Abstract
Objective:
To characterize progressive multifocal leukoencephalopathy (PML) lesions by contrast-enhanced MRI and evaluate their metabolism using proton magnetic resonance spectroscopy (1H- MRS) in the setting of immune reconstitution inflammatory syndrome (IRIS).
Methods:
A total of 42 patients with PML underwent a clinical evaluation as well as brain MRI and 1H-MRS at baseline and 3, 6, and 12 months later. The presence of IRIS was determined based on clinical and laboratory criteria. Ratios of N-acetylaspartate (NAA), choline (Cho), myo-inositol (mI), and lipid/lactate (Lip1 and Lip2) to creatine (Cr) were measured and correlated with the presence of contrast enhancement (CE) in PML lesions.
Results:
IRIS occurred in 16 of 28 (57.1%) PML survivors (PML-S) and 1 of 14 (7.1%) PML progressors (PML-P). Lesions of patients with PML-IRIS showed significantly higher Cho/Cr (p = 0.0001), mI/Cr (p = 0.02), Lip1/Cr (p < 0.0001), and Lip2/Cr (p = 0.002) ratios and lower NAA/Cr (p = 0.02) ratios than patients with PML who did not have IRIS. An elevated Cho/Cr ratio was associated with CE within the 1H-MRS voxel, whereas lipid/Cr ratios were elevated in PML-IRIS lesions independently of CE. Follow-up until 33 months from PML onset showed persistent elevation of the mI/Cr ratio in lesions of patients with PML-IRIS. A Lip1/Cr ratio greater than 1.5 combined with the presence of CE yielded a 79% probability of IRIS compared with 13% in the absence of these criteria.
Conclusion:
1H-MRS is a valuable tool to recognize and track IRIS in PML and may prove useful in the clinical management of these patients.
Treating HIV+ patients with combined antiretroviral therapy (cART) contributes to improved clinical outcome of progressive multifocal leukoencephalopathy (PML). It has also been associated with immune reconstitution inflammatory syndrome (IRIS), causing a paradoxical worsening of neurologic symptoms.1 IRIS can also occur in HIV− individuals after discontinuation of immunosuppressive medications for cancer or autoimmune diseases.2,3
IRIS immunopathogenesis is poorly understood. PML-IRIS is characterized histologically by inflammation and infiltration of the brain by CD8+ T lymphocytes.4 PML lesions may show contrast enhancement (CE) on MRI in 56.7% of patients,1 indicating an inflammatory component and breakdown of the blood-brain barrier as well as edema. There are no biomarkers to define PML-IRIS, and its management represents a therapeutic and prognostic challenge.5,6
We used proton magnetic resonance spectroscopy (1H-MRS) to study the metabolism of the brain within PML lesions in a pilot study. Acute lesions of PML survivors had a higher myo-inositol (mI) concentration than those of PML progressors, who died within a year from disease onset.7 However, longitudinal studies in larger patient groups are lacking. We therefore used a combination of conventional MRI and 1H-MRS to characterize and measure the metabolism of brain lesions of patients with PML and PML-IRIS.
METHODS
Standard protocol approvals, registration, and patient consents.
This observational study has been registered at ClinicalTrials.gov (NCT01132053). Forty-two patients gave written consent to participate in this study according to the institution's guidelines and were enrolled in an outpatient neurology clinic between 2005 and 2011.
Study subjects and design.
PML diagnosis was established according to consensus criteria8: 10 (23.8%) patients had histology-confirmed PML, 24 (57.1%) patients had laboratory-confirmed PML with positive JC virus (JCV) DNA PCR in CSF, 1 (2.4%) patient had both positive biopsy results and JCV detection in CSF, and 7 (16.7%) patients had possible PML based on clinical and radiologic findings. Patients with PML were divided into progressors or survivors, depending on survival shorter or longer than 1 year from onset of neurologic symptoms.9 The presence of IRIS was determined on the basis of clinical and laboratory evaluation, including 1) evidence of immune reconstitution demonstrated by an increase of CD4+ T-cell counts and a decrease in HIV plasma RNA in HIV+ patients or after discontinuation of immunosuppressive medication in HIV− patients, 2) sudden worsening of neurologic signs and symptoms occurring in the context of improved immunologic and virologic response, in contrast with the steady decline of PML progressors, and 3) absence of other pathogens or tumor. These criteria were further supported by the presence of swelling, mass effect, or CE on MRI in some cases. Patients were then classified as PML-simultaneous IRIS when IRIS occurred concomitantly with the initial manifestations of PML or PML-delayed IRIS when IRIS occurred in the setting of a known PML.6
We performed imaging at the time of enrollment (baseline) and after 3, 6, and 12 months. Patients had a full neurologic examination by 2 neurologists (S.G. and I.J.K.). Karnofsky and modified Rankin Scale scores, CD4+ T-cell count, HIV plasma viral load (VL), if applicable, and medications were recorded.
Brain MRI and 1H-MRS.
Brain MRI was done using a 3-T scanner (General Electric, Waukesha, WI). Single voxel 1H- MRS (2 × 2 × 2 cm3, 1.1 minute) of the PML lesions was obtained at the center of the PML lesion. Point-resolved spectroscopy spectra were acquired with a repetition time of 2 seconds, time to echo of 35 msec, spectral width of 5,000 Hz, and 2,048 time points with water-suppressed metabolite signal acquisitions as described previously.7 We defined the combined lactate and lipid peaks at 1.3 ppm as lipid 1 (Lip1) and the combined lipid peak at 0.9 ppm and macromolecules peak at 0.87 ppm as lipid 2 (Lip2). T1-weighted imaging was performed with a magnetization prepared 3-dimensional sequence that provides excellent gray-white matter contrast. This sequence used multiple presaturation pulses followed by a delay and then an adiabatic inversion pulse at 500 msec before image acquisition. A 64-slice encode centric ordered spoiled gradient echo with 15° flip angle was used for acquisition. A 24-cm field of view, 3-mm slice thickness, and 256 × 256 matrix were used. Fluid-attenuated inversion recovery was performed with the product sequence, 3-mm slices, 24-cm field of view, and 256 × 256 matrix. In addition, patients received 0.1 ml/kg Magnevist. A postcontrast sequence was run 5 minutes after administration and as the last sequence of the examination.
Image analysis.
The presence of CE was determined by visual examination by 2 investigators (S.G. and I.J.K.). Spectral analysis was done using the LC model (Stephen Provencher Inc., Oakville, Canada) embedded in SAGE (GE Medical Systems). We measured the concentration of the following metabolites: N-acetylaspartate (NAA), choline (Cho), mI, and mobile lipids/lactate (Lip1 and Lip2). Metabolites were expressed as ratios to creatine (Cr), a marker of basal metabolism.
Statistical analysis.
Using Prism 4 for MacIntosh, we performed Mann-Whitney tests to compare age, CD4+ T-cell count, HIV plasma VL, Karnofsky and modified Rankin Scale scores, time until start of cART, and time until delayed IRIS between PML survivors (PML-S) and PML progressors (PML-P) and between patients with and without IRIS. Fisher exact test was used to compare HIV status, presence of IRIS, gender, and ethnicity between PML-P and PML-S. The Mann-Whitney test was applied on all comparisons of metabolite ratios between different groups. No adjustment for multiple comparisons was performed. A linear mixed-effects model with time as independent variable, thereby controlling for survival, and also controlling for enhancement was used to examine the longitudinal trends between patients with and without IRIS up to 1,000 days after symptom onset using SAS software. The correlation of within-subject measurements was modeled using the variance-covariance structure of compound symmetry, which yielded the equivalent model with random intercept and fixed time effect. The 2 variance components from the compound symmetry variance-covariance matrix of the within-subject metabolites were estimated using the restricted maximum likelihood estimation method. These variance-component estimates were then used iteratively in the estimation of the fixed effect (time effect). In addition, we used a logistic regression model to evaluate IRIS predictors, followed by a stepwise model selection procedure with entry significance level set at 0.05 and stay significance level set at 0.10 to obtain a parsimonious selection of the best predictors. This model yielded Lip1/Cr and CE as best predictors. We subsequently determined the threshold of the Lip1/Cr ratio based on sensitivity and specificity for IRIS and reassigned values as a binary outcome with a cutoff of 1.5. Threshold optimality was determined using the diagnostic measure of overall accuracy, which was defined as the sum of true positives and true negatives divided by the total number of measurements. The sensitivity and specificity that correspond to the maximum accuracy were selected as the optimal sensitivity and specificity.
RESULTS
Study subject characteristics.
Of 42 patients, 66.7% were survivors and 33.3% were progressors (table 1). PML-P were older than PML-S (p = 0.004). There were no differences between the groups in gender or ethnicity. HIV+ patients accounted for 57.1% of PML-S and 21.4% of PML-P (p = 0.05). HIV+ PML-P had nonsignificant lower CD4+ T-cell counts at the time of the first MRI than HIV+ PML-S (p = 0.19). HIV plasma RNA was comparable (p = 0.48). CD4+ T-cell counts in PML-P and PML-S HIV− groups were comparable (p = 0.54) (table 1). Underlying conditions for all HIV− patients are shown in table 1.
Table 1.
Study subject characteristics
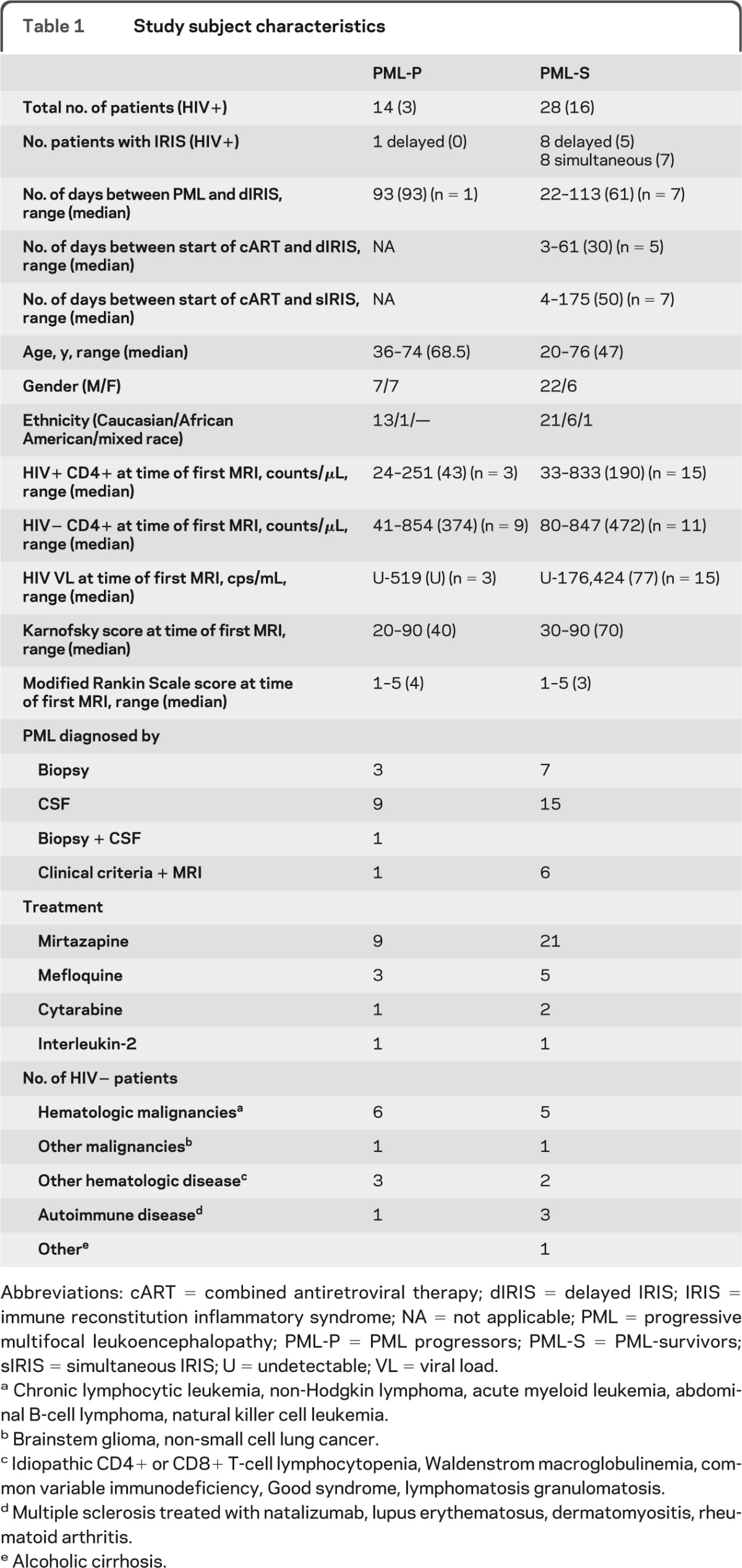
Abbreviations: cART = combined antiretroviral therapy; dIRIS = delayed IRIS; IRIS = immune reconstitution inflammatory syndrome; NA = not applicable; PML = progressive multifocal leukoencephalopathy; PML-P = PML progressors; PML-S = PML-survivors; sIRIS = simultaneous IRIS; U = undetectable; VL = viral load.
Chronic lymphocytic leukemia, non-Hodgkin lymphoma, acute myeloid leukemia, abdominal B-cell lymphoma, natural killer cell leukemia.
Brainstem glioma, non-small cell lung cancer.
Idiopathic CD4+ or CD8+ T-cell lymphocytopenia, Waldenstrom macroglobulinemia, common variable immunodeficiency, Good syndrome, lymphomatosis granulomatosis.
Multiple sclerosis treated with natalizumab, lupus erythematosus, dermatomyositis, rheumatoid arthritis.
Alcoholic cirrhosis.
IRIS was more common in the PML-S group (57.1%) than in the PML-P group (7.1%) (p = 0.002) and was further subdivided into delayed IRIS or simultaneous IRIS (table 1). Of PML-S-IRIS, 75% were HIV+ and had a median CD4+ T-cell increase of 114/μL (44–567 μL) and a median HIV VL decrease of 87,442 copies/mL (undetectable- 228,083) by the time of IRIS onset. Only one patient with PML-IRIS received steroids at the time of the first MRI.
The Karnofsky and modified Rankin Scale scores were better in PML-S than in PML-P, who were first examined within 6 months of symptom onset (p = 0.02 and p = 0.03, respectively) (table 1). There were no differences in Karnofsky and modified Rankin Scale scores between patients with and without IRIS at any of the 4 time points.
All HIV+ patients were treated with cART. Immunosuppressive medications were decreased or stopped when possible. In addition, a total of 30 patients received mirtazapine,10 8 patients were treated with mefloquine,11 3 patients were treated with cytarabine,12 and 2 were treated with interleukin-2 (table 1).
A total of 101 MRIs were performed, of which only 11 were without injection of gadolinium and 2 were without concomitant 1H-MRS, because of technical reasons.
Among PML-P, survival was limited to 70–206 days (median 118.5 days) and, therefore, all patients except one, had only one MRI. Of 15 MRI scans obtained in the PML-P group, 1 occurred during an episode of IRIS. Among PML-S, 16 patients completed 4 MRI scans, 3 patients completed 3 MRI scans, 4 patients completed 2 MRI scans, and 5 patients completed 1 MRI scan. Of the 86 MRI scans in the PML-S group, 25 were obtained during episodes of IRIS.
Contrast enhancement.
To determine whether CE was associated with a better outcome, we compared the incidence of CE on the first MRI of each patient performed within 6 months of symptom onset. There was no difference between the incidence of CE in PML-S and PML-P (11 of 16 [68.8%] vs 5 of 11 [45.5%], p = 0.26).
Of the 25 MRI scans obtained during IRIS, 22 (88%) showed CE compared with only 10 of 65 (15.4%) MRI scans obtained in the absence of IRIS (p < 0.0001).
1H MR spectroscopy.
Metabolite ratios in PML lesions do not differentiate PML-P and PML-S.
All first 1H-MRS examinations of PML-P were obtained within 6 months of symptom onset and were thus compared with those of PML-S within the same time frame. No significant differences were found between the NAA/Cr, Cho/Cr, mI/Cr, Lip1/Cr, and Lip2/Cr ratios of 47 lesions of 16 PML-S and 36 lesions of 14 PML-P (data not shown).
There is a decrease in NAA and increase in Cho/Cr, mI/Cr, Lip1/Cr, and Lip2/Cr ratios in lesions of patients with PML-IRIS.
Examples of the MRI and 1H-MRS spectra of patients with PML-IRIS and patients with PML without IRIS are shown in figure 1. Lesions of PML-IRIS could occur in the cerebrum (figure 1, A–F) and cerebellum (figure 1, G–L) of HIV+ (figure 1, J–L) or HIV− (figure 1, A–I) individuals. Because the latest PML-IRIS episode that was captured on 1H-MRS occurred 309 days after PML symptom onset, all 1H-MRS results obtained during IRIS were compared with those of patients who never experienced IRIS obtained within the same time frame. The metabolite ratios of 69 lesions from 25 1H-MRS scans in 13 patients with IRIS and those of 88 lesions from 34 1H-MRS scans in 23 patients without IRIS are shown in figure 2.
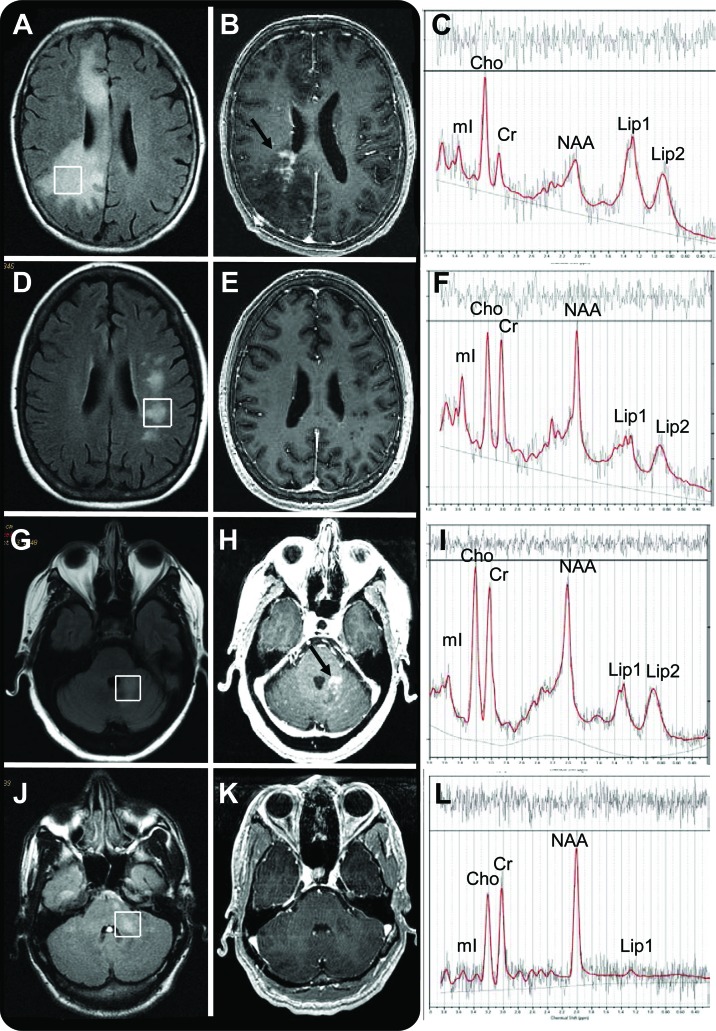
Figure 1. MRI and proton magnetic resonance spectroscopy (1H-MRS) characteristics of progressive multifocal leukoencephalopathy (PML) and PML-immune reconstitution inflammatory syndrome (IRIS) lesions.
(A–F) Cerebral PML lesion of an HIV− patient with PML-IRIS (A–C) and PML without IRIS (D–F) seen on fluid-attenuated inversion recovery (FLAIR) (A, D), and T1-weighted images with gadolinium (B, E). The area of enhancement is marked by an arrow (B), and the 1H-MRS spectrum (C, F) shows elevated choline (Cho) and lipid peaks in the patients with PML-IRIS. (G–L) Cerebellar PML lesion of an HIV− patient with PML-IRIS (G–I) and an HIV+ patient with PML without IRIS (J–L) seen on FLAIR (G, J) and T1-weighted image with gadolinium (H, K). The area of enhancement is marked by an arrow (H), and the 1H-MRS spectrum (I, L) shows elevated Cho and lipid peaks in the patient with IRIS. Cr = creatine; Lip1 = lipid/lactate; Lip2 = lipid/macromolecules; mI = myo-inositol; NAA = N-acetylaspartate.
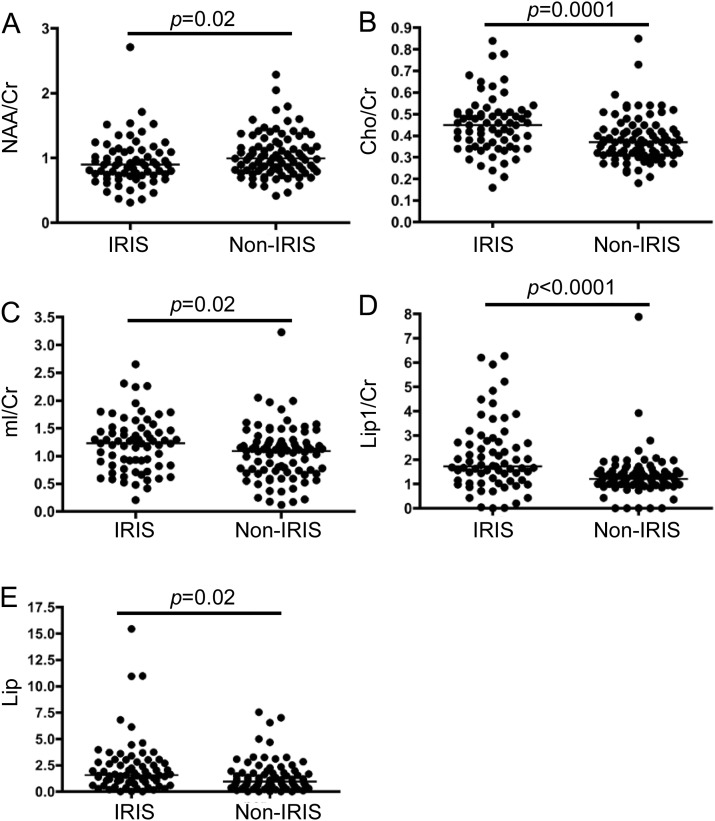
Figure 2. Comparison of metabolite ratios within progressive multifocal leukoencephalopathy (PML) lesions of patients with and without immune reconstitution inflammatory syndrome (IRIS).
All MRIs were obtained within 1 year of onset of PML symptoms. Each dot represents the ratio obtained within one lesion. The bars represent the median ratio: (A) N-acetylaspartate (NAA)/creatine (Cr) ratio; (B) choline (Cho)/Cr ratio; (C) myo-inositol (mI)/Cr ratio; (D) lipid/lactate (Lip1)/Cr ratio and (E) lipid/macromolecules (Lip2)/Cr ratio.
The NAA/Cr ratio was lower in lesions of patients with IRIS (median 0.9 vs 0.99, p = 0.02) (figure 2A). Conversely, the Cho/Cr ratio was markedly higher in lesions of patients with IRIS (median 0.45 vs 0.37, p = 0.0001) (figure 2B). Furthermore, the mI/Cr ratio was elevated in the IRIS group (median 1.23 vs 1.09, p = 0.02) (figure 2C). Finally, both the Lip1/Cr and Lip2/Cr ratios were markedly elevated during IRIS (median 1.73 vs 1.21 and 1.57 vs 0.96, p < 0.0001 and p = 0.002) (figure 2, D and E).
CE is associated with elevated Cho/Cr ratios in PML-IRIS, whereas increases in lipid/Cr ratios are independent of CE.
To determine whether CE was associated with a specific metabolic profile in patients with PML-IRIS, we compared metabolic ratios within PML lesions of patients with IRIS with and without CE within the voxel under consideration. We compared 35 enhancing lesions in 17 MRI scans of 9 patients with IRIS, with 48 nonenhancing lesions in 20 MRI scans of 11 patients with IRIS. Some patients had enhancing and nonenhancing lesions within the same MRI scan. The Cho/Cr ratio was significantly elevated in enhancing lesions (median 0.5 vs 0.39, p < 0.0001), whereas mI/Cr was decreased (median 1.16 vs 1.39, p = 0.007). Lip1/Cr and Lip2/Cr ratios were not significantly different in lesions with or without CE (median 2.17 vs 1.69 and median 1.9 vs 1.94, p = 0.07 and p = 0.46, respectively). No difference was observed in NAA/Cr ratios (median 0.9 vs 0.82, p = 0.39). These results suggest that elevation of lipid/Cr ratios as seen in IRIS was independent of CE, whereas the increase in the Cho/Cr ratio is associated with the presence of CE.
mI/Cr remains elevated in lesions of patients with PML-IRIS over time.
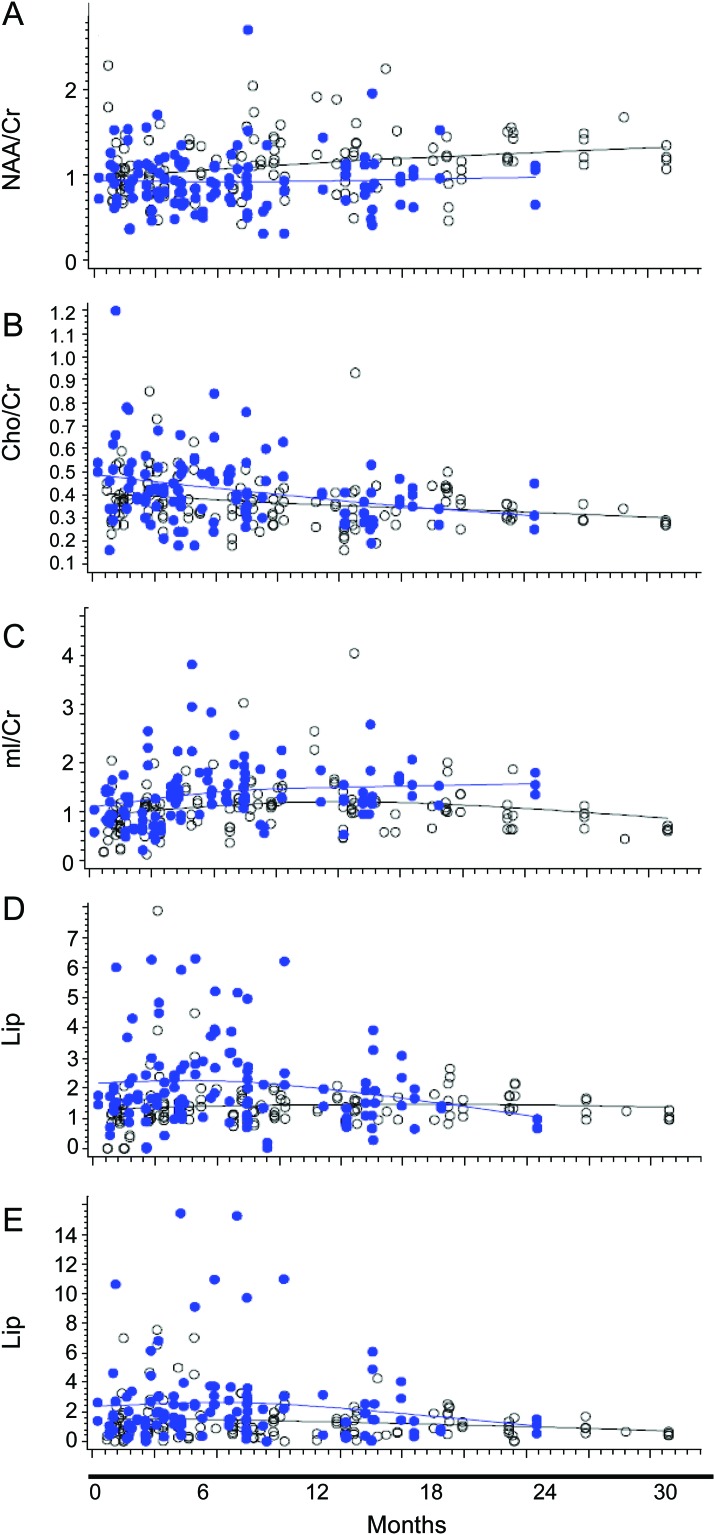
Metabolite ratios were measured in 238 lesions of PML-S and 38 lesions of PML-P over the course of 8 to 4,526 days after symptom onset. Cho, Lip1, and Lip2 ratios decreased over time in the IRIS group, whereas NAA increased (figure 3). Thirty months after symptom onset, there was no significant difference between any of the metabolite ratios in the IRIS and non-IRIS groups, except for the mI/Cr ratio, which remained higher in the IRIS group over time (mean difference 0.3418, p = 0.01) (figure 3).
Figure 3. Longitudinal evolution of metabolite ratios in patients with immune reconstitution inflammatory syndrome (IRIS) vs those without IRIS.
Metabolite/creatine (Cr) ratios obtained in progressive multifocal leukoencephalopathy (PML) lesions of patients with IRIS (solid dots) and patients without IRIS (white dots) up to 30 months after symptom onset are shown. (A) N-Acetylaspartate (NAA)/Cr ratios in patients with IRIS increase over time, but remain overall lower than those in the group without IRIS (not significantly different). (B) The choline (Cho)/Cr ratio decreases quickly over time in the group with IRIS, and after 30 months there is no difference between the groups with and without IRIS. (C) Myo-inositol (mI)/Cr ratios of patients with IRIS remain statistically significantly higher over time compared with those of patients without IRIS. (D) Lipid/lactate (Lip1)/Cr and (E) lipid/macromolecules (Lip2)/Cr ratios decrease quickly over time in the group with IRIS, and after 30 months there is no difference between the groups with and without IRIS.
Lip1/Cr ratio and CE in PML lesions are diagnostic indicators of IRIS.
We reclassified all PML lesions as having a Lip1/Cr ratio either greater than or less than 1.5 (see Statistical Analysis). Combining this measurement with the presence of CE allowed us to determine the probabilities of IRIS as shown in table 2. When a lesion had a Lip1/Cr ratio less than 1.5 and no CE, the likelihood of IRIS was only 13%. Conversely, a Lip1/Cr ratio greater than 1.5 and the presence of CE increased the likelihood of IRIS up to 79%.
Table 2.
Probability of having IRIS based on contrast enhancement and Lip1/Cr ratio
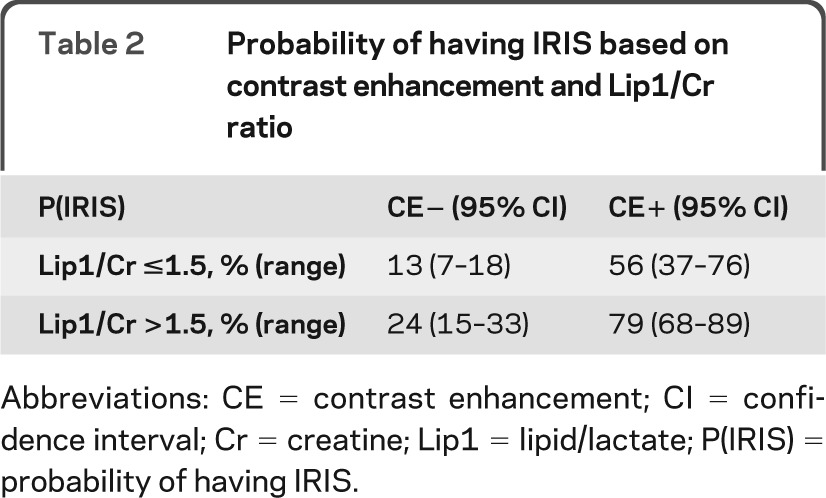
Abbreviations: CE = contrast enhancement; CI = confidence interval; Cr = creatine; Lip1 = lipid/lactate; P(IRIS) = probability of having IRIS.
DISCUSSION
Our study uncovered major differences in the metabolic profile of the brain lesions of patients with PML with IRIS compared with patients with PML without IRIS. These differences subsided over time, except for the mI/Cr ratio, which remained higher in PML-IRIS. We designed a diagnostic model for IRIS using the Lip1/Cr ratio and CE. These findings will be useful in the management of PML. Clinicians are often confronted with patients with PML whose condition worsens when taking cART or when immunosuppressive medications are discontinued, but classic MRI fails to differentiate IRIS from the natural progression of PML, especially in the absence of CE. In our study, some patients with IRIS already had a clinical worsening before CE was recognized on MRI or continued to improve after CE subsided. Therefore, CE by itself is not a reliable parameter to predict or follow IRIS, in line with previous observations.13
Mobile lipids (Lip1 and Lip2) consist of triglyceride and cholesterol esters, which appear as a stress response.14 The Lip1 peak also contains lactate. Both lipid peaks were elevated in IRIS compared with non-IRIS lesions. Higher lipid peaks were also observed in one HIV+ and one HIV− patient with PML-IRIS.15,16 In addition, lipid peaks were identified in spectra obtained in multiple sclerosis (MS) plaques17 and have been studied in brain tumors (reviewed in14). Lipid/lactate peaks are highest in lesions with the most inflammation when 1H-MRS results were correlated with stereotactic biopsy specimens of patients with MS18 and normalize quickly over time.19 These have also been observed in the acute phase of acute disseminated encephalomyelitis (ADEM).20,21 Increased lipid ratios in PML-IRIS are reminiscent of primary CNS lymphoma,22 in which increased lipids were also visualized outside of the areas of CE.23 PML-IRIS lesions are infiltrated by numerous CD8+ T cells.4 On in vitro 1H-MRS, the presence of lipids has been linked to the presence of immune cells, including T cells,24 (reviewed in Delikatny et al.14), and, therefore, lipids have been proposed to be a marker for activated lymphocytes.25 In addition, increased lipid concentrations may promote membrane fluidity, which will facilitate migration of T cells to the site of infection.25 Therefore, the presence of increased lipid/Cr ratios in the setting of PML-IRIS may be caused by infiltration of activated T cells. Combining the Lip1/Cr ratio with CE allowed us to determine the probability of a patient to have IRIS and therefore is a useful clinical tool.
The NAA/Cr ratio was decreased in PML-IRIS lesions. NAA is a marker of neuronal integrity and is decreased in the context of neuronal or axonal damage.18 NAA/Cr ratio reduction has been described in PML26 and in clinically isolated syndrome,27 ADEM,28 and active MS lesions,29 but also in normal-appearing white and gray matter of patients with MS.30 Furthermore, whole-brain NAA is decreased in patients with benign MS31 and is associated with cognitive decline in longitudinal studies.32,33 The NAA/Cr ratio returned to baseline over time in patients with PML-IRIS, suggesting that neuronal dysfunction was in part reversible after inflammation resolved.
Cho is a cell membrane component and is elevated in the setting of increased membrane turnover. An elevated Cho/Cr ratio has been found in other demyelinating and inflammatory processes such as MS, ADEM, and acute experimental allergic encephalomyelitis.17,21,28,34–36 Because elevated Cho was associated with CE, this biomarker by itself is not helpful to distinguish patients with IRIS from patients without IRIS.
mI was higher in lesions of patients with PML-IRIS and is the only metabolite that remained elevated over time. mI is usually thought to be a marker of inflammation, but the mI/Cr ratio was lower in enhancing lesions from patients with IRIS. Similarly, the mI/Cr ratio decreases during the acute phase of ADEM28 and then increases in the chronic phase. In addition, high mI is found in demyelinated and remyelinated MS lesions, suggesting that mI probably reflects accumulation of myelin breakdown products in the acute phase and astrocytosis later on.18 A similar situation might occur in PML.
Our study has several limitations. A few patients with PML had negative JCV PCR in CSF, and PML was diagnosed using clinical and MRI criteria alone.8 This is necessary because PML is a rare disease, and the sensitivity of the JCV CSF PCR has dropped to 58% in cART-treated patients with PML.37 However, we are confident that these patients had the correct diagnosis, because all other infectious or tumoral pathologies were excluded, and the follow-up and immune responses to JCV were indistinguishable from those for patients with virologically or histologically proven cases.5 We could not compare metabolites to the contralateral region, because these were also often affected by PML. Almost all our patients have an identified underlying disease, which itself might cause metabolite changes. Correlation between 1H-MRS findings and histology could not be verified because no patients with PML-IRIS had a biopsy or autopsy at the time of ongoing IRIS. Although we were able to design a diagnostic model for the presence of IRIS, we did not have a second dataset to validate our findings.
This is the largest 1H-MRS study to date examining the features of PML-IRIS. Our observations are consistent with those made in other inflammatory and demyelinating diseases. Lipid/Cr ratios were elevated in patients with PML-IRIS regardless of the presence of CE, which suggest that these are a more consistent biomarker of PML-IRIS and might be explained by T-cell infiltrates in PML-IRIS lesions. Combining the Lip1/Cr ratio with CE allowed us to more accurately determine the probability of IRIS, which up to now constituted a challenge. 1H-MRS may become a valuable tool to diagnose and track IRIS and might help guide its clinical management.
ACKNOWLEDGMENT
The authors thank Evelyn Bord and Elizabeth Norton for excellent clinical coordination.
GLOSSARY
- ADEM
acute disseminated encephalomyelitis
- cART
combined antiretroviral therapy
- CE
contrast enhancement
- Cho
choline
- Cr
creatine
- 1H-MRS
proton magnetic resonance spectroscopy
- IRIS
immune reconstitution inflammatory syndrome
- JCV
JC virus
- Lip1
lipid/lactate
- Lip2
lipid/macromolecules
- mI
myo-inositol
- MS
multiple sclerosis
- NAA
N-acetylaspartate
- PML
progressive multifocal leukoencephalopathy
- PML-P
PML progressors
- PML-S
PML survivors
- VL
viral load
AUTHOR CONTRIBUTIONS
Sarah Gheuens was involved in patient care, imaging and immunologic studies, writing the first draft of the manuscript, and preparing the figures. Long Ngo was involved in statistical analysis and advice and revising the manuscript. Xiaoen Wang was involved in performing the imaging and collecting the MRS data and revising the manuscript. David C. Alsop was involved in performing the imaging and collecting the MRS data and revising the manuscript. Robert Lenkinski was involved in performing the imaging and collecting the MRS data and revising the manuscript. Igor J. Koralnik was involved in patient care, imaging and immunologic studies, supervising, editing, and revising the manuscript, and figure preparation.
DISCLOSURE
S. Gheuens is funded by NIH grant T32-AI07387-21 and is a fellow of the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co. L. Ngo reports no disclosures. X. Wang is supported in part by NIH grant R01-NS047029. D. Alsop is an inventor on several patents related to perfusion MRI (US patents 7,545,142, 7,369,888, 6,980,845, and 6,717,405), for which he has received royalties from GE Healthcare and Siemens Medical; receives research support from GE Healthcare and is also supported by grants from the NIH (CA115745, EB004582, MH80729, MH077073, DC008796, NS047029, CA101942, AG031720, AG028076, and DK084463) and the Congressionally Directed Military Research Program of the Department of Defense (SC090251); and serves as Associate Editor of Magnetic Resonance in Medicine. R. Lenkinski is supported in part by NIH grant R01-NS047029 and receives research support from GE Healthcare. I. Koralnik is funded by NIH grants R56-NS041198, R01-NS047029, and K24-NS060950; has received a research grant from Biogen Idec and the National Multiple Sclerosis Society; served on scientific advisory boards for Hoffmann La-Roche, GlaxoSmithKline, and Merck Serono; received consulting fees from Bristol-Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffmann La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceutical, and Johnson & Johnson; is an editorial board member for the Journal of NeuroVirology; and receives royalties from UpToDate for topics on the management of HIV and CNS mass lesions and on PML. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009; 72: 1458– 1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011; 77: 1061– 1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenning W, Haghikia A, Laubenberger J, et al. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med 2009; 361: 1075– 1080 [DOI] [PubMed] [Google Scholar]
- 4. Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol 2005; 109: 449– 455 [DOI] [PubMed] [Google Scholar]
- 5. Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with Immune reconstitution inflammatory syndrome. J Virol 2011; 85: 7256– 7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson T, Nath A. Neurological complications of immune reconstitution in HIV-infected populations. Ann NY Acad Sci 2010; 1184: 106– 120 [DOI] [PubMed] [Google Scholar]
- 7. Katz-Brull R, Lenkinski RE, Du Pasquier RA, Koralnik IJ. Elevation of myoinositol is associated with disease containment in progressive multifocal leukoencephalopathy. Neurology 2004; 63: 897– 900 [DOI] [PubMed] [Google Scholar]
- 8. Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol 2003; 9 (suppl 1): 88– 92 [DOI] [PubMed] [Google Scholar]
- 9. Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009; 73: 1551– 1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells Science 2004; 306: 1380– 1383 [DOI] [PubMed] [Google Scholar]
- 11. Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009; 53: 1840– 1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aksamit AJ. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol 2001; 7: 386– 390 [DOI] [PubMed] [Google Scholar]
- 13. Harrison DM, Newsome SD, Skolasky RL, McArthur JC, Nath A. Immune reconstitution is not a prognostic factor in progressive multifocal leukoencephalopathy. J Neuroimmunol 2011; 238: 81– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delikatny EJ, Chawla S, Leung DJ, Poptani H. MR-visible lipids and the tumor microenvironment. NMR Biomed 2011; 24: 592– 611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuvinciuc V, Martin-Blondel G, Marchou B, Bonneville F. Proton MR spectroscopy of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol 2010; 31: E69– E71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gheuens S, Smith DR, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Simultaneous PML-IRIS after discontinuation of natalizumab in an MS patient. Neurology 2012; 78: 1390– 1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koopmans RA, Li DK, Zhu G, Allen PS, Penn A, Paty DW. Magnetic resonance spectroscopy of multiple sclerosis: in-vivo detection of myelin breakdown products. Lancet 1993; 341: 631– 632 [DOI] [PubMed] [Google Scholar]
- 18. Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 1999; 20: 1619– 1627 [PMC free article] [PubMed] [Google Scholar]
- 19. De Stefano N, Matthews PM, Antel JP, Preul M, Francis G, Arnold DL. Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol 1995; 38: 901– 909 [DOI] [PubMed] [Google Scholar]
- 20. Kuker W, Ruff J, Gaertner S, Mehnert F, Mader I, Nagele T. Modern MRI tools for the characterization of acute demyelinating lesions: value of chemical shift and diffusion-weighted imaging. Neuroradiology 2004; 46: 421– 426 [DOI] [PubMed] [Google Scholar]
- 21. Gabis LV, Panasci DJ, Andriola MR, Huang W. Acute disseminated encephalomyelitis: an MRI/MRS longitudinal study. Pediatr Neurol 2004; 30: 324– 329 [DOI] [PubMed] [Google Scholar]
- 22. Harting I, Hartmann M, Jost G, et al. Differentiating primary central nervous system lymphoma from glioma in humans using localised proton magnetic resonance spectroscopy. Neurosci Lett 2003; 342: 163– 166 [DOI] [PubMed] [Google Scholar]
- 23. Burtscher IM, Skagerberg G, Geijer B, Englund E, Stahlberg F, Holtas S. Proton MR spectroscopy and preoperative diagnostic accuracy: an evaluation of intracranial mass lesions characterized by stereotactic biopsy findings. AJNR Am J Neuroradiol 2000; 21: 84– 93 [PMC free article] [PubMed] [Google Scholar]
- 24. King NJ, Delikatny EJ, Holmes KT. 1H magnetic resonance spectroscopy of primary human and murine cells of the myeloid lineage. Immunomethods 1994; 4: 188– 198 [DOI] [PubMed] [Google Scholar]
- 25. Dingley AJ, Veale MF, King NJ, King GF. Two-dimensional 1H NMR studies of membrane changes during the activation of primary T lymphocytes. Immunomethods 1994; 4: 127– 138 [DOI] [PubMed] [Google Scholar]
- 26. Simone IL, Federico F, Tortorella C, et al. Localised 1H-MR spectroscopy for metabolic characterisation of diffuse and focal brain lesions in patients infected with HIV. J Neurol Neurosurg Psychiatry 1998; 64: 516– 523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wattjes MP, Harzheim M, Lutterbey GG, Bogdanow M, Schild HH, Traber F. High field MR imaging and 1H-MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis: correlation between metabolic alterations and diagnostic MR imaging criteria. J Neurol 2008; 255: 56– 63 [DOI] [PubMed] [Google Scholar]
- 28. Ben Sira L, Miller E, Artzi M, Fattal-Valevski A, Constantini S, Ben Bashat D. 1H-MRS for the diagnosis of acute disseminated encephalomyelitis: insight into the acute-disease stage. Pediatr Radiol 2010; 40: 106– 113 [DOI] [PubMed] [Google Scholar]
- 29. Simone IL, Tortorella C, Federico F, et al. Axonal damage in multiple sclerosis plaques: a combined magnetic resonance imaging and 1H-magnetic resonance spectroscopy study. J Neurol Sci 2001; 182: 143– 150 [DOI] [PubMed] [Google Scholar]
- 30. Sastre-Garriga J, Ingle GT, Chard DT, et al. Metabolite changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch Neurol 2005; 62: 569– 573 [DOI] [PubMed] [Google Scholar]
- 31. Rigotti DJ, Gonen O, Grossman RI, et al. Global N-acetylaspartate declines even in benign multiple sclerosis. AJNR Am J Neuroradiol 2011; 32: 204– 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaaraoui W, Reuter F, Rico A, et al. Occurrence of neuronal dysfunction during the first 5 years of multiple sclerosis is associated with cognitive deterioration. J Neurol 2011; 258: 811– 819 [DOI] [PubMed] [Google Scholar]
- 33. Aboul-Enein F, Krssak M, Hoftberger R, Prayer D, Kristoferitsch W. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression: a study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One 2010; 5: e11625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnold DL, Matthews PM, Francis GS, O'Connor J, Antel JP. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol 1992; 31: 235– 241 [DOI] [PubMed] [Google Scholar]
- 35. Brenner RE, Munro PM, Williams SC, et al. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 1993; 29: 737– 745 [DOI] [PubMed] [Google Scholar]
- 36. Tartaglia MC, Narayanan S, De Stefano N, et al. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol 2002; 249: 1382– 1390 [DOI] [PubMed] [Google Scholar]
- 37. Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol 2005; 43: 4175– 4177 [DOI] [PMC free article] [PubMed] [Google Scholar]