Abstract
Background
Vascular dysfunction in atherosclerosis and diabetes, as observed in the aging population of developed societies, is associated with vascular DNA damage and cell senescence. We hypothesized that cumulative DNA damage during aging contributes to vascular dysfunction.
Methods and Results
In mice with genomic instability due to the defective nucleotide excision repair genes ERCC1 and XPD (Ercc1d/− and XpdTTD mice), we explored age-dependent vascular function as compared to wild-type mice. Ercc1d/− mice showed increased vascular cell senescence, accelerated development of vasodilator dysfunction, increased vascular stiffness and elevated blood pressure at very young age. The vasodilator dysfunction was due to decreased endothelial eNOS levels as well as impaired smooth muscle cell function, which involved phosphodiesterase (PDE) activity. Similar to Ercc1d/− mice, age-related endothelium-dependent vasodilator dysfunction in XpdTTD animals was increased. To investigate the implications for human vascular disease, we explored associations between single nucleotide polymorphisms (SNPs) of selected nucleotide excision repair genes and arterial stiffness within the AortaGen Consortium, and found a significant association of a SNP (rs2029298) in the putative promoter region of DDB2 gene with carotid-femoral pulse wave velocity.
Conclusions
Mice with genomic instability recapitulate age-dependent vascular dysfunction as observed in animal models and in humans, but with an accelerated progression, as compared to wild type mice. In addition, we found associations between variations in human DNA repair genes and markers for vascular stiffness which is associated with aging. Our study supports the concept that genomic instability contributes importantly to the development of cardiovascular disease.
Keywords: aging, cardiovascular disease, endothelial dysfunction, nitric oxide synthase, vasodilation
Introduction
Vascular and endothelial function deteriorates with age and is considered a key factor in the development and progression of age-related cardiovascular disease (CVD).1–3 The high prevalence of CVD-related mortality due to increasing life expectancy highlights the necessity of understanding how aging influences vascular function. Currently, aging is viewed as a consequence of the prolonged exposure to risk factors e.g., an unfavourable lipid profile, smoking and diabetes, during which accumulation of damage increases the risk to develop vascular dysfunction and associated disease.3 At the cellular level this could be related to the increased production of reactive oxygen species (ROS) and a resulting increase in lipid oxidation or interference with cellular metabolism. This can directly affect vascular function and/or lead to apoptosis or cellular senescence, a state in which the cell remains in cell cycle arrest and has lost its optimal function. In the case of endothelial cells (EC), this functional change results in a pro-vasoconstrictor and pro-inflammatory phenotype.4
Despite extensive research into oxidative stress-induced cellular damage and senescence, a main causative mechanism of aging and age-related CVD remains unknown. It is for instance unclear why vasomotor function declines with aging, even in the absence of apparent risk factors.5 Moreover, in the vast majority of epidemiological studies, aging remains the most significant risk factor for CVD, even after correction for classical cardiovascular risk factors.3
The concept that unrepaired DNA damage has dramatic effects on the aging phenotype stems from multiple lines of evidence, including the fact that the majority of human progeroid syndromes are due to mutations in DNA damage repair and response genes.6 One of the DNA repair systems that is very important in this regard is Nucleotide Excision Repair (NER), which removes a wide class of helix-distorting DNA lesions induced by UV, but also numerous man-made or natural chemical compounds and ROS. The process of DNA damage removal consists of 1) DNA damage recognition by XPC for some lesions assisted by the UV-DDB1/2 (XPE) complex, 2) local unwinding of DNA provided by the multi-subunit TFIIH complex, 3) damage verification by XPA, 4) excision of the damaged DNA section by endonucleases Xpg and Ercc1/Xpf and 5) replacement of the excised DNA using the intact strand as template. Mutation of factors involved in NER can have severe consequences for human health as demonstrated by several human progeroid syndromes. Examples are the rare autosomal recessive, genetic disorders Cockayne syndrome (CS), trichothiodystrophy (TTD) and the recently described Xpf-Ercc1 (XFE1) syndrome.7 For instance, TTD is caused by point mutations in the XPD, XPB or TTDA genes, affecting DNA repair function and stability of the dual functional NER/basal transcription initiation factor TFIIH.8 This causes UV sensitivity and accelerated segmental aging symptoms, including early cessation of growth, cachexia, osteoporosis, progressive neurological abnormalities and premature death.9 Likewise, several mouse models with NER defects show a segmental premature aging phenotype, where the severity depends on the extent to which the DNA repair system is affected.
To investigate whether DNA damage plays a role in age-related vascular dysfunction, we studied vasomotor function and cellular senescence in two NER-defect mouse models, differing in type and severity of the DNA repair defect. In Ercc1d/− animals, one allele of the NER-DNA crosslink repair (XLR) endonuclease ERCC1 is mutated resulting in a truncated protein (lacking the C-terminal 7 amino acids) while the other allele is completely inactivated10. In XpdTTD mice, the XPD helicase of the TFIIH core complex carries a homozygous R722W functional pointmutation as found in a TTD patient.11 The NER-XLR defect in the Ercc1d/− animals is more severe, resulting in very early cessation of growth, very premature liver, kidney, bone marrow and neurological aging phenotype, and a reduced lifespan of approximately 5–6 months. The milder phenotype of XpdTTD mice results in retarded growth, cachexia, an age-related osteoporosis, and a slightly reduced lifespan.
To investigate if NER gene variations could have an impact on human vascular disease and in line with our murine phenotype, we performed genetic studies to examine the association of genetic variation in genes coding for proteins involved in NER with carotid-femoral pulse wave velocity (CFPWV). The associations between genetic variation in selected NER genes and the vascular phenotype were assessed within the framework of the ArotaGen Consortium.12
Materials and Methods
For details of the experimental setup, see the online-only Data Supplement.
Animals
The animals used in experiments were 8- and 16-week-old Ercc1d/− mice, their wild-type littermates of the same age (WT), and 16-, 26- and 52-week-old mice of the same background -F1 hybrid between Fvb and C57Bl/6 and 26- and 52-week-old XpdTTD mice and their WT controls in a C57Bl/6 background. All animal studies were approved by an independent Animal Ethical Committee.
Isolation and culture of endothelial cells
Endothelial cells were isolated from 16-week-old Ercc1d/− mice and cultured under mouse lung endothelial cell medium under atmosphere of normal air enriched with 5% CO2.
Senescence-associated β-galactosidase staining
Senescence was determined by senescence-associated β-galactosidase staining (SA-β-gal staining) at pH 6.0.
Quantitative real-time PCR
Relative expression of cyclin-dependent kinase inhibitor 1A (p21) and tumor protein 53 (p53) genes was measured in thoracic aortas of 16 week old Ercc1d/− and WT mice.
Assessment of blood pressure and vasodilator function in vivo
In vivo hindleg vasodilator function was measured by Laser Doppler perfusion imaging of reactive hyperemia after transient blood flow interruption, in 8-week-old Ercc1d/− and WT mice.
In the same mice blood pressure was measured in conscious Ercc1d/− mice and WT littermates using the tail cuff technique.
Organ bath experiments
The responses of aortic tissue of 8- and 16-week-old Ercc1d/− mice and their, 16-, 26- and 52-week-old WT littermates as well as 26- and 52-week-old XpdTTD mice and their WT littermates were measured in small wire myograph organ baths containing oxygenated Krebs-Henseleit buffer of 37°C. Following preconstriction with 30 nmol/L U46619, relaxation concentration-response curves (CRCs) were constructed to acetylcholine, followed by an exposure to 100 μmol/L sodium nitroprusside. L-NAME 100 μmol/L pretreatment was used to investigate the involvement of nitric oxide (NO). 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) 100 μmol/L and N-acetyl-cysteine (NAC, 30 μmol/L) were used as scavengers of reactive oxygen species (ROS). Tetrahydrobiopterin (BH4, 100 μmol/L) was used to prevent the uncoupling of eNOS. Vinpocetine (100 μmol/L) on was used to investigate phosphodiesterase (PDE) activity.
Measurement of mechanical properties of the vascular wall
Carotid arteries were explanted from 16-week-old Ercc1d/− and WT mice and mounted in the perfusion myograph. The vessel diameter – pressure relationship was determined and stress-strain relationships were constructed.13
Immunoprecipitation and immunoblotting of eNOS
Aortas and hearts were used to investigate the levels of eNOS and the fraction of eNOS phosphorylated at position Tyr-657. Ser-1177 e NOS phosphorylation was investigated in lungs, either at baseline or after 10 minutes of stimulation with 10 μmol/L acetylcholine.
Statistical methods of animal studies
Data are presented as mean±SEM. Statistical analysis between the groups of single values was performed by two-sided t-test (*), two-sided t-test after log transformation of the data (#), or Mann-Whitney U test (†) or 1-way ANOVA followed by Bonferroni’s post-hoc test, where appropriate (*). To test the hypothesis that blood pressure would be increased in Ercc1d/−animals, we employed a one-sided t-test. Differences in dose-response curves were tested by ANOVA for repeated measures (sphericity assumed, *). Differences were considered significant at p<0.05.
Human studies
In accordance with the phenotype observed in mice, we investigated the association of SNPs in NER components with carotid-femoral pulse wave velocity (CFPWV), a measure of vascular stiffness. CFPWV is a well known marker of age-related vascular disease in humans and is strongly associated with increased risk for major CVD events. To investigate this association, we used the data from the AortaGen Consortium which consists of 20,634 participants from 9 cohort studies. A detailed description of the AortaGen Consortium is provided in the online-only DataSupplement.
Statistical methods of human studies
Genes coding for NER components that belong to the machinery that binds the DNA to either recognize or repair damage were selected. These were the following NER components: ERCC8 (CSA) ERCC6 (CSB); DDB1; DDB2 (XPE); ERCC1; GTF2H1 (p62); GTF2H3 (p34); GTF2H4 (p52); GTF2H5 (TTDA, TFB5); RAD23A (hHR23A); RAD23B (hHR23B); ERCC3 (XPB); XPC; ERCC2 (XPD); ERCC4 (XPF); ERCC5 (XPG). To test for association with CFPWV, we selected the tag SNPs that cover the variation in the genes of interest ± 50 kb region around and that were non-redundant at linkage disequilibrium (LD) threshold of r2 ≥ 0.7 using the Tagger program of Haploview. Our selection resulted in 310 SNPs. We decided a priori on a significance threshold of p < 1.61×10−4, which corresponds to the Bonferroni adjusted p value for the number of tested SNPs.
Results
Vascular cell aging in Ercc1d/− mice
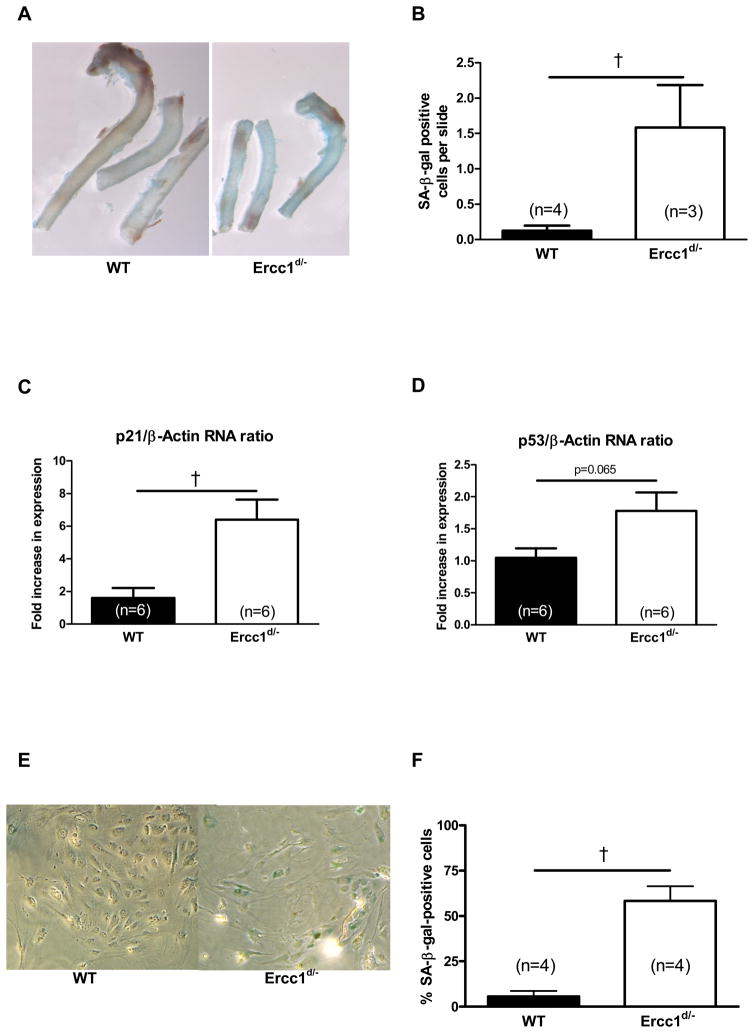
SA-β-gal staining of 16-week-old Ercc1d/− mice versus their wild-type littermates (WT) aorta showed that, macroscopically, senescence staining clearly dominated in aortas from Ercc1d/−(Figure 1A). Microscopically, stained cells were detected in both the endothelium and the media of Ercc1d/− aortas. The quantity and visibility of senescent cells allowed reliable counting in the media, showing a marked increase in Ercc1d/− animals (Figure 1B). Similarly, RNA levels of the genes composing DNA damage-related CDK inhibition and p21 (Cdkn1a) were increased and p53 (Trp53) tended to increase in the aorta of Ercc1d/− (Figures 1C, 1D).
Figure 1.
Senescence in Ercc1d/− mice vascular tissue. The thoracic aorta wall of Ercc1d/− and WT mice stained with SA-β-gal staining (A). Quantification of SA-β-gal positive cells in the lamina media of thoracic aorta (B). Aortic RNA levels of senescence markers p21 (C) and p53 (D). Percentage of SA-β-gal positive cells after prolonged culture of isolated lung endothelial cells in Ercc1d/− compared to WT mice (E, F). †= p<0.05 (Mann-Whitney U test).
To further investigate the effect of defective NER on proliferative senescence in endothelial cells, we measured the percentage of SA-β-gal-positive cells after 20 days in culture. The levels of SA-β-gal-positive endothelial cells in the lung were on average 10.3 times higher in cultures from Ercc1d/− versus WT mice (Figure 1 E, F).
Based on the robust proliferation of freshly cultured lung endothelial cells, we decided to test endothelial-dependent angiogenic outgrowth potential in aortic explants of 16-week-old Ercc1d/− and WT mice. No significant difference in outgrowth was observed (Supplemental Figure 1).
In vivo vascular function of NER-defective and WT mice
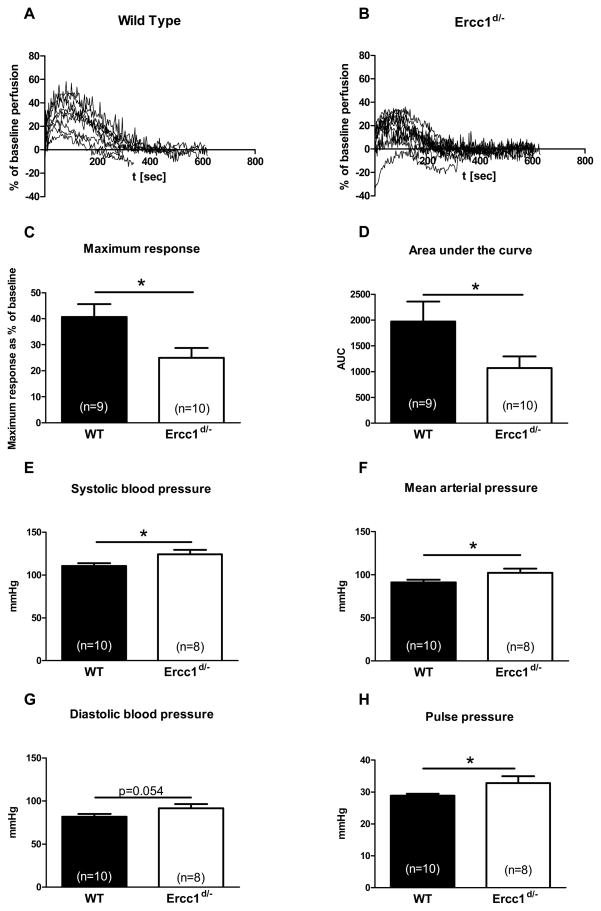
To determine possible functional changes in the vasculature we assessed vasodilator function in response to reactive hyperemia in the hindlimbs of 8-week-old WT (Figure 2A) and Ercc1d/−mice (Figure 2B). In 8-week-old Ercc1d/− animals we observed a decreased reactive hyperemia (Figure 2C, 2D). In addition we observed a significant increase in systolic pressure, mean arterial pressure (MAP) and pulse pressure in Ercc1d/− mice (Figures 2E, 2F, 2G). Diastolic blood pressure tended to increase, albeit without statistical significance (Figure 2H).
Figure 2.
Measures of central and peripheral hemodynamics in 8 week old Ercc1d/− and WT animals. Functional Differences between skin reperfusion after 2 minutes of occlusion between WT (A) and Ercc1d/− (B), average maximum response (C), as well as area under the curve (D). Differences in systolic, mean, diastolic blood pressure (E, F, G) and pulse pressure (H) between 8 week old Ercc1d/− and their WT. *= p<0.05 (t-test).
Age-dependent change of ex vivo vascular function in WT and NER-defective mice
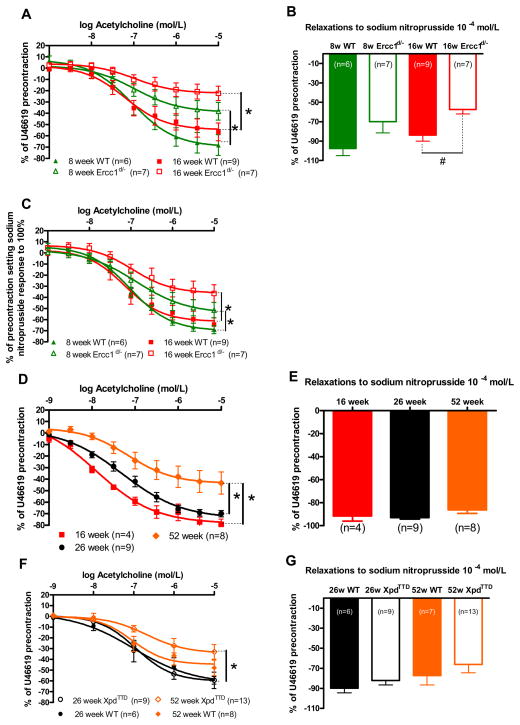
To reveal the mechanisms of vasodilator dysfunction in Ercc1d/− mice and to address the question of age-dependency, we compared vasodilator function in 8- and 16-week-old mice. At both ages, mice show signs of progeria, without obvious deterioration in general health. Ercc1d/− animals showed progressive reduction of acetylcholine-induced aortic relaxation at these ages (Figure 3A). Sodium nitroprusside respones were reduced in 16-week-old Ercc1d/− mice and tended to be decreased in 8-week-old Ercc1d/− mice (Figure 3B). To estimate the contribution of the endothelium to vasodilator dysfunction, acetylcholine-induced relaxations were corrected for sodium nitroprusside responses, revealing that the endothelial contribution to the response to acetylcholine was reduced in Ercc1d/− versus WT at both ages (Figure 3C).
Figure 3.
Ex-vivo vascular function in progeria models and aging models. Vasodilatations to (A) acetylcholine and (B) sodium nitroprusside in U46619-precontracted isolated aortic rings of 8 and 16 week old Ercc1d/− vs. WT, measured in organ bath setups. To express the contribution of the endothelium, acetylcholine responses were expressed as % of the response to sodium nitroprusside (C). Dilator responses to acetylcholine (D) and sodium nitroprusside (E) of WT mouse isolated aortic rings precontracted with 3×10−8 mol/L U46619, measured at 16, 26 and 52 weeks of age. Vasodilatations to (F) acetylcholine and (G) sodium nitroprusside in U46619-precontracted isolated aortic rings of 26 and 52 week old XpdTTD vs. WT. #,*= p<0.05 (t-test on log transformed values, general linear model repeated – measures).
DNA repair competent WT animals of 16, 26 and 52 weeks of age showed a much slower age-dependent reduction in acetylcholine responses than Ercc1d/−, becoming statistically significant after 52 weeks (Figure 3D). Endothelium-independent responses to sodium nitroprusside did not change in WT (Figure 3E).
To determine whether or not a slower onset of progeria could delay the onset of vascular dysfunction, we assessed vascular function in the NER impaired XpdTTD mouse that displays a milder phenotype. Vasodilator responses to acetylcholine in U46619-precontracted aortic rings were significantly reduced in 52-week-old XPDTTD mice compared to those at 26 weeks, and more markedly than in WT littermates (Figure 3F). The noticeable, modest difference between 52-week-old XpdTTD and WT animals did not reach significance. Endothelial-independent responses to sodium nitroprusside were equal (Figure 3G).
Pretreatment with the endothelial nitric oxide synthase (eNOS) inhibitor, L-NAME, abolished all acetylcholine-induced relaxations in all animals (data not shown), indicating that acetylcholine responses entirely depended on eNOS/NO.
Mechanisms of endothelial vasodilator dysfunction in NER-defective mice
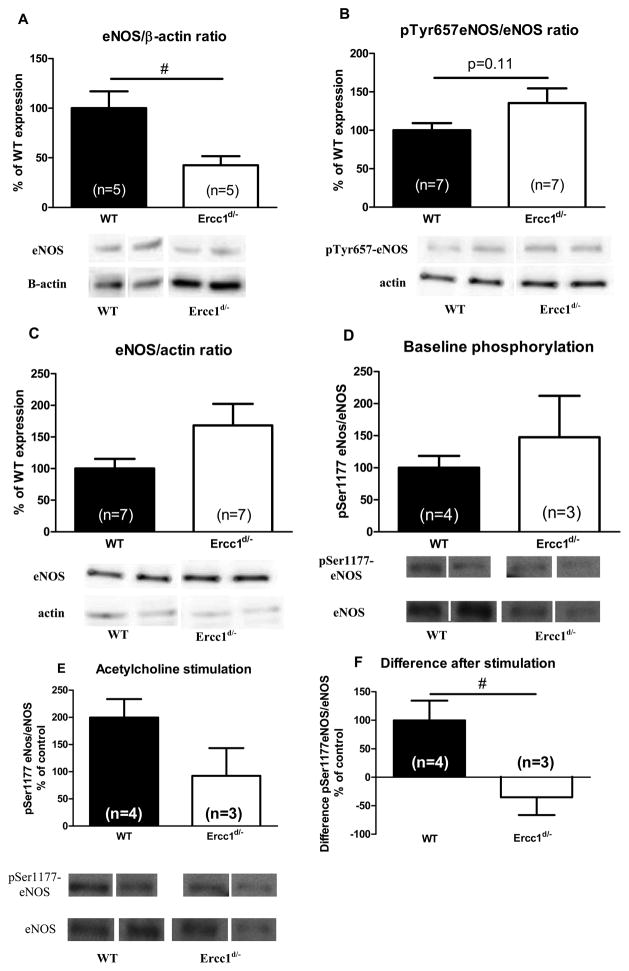
In aortas from 16 week old Ercc1d/− mice eNOS levels were reduced by approximately 67% compared to WT (Figure 4A). Phosphorylation of the tyrosine residue at position 657 (pTyr657-eNOS) inhibits eNOS,14 and this tyrosine phosphorylation tended to be increased in Ercc1d/−hearts (Figure 4B). eNOS-activating phosphorylation of the serine residue at position 1177 (pSer1177-eNOS) was comparable in explanted WT and Ercc1d/− lungs at baseline, but 10 μmol/L acetylcholine only increased pSer1177-eNOS in WT (Figure 4E).
Figure 4.
eNOS in aorta and myocardium of Ercc1d/− mice. Protein levels of eNOS in 16 week old Ercc1d/− animals and their WT littermates, in (A) aorta, and (B, C) cardiac ventricles. Phosphorylation of Ser1177-eNOS in lungs of 16 week old Ercc1d/− animals, and their WT littermates on baseline (D), and upon stimulation with 10−5 mol/L Ach (E). The difference in phosphorylation before and after stimulation (F). # = p<0.05 (t-test on log transformed values). Lanes were run on the same gel but are noncontiguous.
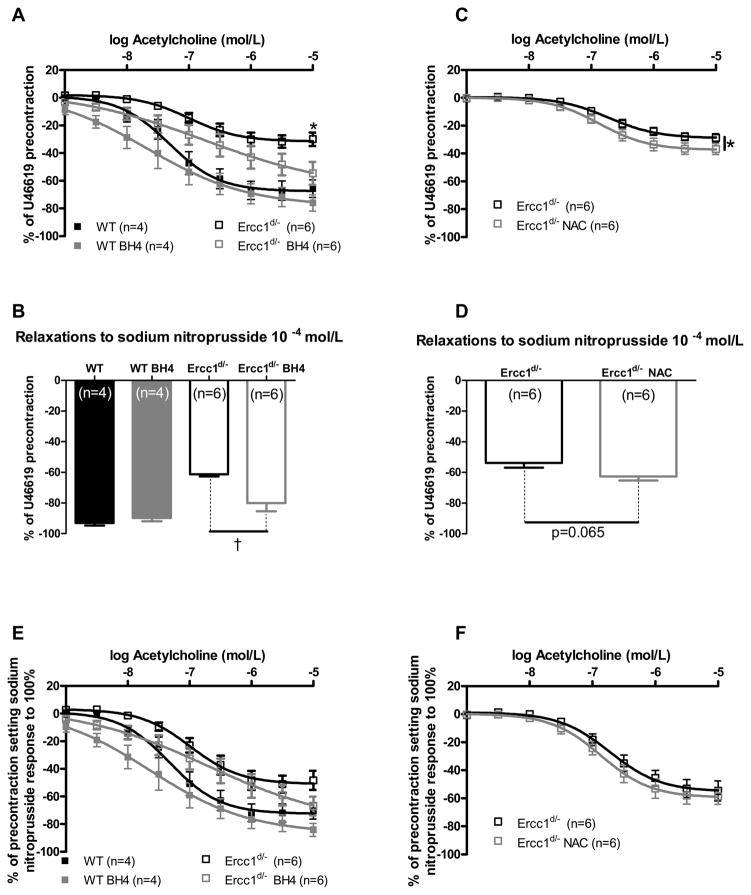
Uncoupling of eNOS results in a switch from NO to ROS production. It can be a consequence of the decreased availability of the essential co-factor tetrahydrobiopterin (BH4), and its reconstitution can restore NO production.15 BH4 had no effect on the acetylcholine-induced relaxation of aortic rings from WT mice but restored that of rings from Ercc1d/− mice (Figure 5A). BH4 also increased endothelium-independent sodium nitroprusside responses (Figure 5B) in Ercc1d/−.
Figure 5.
Effects of ROS scavenging and prevention of eNOS uncoupling on ex-vivo vascular function. Effects of acute BH4 supplementation on responses to acetylcholine (A) and sodium nitroprusside (B) in aortic tissue of 16 week old Ercc1d/− and WT mice. The acetylcholine responses after correction for individual responses to sodium nitroprusside (E). The effect of acute NAC supplementation on responses of Ercc1d/− to acetylcholine (C) and sodium nitroprusside (D). Acetylcholine responses expressed as % of the sodium nitroprusside response (F). †= p<0.05 (Mann-Whitney U test).
ROS coming from various sources can scavenge NO, thereby leading to vasodilator dysfunction that can be rescued by ROS scavengers. Whereas tempol was without effect (Supplemental Figure 2A, B), NAC caused a modest and significant improvement of the acetylcholine and sodium nitroprusside responses in Ercc1d/− aortas (Figure 5C, D). After correction for sodium nitroprusside responses, acetylcholine responses were not changed by BH4 or by NAC (Figure 5E, F). Therefore, the BH4- and NAC-induced improvement of relaxations most likely represents improved VSMC sensitivity.
Mechanisms of VSMC vasodilator dysfunction in NER-defective mice
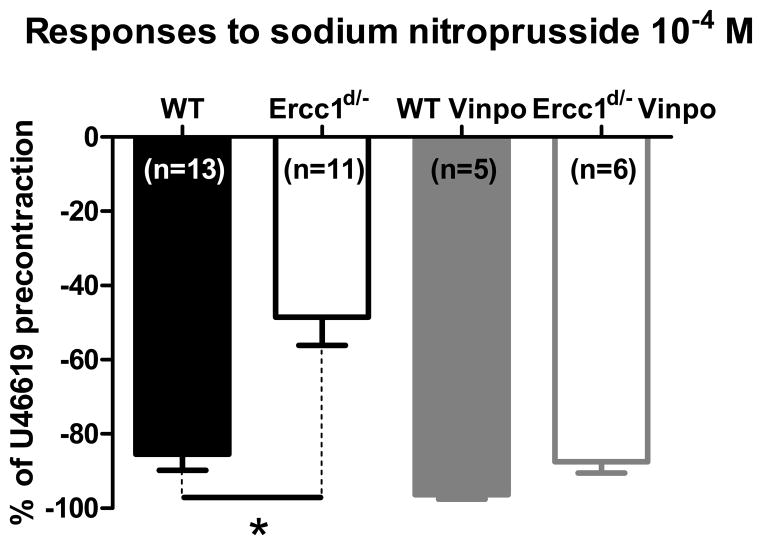
PDE inhibitor vinpocetine improved sodium nitroprusside responses in Ercc1d/− mice (Figure 6). PDE5-specific inhibitor sildenafil had similar effects (data not shown, n=5). The responses to an activator of protein kinase G (PKG) remained unchanged in Ercc1d/− and WT littermates (n=9 per group; logarithm of concentration needed to reach 50% dilation: −5.268±0.2353 vs. −5.569±0.1957 respectively).
Figure 6.
Effect of PDE inhibition vinpocetine on endothelium-independent vasodilatation. Ercc1d/− mice show reduced vasodilatory response to sodium nitroprusside 10−4 mol/L, when compared with WT littermates. PDE blockade abolishes this difference. * = p<0.05 (t-test).
Mechanical properties of conductive vessels in Ercc1d/−
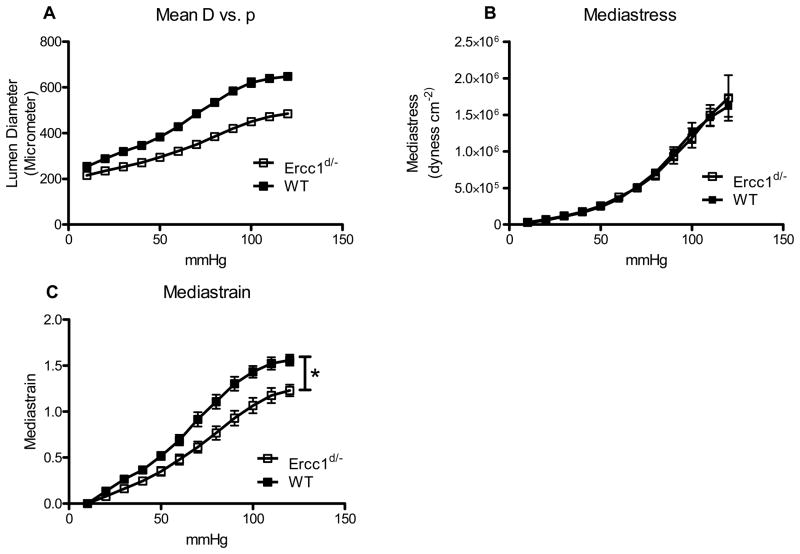
Under similar perfusion pressure increments differential increases in the vessel lumen were observed in Ercc1d/− versus WT mice (Figure 7A). Recalculation of measured values demonstrated a significantly lower strain, indicating lower elasticity, in Ercc1d/− mice under comparable stress as in WT (Figures 7B, 7C). Morphometric analysis on microscopical sections shows that although the vascular wall thickness of Ercc1d/− is significantly lower, the wall-to-lumen ratio was equal (Supplemental Figure 3).
Figure 7.
Mechanical properties of conductive vessels in Ercc1d/−. Relationship of the diameter of carotid artery to the internal pressure (A). Reduced strain of the tissue of Ercc1d/−mice (B) is accompanied by no difference in stress on the wall (C). * = p<0.05 (general linear model repeated – measures)
Human studies
Previous human studies indicate that NER component SNPs influence the risk of developing cancer16, suggesting that these SNPs modulate NER function. Ercc1d/− mice showed an increased vascular stiffness. We hypothesized that genetic variation in the NER components might also influence vascular stiffness in humans. Increased vascular stiffness is an important feature of human vascular aging, represented by the variable CFPWV. Therefore, we studied the effect of SNPs in NER components that, like ERCC1 and XPD, belong to the machinery that binds the DNA to either recognize or repair damage on variation of CFPWV in the cohorts from the AortaGen Consortium.
The AortaGen Consortium used a sex-specific standardized regression residual for 1000/CFPWV, adjusted for age, age2, height and weight for meta-analysis. Genome-wide association analyses were conducted using an additive gene-dose model. The results for the association of the genetic variants in the NER components with CFPWV in humans are presented in Table 1. The allele dose effect is expressed as standard deviations of inverse CFPWV per coded allele. After Bonferroni correction for multiple testing, one locus reached an association with CFPWV at the p-value threshold of 1.61×10−4, namely SNP rs2029298 (Beta: −0.05, SE: 0.01, P-value: 1.04*10−4). The closest gene to this SNP is DDB2 (XPE). Another SNP in this region, rs3781619 located within the DDB2 (XPE) gene, showed a suggestive association with the CFPWV measure (Beta: −0.03, SE: 0.02, p value=3.80*10−2). In addition, we found suggestive associations for 8 other SNPs located within or near ERCC5 (XPG), ERCC6 (CSB), GTF2H3, GTF2H1, ERCC2 (XPD). To summarize, DDB2 (XPE) was the most important gene associated with CFPWV.
Table 1.
Association of the selected tag SNPs in the NER component with Pulse Wave Velocity.
| SNP | Chromosome
|
Allele
|
AortaGen Meta-analysis*
|
Closest Gene | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Position | Coded | Frequency | Beta | SE | P value | ||
| Associations with P<1.61 × 10−4 | ||||||||
| rs2029298 | 11 | 47191294 | C | 0.30 | −0.05 | 0.01 | 1.04E-04 | DDB2 |
| Associations with 1.61 × 10−4<P<5.00 × 10−2 | ||||||||
| rs4772511 | 13 | 102369013 | T | 0.19 | 0.04 | 0.01 | 1.07E-02 | ERCC5 |
| rs4253002 | 10 | 50417344 | T | 0.02 | 0.13 | 0.05 | 1.53E-02 | ERCC6 |
| rs2340693 | 12 | 122700941 | G | 0.03 | −0.09 | 0.04 | 3.12E-02 | GTF2H3 |
| rs4150548 | 11 | 18304112 | A | 0.03 | 0.06 | 0.03 | 3.44E-02 | GTF2H1 |
| rs4253162 | 10 | 50361366 | T | 0.09 | −0.04 | 0.02 | 3.51E-02 | ERCC6 |
| rs3781619 | 11 | 47211893 | A | 0.13 | −0.03 | 0.02 | 3.80E-02 | DDB2 |
| rs50871 | 19 | 50554355 | A | 0.49 | −0.03 | 0.01 | 3.80E-02 | ERCC2 |
| rs9586010 | 13 | 102375850 | T | 0.26 | −0.03 | 0.02 | 3.90E-02 | ERCC5 |
| rs6488885 | 12 | 122685108 | G | 0.02 | −0.09 | 0.04 | 4.92E-02 | GTF2H3 |
Abbreviations: SNP, single nucleotide polymorphism; NER, Nucleotide Excision Repair; SE, standard error.
Coded allele is the minor allele. The analyses were adjusted for age, age2, sex, height and weight. The allele dose effect is the standard deviation of inverse carotid femoral pulse wave velocity (CFPWV) per coded allele. Because of the inverse transformation of CFPWV, a negative beta represents a higher CFPWV for each dose of the minor allele. For all of the SNPs in the table, N is 20634, except for rs2340693, rs9586010 and rs6488885 where N=16418.
Discussion
The present study shows that mice with an increased susceptibility to DNA damage due to a defect in NER as in XpdTTD, and NER and XLR as in Ercc1d/−, display an increased number of senescent vascular cells, an increased susceptibility of EC to become senescent, and accelerated worsening of vasodilator function during aging. The latter involves both EC as well as VSMC dysfunction. Furthermore, Ercc1d/− mice display increased vascular stiffness, systolic blood pressure, and pulse pressure, and reduced reactive hyperemia, which are typical features of vascular aging in humans. In addition, genetic association studies from the AortaGen Consortium suggest that SNPs in NER genes coding for components that participate in DNA recognition and repair contribute to vascular stiffness, as measured by CFPWV, in humans. These results suggest that genomic instability is involved in the development of vascular aging, and warrant further investigations into the involvement of the DNA repair systems in age-related cardiovascular disease.
The involvement of DNA damage and repair in age-related vascular disease is also apparent from previous clinical studies showing that senescent cells and oxidative DNA damage are present in atherosclerotic plaques17–18, the beneficial effects of statins on DNA repair19, the increased levels of 8-hydroxy-2,9-deoxyguanosine in urine of hypertensive subject20, and genome-wide association studies that report an association between SNPs in the 9p21 locus with risk for coronary artery disease, intracranial aneurysm and type 2 diabetes.21 These SNPs, which are located in the ANRIL non-coding RNA, lie in the vicinity of the INK4A genes that code for the cyclin-dependent kinase inhibitors p15/CDKN2B, p16/CDKN2A and p14/ARF, which may affect induction of cellular senescence in response to DNA damage.22 Deletion of ANRIL in mice reduces CDKN2A and CDKN2B expression, and increases cultured smooth muscle cell proliferation23. Vascular function has not been assessed in these mice. No previous studies examined vascular senescence in parallel to vascular function, but the studies cited here17, 19–24 support our hypothesis that DNA damage and cellular senescence contribute the pathogenesis of age-related CVD.
Ercc1d/− mice exhibited reduced reactive hyperemia, suggesting vasodilator dysfunction in resistance vasculature. Increased peripheral resistance may lead to increased MAP, which was observed in Ercc1d/− mice. Since systolic blood pressure also increased, we suspected reduced compliance of conduit vessels. The observation that aortic dilator function was decreased in Ercc1d/− and XpdTTD supports this concept. However, Ercc1d/− dilator function seems to depend both on VSMC as well as endothelial function, whereas XpdTTD showed no impaired VSMC relaxation. Naturally aging mice also showed endothelial dysfunction without VSMC impairment. Thus, the observed VSMC dysfunction in Ercc1d/− might represent an extreme aging phenotype, as might be expected from a combined defect of NER as well as XLR. Although the senescence marker prelamin A is found in aortic VSMC of aged humans outside of atherosclerotic plaques 25, the role of VSMC senescence in human vasodilator function remains unclear, whereas a role in atherosclerosis has been proposed 26. In contrast, the role of endothelial (progenitor) cell senescence has been amply addressed4, 18, 27.
We explored the role of eNOS in vasodilator dysfunction and found that aortic NO function and eNOS levels are decreased in Ercc1d/− mice. This is in agreement with previous observations in 26- to 28-month-old mice, and with a diminished NO release and eNOS expression in cultured senescent endothelial cells and in atherosclerotic plaque samples.18, 28–32 Our pharmacological studies with BH4, tempol and NAC show that ROS production due to eNOS uncoupling does not play a major role in the observed aortic endothelial dysfunction. At most, ROS modestly affected VSMC function. Interestingly, BH4 and NAC improved the vasodilator function to a similar extent. This might suggest a similar action of both compounds, which would be in line with ROS scavenging properties of BH4.33
Our observation that BH4 mainly improved VSMC instead of EC vasodilator function in aorta is in sharp contrast with the observation that the BH4 precursor sepiapterin improved dilations to acetylcholine but not to NaNO2 in mesenteric arteries of 24-month-old mice,34 and warrants exploration of vasodilator mechanisms in resistance vessels from our mice.
Exploration of the endothelium-independent vasodilator dysfunction revealed that PDE inhibition almost completely rescued VSMC vasodilator function. This strongly suggests the existence of a relative PDE overactivity in Ercc1d/− mice. Interestingly, the substrate of several PDE enzymes, cyclic guanosine monophosphate (cGMP), also regulates VSMC proliferation35 and extracellular matrix composition36. Therefore, long-term changes in cGMP levels could lead to vascular changes including increased stiffness and a higher number of senescent cells. Prolonged increase of PDE activity and reduction in NO production could thus lead to an increased vascular stiffness, as supported by the increased vascular stiffness, despite an increased sensitivity to NO, in eNOS knockout mice.37 In Ercc1d/− mice the reduced sensitivity to NO will even further contribute to reduced cGMP levels.
Our human genetic study intended to explore if there is a possible relationship between genetic variants in NER genes and risk for increased vascular stiffness, a typical marker for human vascular aging that was recapitulated in the Ercc1d/− mice. Besides finding one SNP, rs2029298, upstream of the start codon of DDB2 (XPE) that passed the Bonferroni-corrected significance level, we found suggestive associations of SNPs in or close to ERCC5, ERCC6, GTF2H3, GTF2H1, and XPD for CFPWV. The finding that CFPWV is significantly associated with DDB2 is compelling since CFPWV is the human equivalent of the mouse variables for vascular stiffness that were measured, and is negatively associated with vasodilator function in aging mice32 and humans.38 DDB2 encodes the smaller subunit of a heterodimeric DNA binding protein with high affinity to UV-damaged DNA (UV-DDB), and has been studied both in rodents as well as human. XPE is a component of NER and its activity is not tied to transcription, but it acts in the whole genome. The role of XPE is to identify UV induced lesions – cyclobutane pyrimidine dimers and also polycyclic aromatic hydrocarbon adducts.39 The function of the upstream region in which the SNP for DDB2 was found has not been characterized, although the gene in humans has been shown to be under control of p53, the guardian of the genome, and to be inducible after genotoxic stress. Hence DDB2 (XPE) may at least in part control global genome NER.
As usual for common genetic variants, the effect sizes were small. The effect size of our top SNP, rs2029298, is −0.05 ± 0.01 SD/allele, after correction for age, age2, sex, height, and weight. Extrapolation of this value of −0.05 SD/allele to an absolute age value for an individual with the use of CFPWV-age association graphs is hard since these relations are not linear. Moreover the impact of the SNP will depend on other risk factors that are present. It is, however, possible to compare the relative contribution of the risk allele of SNP rs2029298 to others found in GWA studies. In the AortaGen Consortium study, the locus, found in a BCL11B gene desert, that was strongest associated with CFPWV, had an effect size of −0.075±0.012 SD/allele. Clearly, the effect size of the DDB2 SNP found in the present study is in a comparable range.
More detailed sequencing might pinpoint genetic variants with stronger associations. This is an important consideration in view of the evolutionary pressure against genetic variations that severely impair the function of DNA repair systems. Severe DNA repair dysfunctions are often lethal or lead to infertility, which makes them more likely to be present only as minor alleles. Furthermore, the involvement of a DNA repair defect in vascular damage might depend strongly on the presence of factors that induce DNA damage. Nonetheless, increased PWV is prominently present in Hutchinson-Gilford progeria, a very rare syndrome caused by genomic instability due to mutation of the Lmna gene, which is featured by premature cardiovascular death 12, 40.
In summary, we explored the possibility whether reduced efficiency of two DNA repair pathways, Nucleotide Excision and interstrand cross link repair, contributes to vascular aging in mice. We observed functional changes of the vasculature (worsened vasodilator function and increased vascular stiffness) and hypertension, which are reminiscent of changes in aging humans and animals. We also found an association between SNPs of genes that encode for relevant NER components with increased vascular stiffness. Based on these observations we conclude that DNA repair capacity is associated with accelerated vascular aging in mice, and that there may be implications for risk stratification in humans with respect to age-dependent cardiovascular disease. Whether this relates to oxidative stress, classical risk factors and/or local damage, or even extends beyond these limits, will be a central question in studies to come.
Supplementary Material
Clinical implications.
Ageing strongly contributes to cardiovascular disease. It prolongs exposure to classical cardiovascular risk factors such as hypertension and diabetes, but also acts as independent risk factor. Recent evidence suggests that gradually accumulating DNA damage, leading to genomic instability, is a main cause of ageing. This study is the first to show that mice with a defective DNA repair system not only age fast, but also display accelerated development of vascular problems mimicking those in ageing humans: increased blood pressure, increased vascular stiffness, decreased vascular relaxation, and cellular ageing. Of interest, phosphodiesterase (PDE) inhibition acutely improved the diminished relaxation in vitro, suggesting that enhanced breakdown of cyclic guanosine monophosphate (cGMP) may underlie this phenomenon. Furthermore, in humans, variations in DNA repair genes were associated with markers for vascular ageing. Taken together, genomic instability plays a central role in vascular ageing. It may also explain the high prevalence of cardiovascular death in Hutchinson-Gilford progeria and Werner’s progeroid syndrome, which are both featured by genomic instability. Since oxidative stress is an important inductor of DNA damage, future ageing-suppressor agents may involve drugs that improve genomic integrity (e.g., statins and rapamycin) as well as drugs that prevent oxidative stress (e.g., renin-angiotensin system blockers and antioxidants). In addition, drugs facilitating the NO-soluble guanylate cyclase-cGMP-PDE pathway might be of value. The successful application of such treatments requires proper risk stratification, preferably at younger ages. This might include analyses of genetic variations in DNA repair genes and the identification of all possible sources of cardiovascular DNA damage.
Acknowledgments
The authors are indebted to Prof. Dr. Bert van der Horst (EMC Rotterdam, dept. of Genetics) for fruitful discussions on the mouse progeria models, and to Mrs. Mechtild Piepenbrock and Mrs. Katharina Bruch (JWG University, Frankfurt) for expert technical assistance. We are grateful to the participants and staff from the Rotterdam Study, the participating general practitioners and the pharmacists.
Funding Sources: This study was supported by European commission FP6 Linkage (FP6-513866), Markage (FP7-Health-2008-200880), DNA Repair (LSHG-CT-2005-512113) and LifeSpan (LSHGCT- 2007-036894), NIH/National Institute of Ageing (NIA) (1PO1 AG-17242-02), National Institute of Environmental Health Sciences (NIEHS, 1UO1 ES011044), and Netherlands Organization for Scientific Research (NWO), including the foundation of the Research Institute Diseases of the Elderly (014-93-015; RIDE2), NWO investment grant (nr. 175.010.2005.011, 911-03-012) to AGU, NWO/Netherlands Organization for the Health Research and Development grant (ZonMw) (Vici 918.76.619) to JCMW, Dutch Heart Foundation grant 2007B024 to DJD, and the Deutsche Forschungsgemeinschaft (SFB 834-A4 to AEL & IF), and by the Netherlands Genomics Initiative (NGI)/Netherlands Consortium for Healthy Ageing (NCHA) project nr. 050-060-810. For the full listing of investigators, sources of funding and acknowledgments for the AortaGen consortium please see reference 12.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 2.Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 7.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 8.Andressoo JO, Hoeijmakers JH. Transcription-coupled repair and premature ageing. Mutat Res. 2005;577:179–194. doi: 10.1016/j.mrfmmm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Dolle ME, Kuiper RV, Roodbergen M, Robinson J, de Vlugt S, Wijnhoven SW, Beems RB, de la Fonteyne L, de With P, van der Pluijm I, Niedernhofer LJ, Hasty P, Vijg J, Hoeijmakers JH, van Steeg H. Broad segmental progeroid changes in short-lived Ercc1-/Δ7 mice. Pathobiology of Aging & Age-related Diseases. 2011;1:7219–7232. doi: 10.3402/pba.v1i0.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV, Yasmin, Rietzschel ER, Tanaka T, Liu Y, Parsa A, Najjar SS, O’Shaughnessy KM, Sigurdsson S, De Buyzere ML, Larson MG, Sie MP, Andrews JS, Post WS, Mattace-Raso FU, McEniery CM, Eiriksdottir G, Segers P, Vasan RS, van Rijn MJ, Howard TD, McArdle PF, Dehghan A, Jewell E, Newhouse SJ, Bekaert S, Hamburg NM, Newman AB, Hofmann A, Scuteri A, De Bacquer D, Ikram MA, Psaty B, Fuchsberger C, Olden M, Wain LV, Elliott P, Smith NL, Felix JF, Erdmann J, Vita JA, Sutton-Tyrrell K, Sijbrands EJ, Sanna S, Launer LJ, De Meyer T, Johnson AD, Schut AF, Herrington DM, Rivadeneira F, Uda M, Wilkinson IB, Aspelund T, Gillebert TC, Van Bortel L, Benjamin EJ, Oostra BA, Ding J, Gibson Q, Uitterlinden AG, Abecasis GR, Cockcroft JR, Gudnason V, De Backer GG, Ferrucci L, Harris TB, Shuldiner AR, van Duijn CM, Levy D, Lakatta EG, Witteman JC. Common genetic variation in the 3-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: The AortaGen Consortium. Circ Cardiovasc Genet. 2012;5:81–90. doi: 10.1161/CIRCGENETICS.111.959817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resch M, Wiest R, Moleda L, Fredersdorf S, Stoelcker B, Schroeder JA, Scholmerich J, Endemann DH. Alterations in mechanical properties of mesenteric resistance arteries in experimental portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2009;297:G849–857. doi: 10.1152/ajpgi.00084.2009. [DOI] [PubMed] [Google Scholar]
- 14.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med. 2009;206:2889–2896. doi: 10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katusic ZS, d’Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 17.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 18.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudi M, Gorenne I, Mercer J, Figg N, Littlewood T, Bennett M. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ Res. 2008;103:717–725. doi: 10.1161/CIRCRESAHA.108.182899. [DOI] [PubMed] [Google Scholar]
- 20.Negishi H, Ikeda K, Kuga S, Noguchi T, Kanda T, Njelekela M, Liu L, Miki T, Nara Y, Sato T, Mashalla Y, Mtabaji J, Yamori Y. The relation of oxidative DNA damage to hypertension and other cardiovascular risk factors in Tanzania. J Hypertens. 2001;19:529–533. doi: 10.1097/00004872-200103001-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pasmant E, Sabbagh A, Vidaud M, Bieche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2010:444–448. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- 22.Samani NJ, Schunkert H. Chromosome 9p21 and cardiovascular disease: the story unfolds. Circ Cardiovasc Genet. 2008;1:81–84. doi: 10.1161/CIRCGENETICS.108.832527. [DOI] [PubMed] [Google Scholar]
- 23.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 25.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 26.Gorenne I, Kavurma M, Scott S, Bennett M. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res. 2006;72:9–17. doi: 10.1016/j.cardiores.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Durik M, Seva Pessoa B, Roks AJ. The renin-angiotensin system, bone marrow and progenitor cells. Clin Sci (Lond) 2012;123:205–223. doi: 10.1042/CS20110660. [DOI] [PubMed] [Google Scholar]
- 28.Sato I, Morita I, Kaji K, Ikeda M, Nagao M, Murota S. Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. 1993;195:1070–1076. doi: 10.1006/bbrc.1993.2153. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 30.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Luscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res. 1995;23:419–430. doi: 10.3109/10715769509065263. [DOI] [PubMed] [Google Scholar]
- 34.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kariya K, Kawahara Y, Araki S, Fukuzaki H, Takai Y. Antiproliferative action of cyclic GMP-elevating vasodilators in cultured rabbit aortic smooth muscle cells. Atherosclerosis. 1989;80:143–147. doi: 10.1016/0021-9150(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 36.Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76:305–309. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- 37.Brandes RP, Kim D, Schmitz-Winnenthal FH, Amidi M, Godecke A, Mulsch A, Busse R. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension. 2000;35:231–236. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- 38.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 39.Hanawalt PC, Ford JM, Lloyd DR. Functional characterization of global genomic DNA repair and its implications for cancer. Mutat Res. 2003;544:107–114. doi: 10.1016/j.mrrev.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Gerhard-Herman M, Smoot LB, Wake N, Kieran MW, Kleinman ME, Miller DT, Schwartzman A, Giobbie-Hurder A, Neuberg D, Gordon LB. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension. 2012;59:92–97. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.