Abstract
Renal angiomyoadenomatous tumour is a rare, recently described neoplasm with a distinctive histological appearance. Although reported in the pathology literature, to our knowledge, no prior reports have described its imaging appearance. We describe the computed tomography and magnetic resonance imaging features of an incidentally detected renal angiomyoadenomatous tumour that appeared as a well-marginated, solid T2-hypointense enhancing mass, in a 50-year-old woman. It is indistinguishable from a variety of benign and malignant renal neoplasms.
Introduction
Renal angiomyoadenomatous tumour is a rare neoplasm with a distinct histological appearance that differentiates it from other neoplasms of the kidney.1,2 It is typically composed of a characteristic clear cell epithelial component admixed with a prominent smooth muscle stroma.2 There are other renal neoplasms that may demonstrate epithelial and stromal elements, including a subset of clear cell renal cell carcinoma (RCC),3 angiomyolipoma2 and mixed epithelial and stromal tumor of the kidney.4 The stromal component, especially in angiomyolipoma, may overlap morphologically with the renal angiomyoadenomatous tumour. However, a renal angiomyoadenomatous tumour has a distinct epithelial component characterized architecturally by nests and tubules of epithelial cells that are embedded in a characteristic stroma, and individually surrounded by a distinct vascular pattern and a prominent capsule.1,2 The epithelial cells consist of cuboidal to columnar clear cells containing basally located nuclei.
Renal angiomyoadenomatous tumour was originally reported in 2000;1 since then a single case series and case report have described the histological features.2,5 To the best of our knowledge, the imaging appearance of renal angiomyoadenomatous tumour has not been formally described. The objective of this report was to present the computed tomography (CT) and magnetic resonance imaging (MRI) findings in a patient with renal angiomyoadenomatous tumour.
Case report
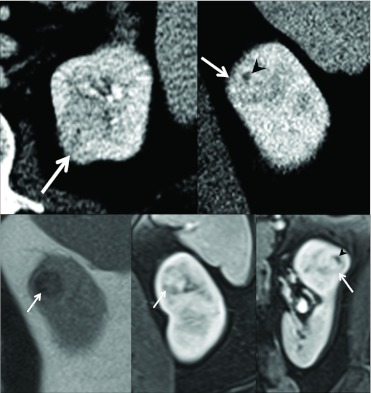
A 50-year-old woman was found to have an incidental renal mass on an abdominal CT scan performed for chronic abdominal pain at an outside institution (Fig. 1). A well-marginated 2.9 × 2.4 × 2.3 cm mass arose from the upper pole of the left kidney. The mass was centrally and exophytically located and abutted renal sinus fat. No calcification or fat was evident. A small area of low density within it measuring 0.9 × 0.7 cm represented either cystic change or necrosis. There was no involvement of the ipsilateral renal vein or lymphadenopathy, nor was there distant metastases. No other solid lesions were seen in either kidney. After the administration of intravenous iodinated contrast material, the mass was slightly hypodense (147 HU) to renal parenchyma in the nephrographic phase.
Fig. 1.
A 50-year-old woman with renal angiomyoadenomatous tumor of left kidney. A and B, Axial (A) and coronal (B) CT scan during nephrographic phase demonstrates 2.9 cm intraparenchymal enhancing mass (arrows). Central low density likely represents cystic change (arrowhead, B). C, Coronal T2-weighted magnetic resonance image shows T2-hypointense intraparenchymal lesion (arrow). D and E, Coronal (D) and sagittal (E) T1-weighted sequence in nephrographic phase shows enhancement of the mass (arrows). Central area of non enhancement likely represents cystic change (arrowhead, E).
On MRI, the mass appeared isointense on T1-weighted images and hypointense on T2-weighted images. After administration of intravenous gadolinium chelate, the mass enhanced heterogeneously by 245% and 83% in the nephrographic and excretory phases, respectively. The small area of low density seen on the CT scan demonstrated no enhancement.
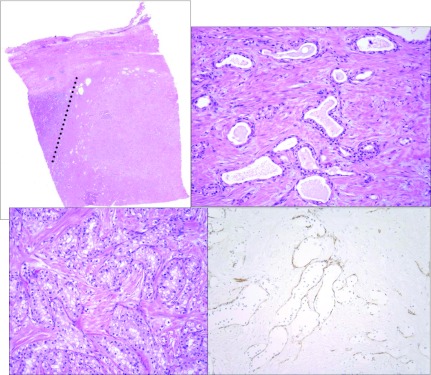
The patient initially had a CT-guided percutaneous fine and core needle biopsy that revealed only fibromuscular stroma and unremarkable renal parenchyma. The smooth muscle marker, desmin, was immunoreactive in the stroma, but human melanosme B-45 (HMB45) immunostain, typically immunoreactive in angiomyolipomas, was negative. The possibility of a smooth muscle predominant angiomyolipoma was raised, however, a RCC was also considered as rare clear epithelial cells were found on the concurrent fine needle biopsy specimens. Subsequently, the patient underwent partial nephrectomy. A 5.0 cm surgical specimen was received which contained a 3.1 cm well-circumscribed mass with a white whorled surface; no hemorrhage, fat or necrosis was identified (Fig. 2). Morphologically the tumour contained branching tubules and nests of renal epithelial cells with clear cytoplasm. These epithelial structures were dispersed in an associated smooth muscle stroma that also formed a prominent capsule around the tumour. Epithelial nests, tubules and microcysts (up to 0.2 cm) were each characteristically surrounded by individual small capillaries that were revealed by hematoxylin and eosin stain and with the vascular marker CD31. Normal renal structures (glomeruli and tubules) were entrapped by the tumour.
Fig. 2.
Morphologic features of the renal angiomyoadenomatous tumor. A, Low power image of the tumour demonstrates the solid growth pattern and the overlying well-circumscribed capsule. Unremarkable renal parenchyma is present to the left of the dotted line. B and C, Epithelial structures lined by cuboidal to columnar cells with clear cytoplasm embedded in a smooth muscle stroma. The ratio of epithelial to stromal components varies throughout the tumour (hematoxylin and eosin stain). D, CD31 immunostain highlights the prominent vascular pattern that surrounds the epithelial structures (this can also be seen in B and C).
Discussion
Renal angiomyoadenomatous tumour is a rare neoplasm of the kidney. Although relatively recently reported in the pathology literature,1,2,6,7 to the best of our knowledge, its imaging appearance has not been formally described.
Radiologically, no specific feature was identified that was unique to this entity. Our patient demonstrated a markedly enhancing mass that was isointense and hypointense on T1 and T2-weighted imaging, respectively. The pronounced enhancement seen on imaging likely correlated to the distinct vascular network seen pathologically. We did not identify a capsule on CT or MRI despite the presence of a prominent capsule on histological evaluation.
The imaging appearance of renal angiomyoadenomatous tumour was not specific as several other renal neoplasms may have a similar appearance. The two most common are papillary RCC and angiomyolipoma with minimal fat. The low T2 signal of papillary carcinoma is well-documented8–10 and has been shown to be due to papillary architecture with contribution from the presence of hemosiderin in some cases.8 However, papillary renal cell carcinoma typically is hypovascular and does not enhance markedly as seen in this case.11 Renal angiomyoadenomatous tumour is more likely to be confused with angiomyolipoma with minimal fat. It is also T2 hypointense due to its predominant smooth muscle content12 and like renal angiomyoadenomatous tumour, angiomyolipoma with minimal fat typically enhances avidly.13 Other enhancing masses that may demonstrate a low signal on T2-weighted imaging include capsular leiomyoma,14,15 metanephric adenoma,16 solitary fibrous tumour17 and mucinous tubular and spindle cell carcinoma of the kidney.18 Finally, clear cell RCC is typically hyperintense on T2-weighted imaging,19 but may demonstrate T2 hypointensity if it has undergone internal hemorrhage. Therefore, when an enhancing T2 hypointense mass is encountered, a percutaneous biopsy is recommended to differentiate between benign and malignant pathologies.20 Obtaining a prospective diagnosis prior to surgery would be useful for guiding management.
Mixed epithelial and stromal tumour of the kidney is another neoplasm that demonstrates both epithelial and stromal components. Unlike renal angiomyoadenomatous tumour, mixed epithelial and stromal tumours often demonstrate significant cyst formation as well as interspersed areas of solid growth and presents as a multiloculated cystic mass. Not surprisingly, given the contrasting gross and histologic features of these two entities, the imaging appearances are very different. In our experience, mixed epithelial and stromal tumour of the kidney typically manifests as a multiseptated cystic lesion usually classified as either Bosniak category III or IV.21
Little is known about the epidemiology and natural history of this entity due to its rarity, although an association with clear cell tubulopapillary RCC (also referred to as clear cell papillary RCC) and end stage kidney disease has been raised. Michal and colleagues reported the pathological features of 5 patients;2 there was male predominance (4 patients) with a mean age of 64.6 years (range: 49 to 93). The mean tumour size was slightly larger than the current case at 4.1 cm (range: 2.3 to 8.5). At gross pathological examination all tumours demonstrated microcystic change without necrosis, with 1 patient demonstrating marked cystic change. Microcysts up to 0.2 cm in greatest dimension were noted in the current case.
Venugopal and colleagues reported a single case of renal angiomyoadenomatous tumour.5 No formal evaluation of the radiological appearances was presented; however, from limited images, there appeared to be some differences in imaging features. The mass appeared ill-defined with peripheral cystic elements and was hyperintense on T2-weighted MRI. No comment on enhancement after intravenous contrast material administration was made. Pathologically, similar to our case, there were no malignant features, such as cellular atypia, mitoses, necrosis or vascular invasion.
Renal angiomyoadenomatous tumour is felt to represent a benign neoplasm, but there has been reluctance to characterize it definitively as such due to the few reported cases in the literature and the limited follow-up available. All cases reported by Michal and colleagues2 showed no atypia or mitoses on microscopic examination. Four of the patients were followed. One patient died of metastatic colonic adenocarcinoma 29 months after nephrectomy with no evidence of recurrence of the renal tumour. The other 3 patients were without recurrence 8, 9 and 12 months post-resection, respectively. Our patient also demonstrated pathological features of a benign neoplasm. Clinical follow-up 6 months after resection demonstrated no evidence of recurrence.
Conclusion
Renal angiomyoadenomatous tumour appears as a well-marginated, solid enhancing renal mass. It should be included in the differential diagnosis of a solid renal neoplasm. Although it is difficult to make recommendations based on a single case report when encountering a small (<3 cm) markedly enhancing T2 hypointense mass, angiomyolipoma with minimal fat and renal angiomyoadenomatous tumor are both considered and a biopsy is indicated. The imaging appearances of additional patients with this type of tumour need to be reviewed to determine whether the appearance described herein is typical.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Michal M, Hes O, Havlicek F. Benign renal angiomyoadenomatous tumor: a previously unreported renal tumor. Ann Diagn Pathol. 2000;4:311–5. doi: 10.1053/adpa.2000.17890. [DOI] [PubMed] [Google Scholar]
- 2.Michal M, Hes O, Nemcova J, et al. Renal angiomyoadenomatous tumor: morphologic, immunohistochemical, and molecular genetic study of a distinct entity. Virchows Arch. 2009;454:89–99. doi: 10.1007/s00428-008-0697-3. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn E, De Anda J, Manoni S, et al. Renal cell carcinoma associated with prominent angioleiomyoma-like proliferation. Report of 5 cases and review of the literature. Am J Surg Pathol. 2006;30:1372–81. doi: 10.1097/01.pas.0000213277.45715.82. [DOI] [PubMed] [Google Scholar]
- 4.Michal M, Hes O, Bisceglia M, et al. Mixed epithelial and stromal tumors of the kidney. A report of 22 cases. Virchows Arch. 2004;445:359–67. doi: 10.1007/s00428-004-1060-y. [DOI] [PubMed] [Google Scholar]
- 5.Venugopal S, Hamid B, Doyle G, et al. Renal angiomyoadenomatoid tumor. Urology. 2011;78:327–8. doi: 10.1016/j.urology.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda N, Michal M, Hes O, et al. Renal angiomyoadenomatous tumor: fluorescence in situ hybridization. Pathol Int. 2009;59:689–91. doi: 10.1111/j.1440-1827.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda N, Hosokawa T, Michal M, et al. Clear cell renal cell carcinoma with focal renal angiomyoadenomatous tumor-like area. Ann Diagn Pathol. 2011;15:202–6. doi: 10.1016/j.anndiagpath.2010.03.003. Epub 2010 Jun 17. [DOI] [PubMed] [Google Scholar]
- 8.Oliva MR, Glickman JN, Zou KH, et al. Renal cell carcinoma: T1 and T2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am J Roentgenol. 2009;192:1524–30. doi: 10.2214/AJR.08.1727. [DOI] [PubMed] [Google Scholar]
- 9.Shinmoto H, Yuasa Y, Tanimoto A, et al. Small renal cell carcinoma: MRI with pathological correlation. J Magn Reson Imaging. 1998;8:690–4. doi: 10.1002/jmri.1880080327. [DOI] [PubMed] [Google Scholar]
- 10.Sussman SK, Glickstein MF, Krzymowski GA. Hypointense renal cell carcinoma: MR imaging with pathological correlation. Radiology. 1990;177:495–7. doi: 10.1148/radiology.177.2.2217790. [DOI] [PubMed] [Google Scholar]
- 11.Jinzaki M, Tanimoto A, Mukai M, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathological findings and tumor angiogenesis. J Comput Assist Tomogr. 2000;24:835–42. doi: 10.1097/00004728-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jinzaki M, Tanimoto A, Narimatsu Y, et al. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997;205:497–502. doi: 10.1148/radiology.205.2.9356635. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Park SY, Shon JH, et al. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology. 2004;230:677–84. doi: 10.1148/radiol.2303030003. [DOI] [PubMed] [Google Scholar]
- 14.Sahni VA, Ly A, Silverman SG. Usefulness of percutaneous biopsy in diagnosing benign renal masses that mimic malignancy. Abdom Imaging. 2010;36:91–101. doi: 10.1007/s00261-009-9597-5. [DOI] [PubMed] [Google Scholar]
- 15.Katabathina VS, Raghunandan V, Nagar AM, et al. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525–40. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 16.Bastide C, Rambeaud J-J, Bach AM, et al. Metanephric adenoma of the kidney: clinical and radiological study of nine cases. BJU Int. 2009;103:1544–8. doi: 10.1111/j.1464-410X.2009.08357.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TR, Pedrosa I, Goldsmith J, et al. Magnetic resonance imaging findings in solitary fibrous tumor of the kidney. J Comput Assist Tomogr. 2005;29:481–3. doi: 10.1097/01.rct.0000166637.24037.41. [DOI] [PubMed] [Google Scholar]
- 18.Noon AP, Smith DJ, McAndrew P. Magnetic resonance imaging characterization of a mucinous tubular and spindle cell carcinoma of the kidney detected incidentally during an ectopic pregnancy. Urology. 2010;75:247–8. doi: 10.1016/j.urology.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Shinmoto H, Yuasa Y, Tanimoto A, et al. Small renal cell carcinoma: MRI with pathological correlation. J Magn Reson Imaging. 1998;8:690–4. doi: 10.1002/jmri.1880080327. [DOI] [PubMed] [Google Scholar]
- 20.Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9:44–55. doi: 10.1102/1470-7330.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahni VA, Mortele KJ, Glickman J, et al. Mixed epithelial and stromal tumour of the kidney: imaging features. BJU Int. 2010;105:932–9. doi: 10.1111/j.1464-410X.2009.08918.x. [DOI] [PubMed] [Google Scholar]