the LRRK2 gene in cognitive indices on the computerized cognitive battery.
Both groups demonstrated normal cognitive function but noncarriers (n = 30) performed better on all tests examined than the noncarriers (n = 30) with significant between groups differences in the executive function index.
Figure 2. Differences between groups in the performance on the Stroop test.
Total performance score on the interference level of the test and response time were significantly different between the groups. Means and SEs are presented; noncarriers n = 29; carriers n = 27.
Of the subjects in this cohort, 38 were related to each other, which corresponded to 16 families. The other 22 individuals did not have a first-degree relative in this cohort; therefore, there were a total of 38 families of patients with PD represented in this study. No significant cluster effects were found between families based on cognitive scores (p > 0.12) or any of the factors assessed (GDS: p > 0.64, UPDRS: p > 0.31).
DISCUSSION
Asymptomatic carriers of the G2019S mutation demonstrated poorer performance on one computerized measure of executive functioning compared with that of noncarriers. Differences were not observed in any other cognitive domain, suggesting subtle specific differences in performance of EF.
The cognitive decline in PD is characterized by executive dysfunction and visuospatial, memory, language, planning, and attentional set shifting impairments.16 However, the executive domain, a theorized cognitive system that controls and manages other cognitive processes, is affected in early stages of PD7,8
In the Stroop task, the strong interference of word reading on color naming is quantified in terms of increased RT and decreased accuracy rate to color naming when noun and presentation color are incongruent compared with when they are congruent. Greater Stroop interference and slower RT on the Stroop task in patients with PD compared with healthy control subjects has been previously demonstrated.17 This slowness is mainly due to deficits in response inhibition. Several studies have found the Stroop task to be one of the best predictors of cognitive deterioration in patients with both early- and late-stage PD.18–20 Our present report extends these findings to the premotor stage of the disease and raises the possibility that cognitive changes can also be demonstrated at least in some populations at risk.
No significant differences in other domains of cognitive capabilities known to be impaired in PD could be demonstrated between carriers and noncarriers of the mutation. This finding could indicate either that these cognitive domains are relatively preserved or that the tools used were not sensitive enough to detect subtle changes in mutation carriers.
As opposed to a previous study,21 we could not detect any motor differences within our study population even when breaking the UPDRS into its different motor components or stratifying our population according to age. This could be due to the different sizes of our cohorts, the fact that our groups were evenly distributed between carriers and noncarriers, the younger age of our cohort (by 2–4 years), or the fact that we only tested carriers of the G2019S mutation and did not include carriers of the N1437H mutation.
The latency period between the beginning of the pathologic changes and motor manifestations of PD is currently unknown and so is the timeline of the premotor symptoms, which include constipation, olfactory impairment, REM sleep behavior disorder, and anxiety disorders.22,23 Although many patients with PD show cognitive decline over time, the possibility of cognitive changes at the prediagnosis stage has not been demonstrated before.
It is currently accepted that dopamine depletion in early PD is restricted to the putamen and the dorsal caudate nucleus, which are connected to the dorsolateral regions of the frontal lobe, areas that have been implicated in EF.24 However, nondopaminergic pathology including cholinergic, noradrenergic, and serotoninergic deficiencies may also play a role in some of the cognitive deficits observed in PD25,26 possibly even before motor symptoms appear.
Nonmanifesting Parkin carriers did not demonstrate any differences on 5 cognitive domains (psychomotor speed, attention, memory, visuospatial function, and EF) compared with noncarriers.27 However, the use of pen and paper tests as opposed to the computerized assessment performed by our group might be responsible for the lack of findings in this group. In a population-based study assessing risk of cognitive impairment in relatives of patients with PD, the risk of cognitive impairment was modestly increased in first-degree relatives of patients with PD and was sizably increased for relatives of patients with younger age at onset of disease.28 A study assessing first-degree relatives of patients with PD with the G2019S LRRK2 mutation found that regardless of genetic status, first-degree relatives demonstrated higher rates of constipation and worse color discrimination than first-degree relatives of patients with PD who were noncarriers of LRRK2 but could not demonstrate cognitive impairments.29
Although our sample is the largest cohort of healthy nonmanifesting carriers of the G2019S mutation in the LRRK2 gene to be published to date, it is still rather small. Therefore, findings need to be considered with caution and confirmed by additional longitudinal studies and in other populations. Our consortium is currently assessing the cognitive capabilities of a large cohort of healthy first-degree relatives of patients with PD with the G2019S LRRK2 mutation, using a standard battery of neuropsychological tests.
In addition, subjects in different age range strata should be evaluated to understand whether our findings represent degenerative cognitive capabilities or congenital differences that are related to the mutation but not necessarily to the risk of future development of PD. Our cohort was constructed by family members of patients who were aware of their mutation status; this may have created bias toward participating in this study.
Another limitation of the study is that the results of the MindStreams tests were not adjusted for multiple comparisons. However, because they were all adjusted to age and years of education and compared as index scores, this would have had negligible impact on the outcomes.
The findings of this study together with previous work done by our group30 indicate that healthy nonmanifesting carriers of the G2019S mutation perform differently on motor as well as cognitive tasks when tested with sensitive tools. The significance of our present observation is not clear in terms of early markers for the presymptomatic state of PD. It is to be determined whether the differences between groups represent a disease state with a progressive course or a congenital state due to genotype. Only a prospective follow-up of a cohort of LRRK2 carriers, which is currently in progress by our consortium, will provide the necessary information to resolve this fundamental question.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants of this study for their time and commitment.
GLOSSARY
- AJ
Ashkenazi Jewish
- EF
executive function
- GDS
Geriatric Depression Scale
- MoCA
Montreal Cognitive Assessment test
- PD
Parkinson disease
- RT
reaction time
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
This work was performed in partial fulfillment of the requirements of a PhD degree of Avner Thaler, Sackler Faculty of Medicine, Tel Aviv University, Israel.
AUTHOR CONTRIBUTIONS
Avner Thaler: conception, study design, data collection, statistical analysis, and manuscript composition. Anat Mirelman: conception, study design, data collection, manuscript composition, and statistical analysis. Tanya Gurevich: review of manuscript. Ely Simon: review of manuscript. Avi Orr-Urtreger: review of manuscript and obtaining funding. Karen Marder: conception, review of manuscript, and obtaining funding. Susan Bressman: conception, review of manuscript, and obtaining funding. Nir Giladi: conception, review of manuscript, and obtaining funding.
DISCLOSURE
A. Thaler reports receiving a travel grant from the 14th and 16th MDS Congress. A. Mirelman reports receiving funding from the National Parkinson Foundation. T. Gurevich reports receiving funding for travel and speaker honoraria from the National Parkinson Foundation, Solvay Pharmaceuticals, TEVA, RAFA, Medtronic, Novartis, Medison, Allergan, GlaxoSmithKline, Perrigo and Intec Pharma. E. Simon reports being employed by the NeuroTrax Corporation; being on the advisory board of Takeda Pharmaceuticals; and holding stock in the NeuroTrax Corporation. A. Orr-Urtreger reports receiving research support from the Israeli Science Foundation Legacy Heritage Fund, the Chief Scientist Department of Health, Israel, the ALS Association USA. and the Kahn Foundation Israel. K. Marder reports receiving research support through Columbia University from Neurosearch, NeuroSearch Sweden AB, and Medivation, as well as from the PDF, MJFF, Huntington Disease Society of America, Parkinson Study Group, and CHDI and being a section editor for Current Neurology and Neuroscience. S. Bressman reports being on the scientific advisory board of Bachmann Strauss Dystonia and Parkinson's Foundation, MJFF, and Dystonia Medical Research Foundation. N. Giladi reports serving on the advisory boards of UCB, Teva-Lundbeck, NeuroDerm, and Intec Pharma and chairing the data monitoring safety committee of Teva LTD; receiving funding for travel and honoraria from Teva-Lundbeck, UCB, NeuroDerm, Schwartz Pharma, and Merz; serving on the editorial board of the Journal of Neural Transmission, Current Treatment Opinion in Neurology, and Journal of Parkinson's Disease; being a consultant for GSK, NeuroDerm, and Intec Pharma; and receiving grants from the NPF, Israeli Science Foundation, NIH, and MJFF. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Thaler A, Ash E, Gan-Or Z, Orr-Urtreger A, Giladi N. The LRRK2 G2019S mutation as the cause of Parkinson's disease in Ashkenazi Jews. J Neural Transm 2009;116:1473–1482. [DOI] [PubMed] [Google Scholar]
- 2. Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med 2006;354:424–425. [DOI] [PubMed] [Google Scholar]
- 3. Orr-Urtreger A, Shifrin C, Rozovski U, et al. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology 2007;69:1595–1602. [DOI] [PubMed] [Google Scholar]
- 4. Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 2008;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet 2005;76:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siderowf A, Stern MB. Premotor Parkinson's disease: clinical features, detection, and prospects for treatment. Ann Neurol 2008;64(suppl 2):S139–S147. [DOI] [PubMed] [Google Scholar]
- 7. Tomer R, Fisher T, Giladi N, Aharon-Peretz J. Dissociation between spontaneous and reactive flexibility in early Parkinson's disease. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:106–112. [PubMed] [Google Scholar]
- 8. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–392. [DOI] [PubMed] [Google Scholar]
- 9. Tomer R, Fisher T, Fray M, Gajewski BJ. Dissociation between spontaneous and reactive flexibility in Parkinson's disease. Int J Neurosci 2010;15:538–543. [Google Scholar]
- 10. Hanna-Pladdy B, Enslein A, Fray M, Gajewski BJ, Pahwa R, Lyons KE. Utility of the NeuroTrax computerized battery for cognitive screening in Parkinson's disease: comparison with the MMSE and the MoCA. Int J Neurosci 2010;120:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fahn S, Elton R. Unified Parkinson's Disease Rating Scale. Florham Park, NJ: Macmillan Health Care Information; 1987. [Google Scholar]
- 13. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 14. Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2007;22:1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: the prominence of impaired executive function and attention. Exp Aging Res 2006;32:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord 2007;22:1272–1277. [DOI] [PubMed] [Google Scholar]
- 17. Hsieh YH, Chen KJ, Wang CC, Lai CL. Cognitive and motor components of response speed in the Stroop test in Parkinson's disease patients. Kaohsiung J Med Sci 2008;24:197–203. [DOI] [PubMed] [Google Scholar]
- 18. Levy G, Jacobs DM, Tang MX, et al. Memory and executive function impairment predict dementia in Parkinson's disease. Mov Disord 2002;17:1221–1226. [DOI] [PubMed] [Google Scholar]
- 19. Dujardin K, Defebvre L, Duhamel A, et al. Cognitive and SPECT characteristics predict progression of Parkinson's disease in newly diagnosed patients. J Neurol 2004;251:1383–1392. [DOI] [PubMed] [Google Scholar]
- 20. Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol 2005;18:149–154. [DOI] [PubMed] [Google Scholar]
- 21. Johansen KK, White LR, Farrer MJ, Aasly JO. Subclinical signs in LRRK2 mutation carriers. Parkinsonism Relat Disord 2011;17:528–532. [DOI] [PubMed] [Google Scholar]
- 22. Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol 2010;67:798–801. [DOI] [PubMed] [Google Scholar]
- 23. Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord 2012;27:54–60. [DOI] [PubMed] [Google Scholar]
- 24. Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease: pathophysiologic and clinical implications. N Engl J Med 318:876–880, 1988. [DOI] [PubMed] [Google Scholar]
- 25. Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 2010;20:199–204. [DOI] [PubMed] [Google Scholar]
- 26. Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol 2006;5:974–983. [DOI] [PubMed] [Google Scholar]
- 27. Caccappolo E, Alcalay RN, Mejia-Santana H, et al. Neuropsychological profile of parkin mutation carriers with and without Parkinson disease: The CORE-PD study. J Int Neuropsychol Soc 2011;17:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rocca WA, Bower JH, Ahlskog JE, et al. Risk of cognitive impairment or dementia in relatives of patients with Parkinson disease. Arch Neurol 2007;64:1458–1464. [DOI] [PubMed] [Google Scholar]
- 29. Marras C, Schule B, Munhoz RP, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology 2011;77:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol 2011;69:193–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.