Abstract
Oxidative stress is a putative factor responsible for reducing function and increasing apoptotic signaling in skeletal muscle with aging. This study examined the contribution and functional significance of the xanthine oxidase enzyme as a potential source of oxidant production in aged skeletal muscle during repetitive in situ electrically stimulated isometric contractions. Xanthine oxidase activity was inhibited in young adult and aged mice via a subcutaneously placed time release (2.5 mg/day) allopurinol pellet, 7 days prior to the start of in situ electrically stimulated isometric contractions. Gastrocnemius muscles were electrically activated with 20 maximal contractions for three consecutive days. Xanthine oxidase activity was 65% greater in the gastrocnemius muscle of aged mice compared to young mice. Xanthine oxidase activity also increased after in situ electrically stimulated isometric contractions in muscles from both young (33%) and aged (28%) mice, relative to contralateral non-contracted muscles. Allopurinol attenuated the exercise-induced increase in oxidative stress, but it did not affect the elevated basal levels of oxidative stress that was associated with aging. In addition, inhibition of xanthine oxidase activity decreased caspase 3 activity, but it had no effect on other markers of mitochondrial associated apoptosis. Our results show that compared to control conditions, suppression of xanthine oxidase activity by allopurinol reduced xanthine oxidase activity, H2O2 levels, lipid peroxidation and caspase-3 activity, prevented the in situ electrically stimulated isometric contraction-induced loss of glutathione, prevented the increase of catalase and copper-zinc superoxide dismutase activities, and increased maximal isometric force in the plantar flexor muscles of aged mice after repetitive electrically evoked contractions.
Keywords: Oxidative stress, aging, electrically evoked isometric contractions, sarcopenia, apoptosis, muscle atrophy
Introduction
The fundamental mechanisms contributing to aging-associated deterioration in muscle function and muscle mass are poorly understood, but a large body of evidence supports the hypothesis that oxidative stress contributes to aging in many tissues, including muscle [1–4]. Oxidative stress occurs when the cellular production of oxidants exceeds the capacity of the cell to inhibit or terminate oxidizing reactions. Increases in oxidative stress have been proposed as a principal component leading to skeletal muscle loss with aging (sarcopenia). Loss of myonuclei via apoptosis is another likely contributor to sarcopenia. However, oxidative stress and apoptosis may not be mutually exclusive events with aging. Rather, the elevation in oxidative stress that occurs with aging can regulate redox-sensitive signaling pathways [5, 6], increase catabolic gene expression [7–9], and activate apoptotic pathways [10, 11], thereby contributing to the progression of sarcopenia.
Mitochondria are a major source of oxidant production in skeletal muscle [12, 13]. The consequence of prolonged exposure to relatively high levels of oxidants reduces mitochondrial membrane integrity and antioxidant enzyme activity [13]. In addition, oxidants can lead to increased mitochondria permeability and the release of mitochondria specific proteins including, apoptosis inducing factor (AIF) and cytochrome c into the cytosol through the mitochondrial transition pore. Cytosolic AIF initiates a caspase independent pathway, while cytosolic cytochrome c initiates the caspase cascade resulting in DNA fragmentation and myonuclear apoptosis. Thus, mitochondria may be important for regulating both oxidative stress and apoptotic signaling in aging skeletal muscle.
The functional implications of elevated oxidative stress in skeletal muscle include reduced specific force [14], altered myofilament function [15], and elevated muscle fatigue [16]. Although exercise is used as a strategy to attempt to reduce sarcopenia and improve muscle function, acute exercise can also increase free radical generation in skeletal muscle [17]. This has important implications in a highly metabolic tissue such as skeletal muscle, where basal oxidant production is already elevated with aging, and exercise has the potential to further increase oxidant production by as much as 80% [12].
There are three major sources of oxidant production with exercise. These include infiltrating immune cells, mitochondrial respiration and xanthine oxidase activity [18]. The magnitude and the sources of oxidant production are dependent on the mode, duration, and intensity of exercise.
Under normal physiological conditions, xanthine dehydrogenase is the principal form of the enzyme, which catalyses the oxidization of both hypoxanthine and xanthine to form uric acid via the reduction of NAD+ to NADH. Both reactions result in the generation of hydrogen peroxide. However, during repetitive muscle contractions, the increased ATP utilization and a brief localized period of ischemia will facilitate adenine nucleotide degradation, thus breaking down ATP to AMP and eventually to hypoxanthine. Xanthine dehydrogenase can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modifications [19, 20]. Xanthine dehydrogenase catalyzes the oxidation of xanthine to uric acid using NAD as a substrate without the formation of hydrogen peroxide or other oxidants.
Increased xanthine oxidase activity within the vascular endothelium [21], is a contributing factor associated with oxidative stress and damage during exhaustive exercise [1, 22–25]. Allopurinol, which is a structural isomer of hypoxanthine, acts as a competitive inhibitor to xanthine oxidase protecting cells from oxidative damage associated with exhaustive exercise [24]. It has been hypothesized that the activation of the enzyme, xanthine oxidase, during exhaustive exercise is similar to the process observed during ischemia–reperfusion injury [24, 26, 27]. During repetitive muscle contractions, the combination of increased ATP utilization and intermittent localized periods of ischemia due to muscle contractions will facilitate adenine nucleotide degradation and accumulation of hypoxanthine.
Conversion of xanthine dehydrogenase to xanthine oxidase has been shown to be dependent on both calcium and oxidant concentrations [28]. During muscle contractions, intracellular calcium concentrations are elevated, which in turn, activate proteases that cause the irreversible conversion of xanthine dehydrogenase to xanthine oxidase. Furthermore, increased oxidant production may lead to the oxidation of cysteine residues on xanthine dehydrogenase forming disulfide bonds resulting in the reversible conversion to xanthine oxidase [20].
During muscle relaxation, the influx of oxygen rich blood catalyzes the reaction of xanthine oxidase to form hypoxanthine and oxygen to form xanthine and superoxide. Within the muscle environment, H2O2 concentrations are expected to increase, via the accumulation of superoxide formed by xanthine oxidase activity, mitochondrial sources and NADPH oxidase activity, since the superoxide anion is quickly dismutated to H2O2 by SOD. Decreases in antioxidant capacity with aging and exercise may lead to an increase in contractile protein and mitochondrial damage caused by an augmented duration and exposure to oxidants thus potentially accelerating muscle loss [29, 30].
Xanthine oxidase has been reported to make important contributions to oxidative stress in the heart [31] and gastrocnemius muscles [32–34] from aged rodents; however, this age-dependent elevation in xanthine oxidase activity is not observed universally [35]. Xanthine oxidase activity contributes, at least in part, to an increase in oxidant production during exhaustive exercise, but it is not known if xanthine oxidase is an important source of oxidant production with more moderate exercise in aged animals. Therefore, the purpose of this investigation was to determine the contribution of the xanthine oxidase enzyme as a source of oxidant production during repetitive isometric contractions, and to determine if it further contributes to oxidative stress in aged skeletal muscle. A second aim of this study was to determine if increased xanthine oxidase levels plays a role in regulating the decreased functional capacity and increased apoptotic signaling in aged muscles. We tested the hypothesis that the inhibition of xanthine oxidase will improve the redox environment within muscle by reducing oxidative stress and thus preserving functional capacity in aged animals after in situ electrically stimulated isometric contractions. The second hypothesis tested was that xanthine oxidase-associated oxidative stress will exacerbate the release of pro-apoptotic mitochondrial proteins into the cytosol, thereby increasing apoptotic signaling in aged skeletal muscle after in situ electrically evoked contractions, whereas, reducing xanthine oxidase by allopurinol will prevent these negative changes in aging muscles. Our rationale was that if acute contractions induced detrimental changes in aged muscle (e.g., as a result of increased oxidant production), where oxidant levels are already high relative to muscle conditions in young animals [34, 36], and if allopurinol could suppress xanthine oxidase induced oxidative stress that occurs as a result of muscle contractions, then acutely, muscle redox and function (e.g., force) would be improved and oxidant damage in loaded muscles would be reduced.
Materials and Methods
Suppression of xanthine oxidase during in situ electrically stimulated isometric contractions
All experimental procedures were carried out with approval from the Institutional Animal Use and Care Committee from West Virginia University School of Medicine. The animal care standards were followed by adhering to the recommendations for the care of laboratory animals as advocated by the American Association for Accreditation of Laboratory Animal Care.
A subcutaneous 2.5 mg 21 day release allopurinol pellet (Innovative Research of America Inc., Sarasota, FL) was implanted subcutaneously over the dorsal cervical column in anesthetized mice (Isotec 5, Ohmeda; 3% isoflurane/97% O2), seven days prior to the start of the in situ electrically stimulated isometric contraction protocol. The incision was closed with a 9 mm wound clip. A sham surgery was performed on control animals. Forty-eight young adult (3–5 months) and 48 aged (26–28 months) C57BL/6 mice were randomly separated into groups receiving the allopurinol pellet, or only the sham surgery (n= 24 per treatment group). In each treatment group, one half of the gastrocnemius muscles was processed for whole muscle homogenate and RNA isolation, while the gastrocnemius muscle from the other half of the treatment group was homogenized and separated into a mitochondrial fraction and a mitochondrial free cytosolic fraction. Mitochondria were isolated from gastrocnemius in other animals in each group.
In situ electrically stimulated isometric contractions were conducted on a custom-built mouse dynamometer that has been previously described [34]. Briefly, mice were anesthetized with a mixture of oxygen (97%) and isoflurane gas (3%) and placed on a plate that was heated to 37°C. The left ankle was positioned at 90° of flexion and was aligned with the axis of rotation of the servomotor (Model 6350*350; Cambridge Technology Inc., Cambridge, MA). The foot was secured to the foot plate that was connected to the servomotor. Commercially available software (Dynamic Muscle Control; Aurora Scientific Inc., Aurora, Ontario, Canada) was used to control the servomotor providing for the angular position of the foot. Muscle contractions of the plantar flexor muscles were stimulated via subcutaneously placing platinum electrodes that were placed on either side of the tibial nerve. Electrode placement was tested via a short stimulation of the nerve to cause plantar flexion. When stimulated, the foot plantar flexed without any visible appearance of eversion, or inversion, of the foot. Twenty electrically evoked (10v, 100 Hz, 200 µs pulses) isometric contractions of the plantar flexor muscle group were obtained in one limb. Each contraction train lasted for five seconds, and a 25-second recovery period occurred between subsequent contractions. Isometric contractions were conducted over three consecutive days in the left limb, while the contralateral limb served as the intra-animal control. Muscle functional data was collected as a force × time curve during isometric contractions for each session and values were normalized to each animal’s body weight. The contractile data were analyzed by off-line (Dynamic Muscle Analysis software, Aurora Scientific Inc., Aurora, Ontario, Canada).
Mitochondrial isolation
Gastrocnemius muscles were dissected with the mice under deep anesthesia (5% isoflurane / 95% oxygen). Careful precautions were made to keep the blood supply to the gastrocnemius intact until it was removed. Mitochondria and mitochondria free cytosolic muscle fractions were obtained by protease digestion from the myofibrils, followed by centrifugation, using modifications of the manufacture’s recommendations (MITOISO1-1KT; Sigma-Aldrich Co., St Louis, MO). Briefly, the gastrocnemius muscle was placed on ice and minced in a 1.5ml Eppendorf tube. Samples were washed and re-suspended in an extraction buffer containing 0.25 mg/ml trypsin. After a 20-minute incubation period, albumin was added to a final concentration of 10 mg/ml to quench the proteolytic reaction. Samples were washed and re-suspended in the extraction buffer, then homogenized with a Teflon pestle for two strokes of five seconds each. The homogenate was then centrifuged at 1100g for 5 minutes. The supernatant was transferred to a new tube and centrifuged at 11,000 g for 10 minutes. The supernatant was collected as mitochondrial free, cytosolic fraction. The mitochondrial pellet was suspended in a storage buffer containing sucrose.
H2O2 concentration in whole muscle homogenates and isolated mitochondria
Muscle hydrogen peroxide (H2O2) levels were determined with a fluorescent H2O2 assay (FLOH 100-3; Cell Technology, Mountain View, CA). This assay utilizes a non-fluorescent detection reagent to detect H2O2. H2O2 oxidizes the detection reagent in a 1:1 stoichiometry to produce a fluorescent product resorufin. This oxidation is catalyzed by peroxidase. The change in fluorescent signals is detected with an excitation of 530 nm and an emission of 590 nm and plotted against at standard curve of known concentrations of H2O2. Whole muscles were homogenized in phosphate-buffered saline (PBS) (pH=7.4). Isolated mitochondria were obtained as described above. Reagents and standards were prepared as recommended by the manufacturer with slight modifications and have been previously described [34]. H2O2 was normalized to the muscle protein concentration of each sample as determined by a DC protein concentration assay (Bio-Rad). All analyses were done in duplicate.
Electron paramagnetic resonance spectroscopy (EPR) to measure reactive oxygen species (ROS) in isolated mitochondria
EPR spin trapping was used to detect short-lived free radicals including hydroxyl radicals (•OH) and superoxide radicals (O2•−) [37]. This method utilizes the reaction of a short-lived radical binding with a paramagnetic compound to form a spin adduct, which is a relatively long-lived free radical product. The spin adduct can be measured with conventional EPR. The intensity of the EPR signal is used to estimate the amount of short-lived radicals that were trapped. The hyperfine structure of the spin adducts represent characteristics of the original trapped radicals. The specificity and sensitivity of this assay makes it optimal for detecting and identifying free radicals. All EPR measurements were conducted using a Bruker EMX spectrometer (Bruker Instruments, Billerica, MA) and a flat cell assembly. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetraperoxochromate (K3CrO8) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) as reference standards [37]. Data acquisitions and analyses were conducted using the Acquisit data analysis program (Bruker Instruments). The assay was conducted as reported in detail previously [38]. Briefly, isolated mitochondria samples were suspended in phosphate buffered saline then incubated with the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (200 mM) in the presence or absence of the excess complex I respiratory substrates glutamate and malate. The samples were incubated at 37°C and measured at 20°C in an EPR flat cell. Three scans were made of each cell. The reaction obtained with xanthine and xanthine oxidase was used as a reference. The relative radical concentration was estimated by measuring the peak to peak height (mm) of the observed spectra.
Concentration of reduced and oxidized glutathione
Glutathione (GSH), and oxidized glutathione (GSSG) were measured in muscle homogenates using commercially available substrates (21040, Percipio Biosciences, Inc., Burlingame, CA). The data were expressed as the ratio of reduced to oxidized glutathione (GSH/GSSG). The assay was conducted as previously described [34]. Briefly, muscle tissue (~40 mg) was homogenized immediately after dissection in 530 µl of cold buffer (5% metaphosphoric acid for the GSH or 5% metaphosphoric acid and M2VO scavenger for the GSSG sample). The GSH/GSSG assay uses the thiol-scavenging reagent, 1-methyl-2-vinylpyridinium trifluoromethanesulfonate to rapidly scavenge GSH but it does not interfere with the glutathione reductase assay. The method employs Ellman’s reagent (5,5'- dithiobis-2-nitrobenzoic acid), which reacts with GSH to form a spectrophotometrically detectable product at 412 nm. The appropriate amounts of sample chromogen (5,5’-Dithiobis-(2-nitrobenzoic acid)) and enzyme (glutathione reductase) were mixed and incubated at room temperature. NADPH was added and the absorbance (412 nm) of each sample was read for three consecutive minutes. GSSG was determined by the reduction of GSSG to GSH, which is then determined by the reaction with Ellman’s reagent. This assay measures the change in color development during the reaction, and the reaction rate is proportional to the GSH and GSSG concentrations. The concentration for each sample was determined via a DC protein concentration assay (BIORAD). The signals from each sample were normalized to the corresponding protein content of that sample.
Xanthine Oxidase activity, hypoxanthine and xanthine concentration in the whole muscle homogenate
Xanthine and hypoxanthine concentrations and xanthine oxidase activity were measured in muscle homogenates using commercially available reagents (A22182; Invitrogen, Eugene, OR). In this assay, xanthine oxidase catalyzes the oxidation of purine bases, hypoxanthine or xanthine, to uric acid and superoxide. The superoxide spontaneously degrades to H2O2, and the H2O2, in the presence of horseradish peroxidase, reacts stoichiometrically with Amplex Red to generate the red-fluorescent oxidation product, resorufin. The methods have been described previously in our laboratory [34]. Fluorescence was measured in a microplate reader using an excitation of 530 nm and emission detection at 590 nm. Each sample was corrected for background fluorescence by subtracting the values derived from the non-xanthine containing wells. Hypoxanthine and xanthine concentrations were determined by comparing sample values to values obtained from a standard curve. Relative concentrations of hypoxanthine and xanthine concentrations were determined by comparing sample values relative fluorescent units (RFU). Each sample and standard was performed in duplicate and then normalized to protein concentrations (Bio-Rad Hercules, CA) of the samples.
Lipid peroxidation in the whole muscle homogenates
Malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) were measured as an indicator of lipid peroxidation (BIOXYTECH LPO-586; Percipio Biosciences, Inc., Burlingame, CA). This assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole, with MDA and HAE at 45°C. One molecule of either MDA or HAE reacts with 2 molecules of N-methyl-2-phenylindole to yield a stable chromophore with a maximal absorbance at 586 nm. Methanesulfonic acid was used as the acid solvent. The methods have been previously described [34]. Briefly, 75–100 mg of each muscle was homogenized in ice-cold PBS and 5 µL 0.5 M butylated hydroxytoluene (BHT) in acetonitrile. Absorbance of the supernatant was obtained at 586nm. The samples were normalized for differences in the amount of protein in each sample as determined by a DC protein concentration assay (Bio-Rad, Hercules, CA).
Glutathione Peroxidase (GPx) in the whole muscle homogenate
A commercially available cellular GPx assay (353919; EMD/Calbiochem, San Diego, CA) was used to measure GPx activity in muscle homogenates. This assay measures GPx activity indirectly by a coupled reaction with glutathione reductase. Oxidized glutathione (GSSG), produced upon reduction of hydroperoxide by glutathione peroxidase, is recycled to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ results in a decrease in absorbance at 340 nm. Under conditions in which the glutathione peroxidase activity is rate limiting, the rate of decrease in the A340 is directly proportional to the glutathione peroxidase activity in the muscle sample. We have previously reported the methods to measure GPx [34]. Briefly, a portion of each muscle was homogenized in PBS (pH 7.5) containing 5mM EDTA and 1mM DTT. The homogenate was centrifuged at 10,000g and the supernatant was used for the assay. All reagents and samples were equilibrated to 25°C. The absorbance was measured at 340 nm using a 96-well plate reader (DYNEX technologies, Chantilly Va., USA). Each sample and standard was performed in duplicate.
Catalase Activity in whole muscle homogenate
Commercially available reagents (#A22180, Invitrogen, Eugene, OR) were used to measure the activity of the catalase enzyme in whole muscle homogenates. In this assay, catalase reacts with H2O2 to produce water and oxygen. The Amplex Red reagent reacts with a 1:1 stoichometry with any unreacted H2O2 in the presence of horseradish peroxidase to produce the highly fluorescent oxidation product, resorufin. As catalase activity increases, the signal from resorufin decreases. The methods have been previously reported [36]. Briefly, 25µl of muscle homogenates were mixed with 25µl of 40 µM H2O2 solution and allowed to incubate in the dark for 30mins at room temperature. After 30 minutes, the sample containing solution was mixed with 50 µM Amplex® Red, 0.4 U/mL horseradish peroxidase and incubated at 37°C in the dark. Fluorescence was measured in a microplate reader using an excitation of 530 nm and emission detection at 590 nm. The change in fluorescence was determined by subtracting the sample value from that of the no-catalase control. The concentration of catalase was determined by comparing the sample to a standard curve. All analyses were measured in duplicate and the samples were normalized to the protein concentration in each sample as assessed using a DC protein concentration assay (Bio-Rad, Hercules, CA).
Mitochondrial Manganese Superoxide Dismutase (MnSOD)
MnSOD activity was measured in the mitochondrial fraction of each muscle sample using commercially available reagents (574601; EMD/Calbiochem, San Diego, CA). The assay was performed with slight modifications to the manufacturer’s directions and all samples and standards were measured in duplicate. The assay utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. Mitochondrial MnSOD was measured with 1 mM potassium cyanide added to the assay to inhibit CuZnSOD and extracellular SOD, resulting in the detection of only MnSOD activity. The assay was performed in a 96-well plate with each sample being treated with 10µL of 12 mM potassium cyanide to inhibit any residual CuZnSOD and extracellular SOD activities. The absorbance was measured at 450 nm using a 96-well plate reader (Dynex Tech., Chantilly VA., USA). The samples were normalized to the protein concentration in each sample as assessed using a DC protein concentration assay (Bio-Rad, Hercules, CA).
Cytosolic Copper-Zinc Superoxide Dismutase (CuZnSOD) Activity
CuZnSOD activity was measured in the mitochondrial free fraction of muscle homogenates using reagents and methods (574601; EMD/Calbiochem, San Diego, CA) as described previously [34]. All samples and standards were measured in duplicate. This assay is based on the same principles as that which was used to measure MnSOD, except that potassium cyanide was not added to the assay buffer. This results in the detection of both CuZnSOD and MnSOD activity. MnSOD activity was then subtracted from total activity to obtain CuZnSOD activity. The assay was performed in a 96-well plate and the absorbance was measured at 450 nm using a 96-well plate reader (Dynex Tech., Chantilly VA., USA). The samples were normalized to the protein concentration in each sample as assessed using a DC protein concentration assay (Bio-Rad, Hercules, CA).
Measuring mRNA concentrations of antioxidant enzymes
CuZnSOD, MnSOD, catalase and GPx-1 mRNA were determined by means of reverse transcription-polymerase chain reaction (RT-PCR) according previously published procedures from our laboratory [34, 36, 39]. Briefly, RNA was isolated from sixty micrograms of the gastrocnemius muscle homogenized in 1ml of Tri-Reagent (Molecular Research Center, Cincinnati, OH). RNA purity was accessed using a minimum 260:280 ratio of 1.7. RNA was reverse transcribed using random primers, dNTP, and SuperScript II reverse transcriptase (Invitrogen/Life Technologies, Bethesda MD). The primers for CuZnSOD, MnSOD, GPx-1, and catalase have been previously published [36]. The signal from the gene was expressed as a ratio to the 18S signal from the same PCR product. The PCR product from each reaction was separated on a 1.5% agarose gel containing ethidium bromide via electrophoresis. The resulting signals were digitally captured (Kodak DC290, Eastman Kodak Company, Rochester, NY) and quantified using 1D Kodak image analysis software (Eastman Kodak Company, Rochester, NY).
Fluorometric Caspase-Activity Assay
The proteolytic activities of caspase-3 was determined by using the fluorogenic substrate AC-DEVD-AFC (Alexis Biochem/Enzo Life Sciences, PA) as a substrate. Ac-LEHD-AFC (Alexis Biochem/Enzo Life Sciences, PA) was used as the flurogenic caspase-9 substrate. Briefly, 50µl of caspase activity buffer (50mM PIPES, 0.1 mM EDTA, 10% glycerol, 1mM DTT), 50µl of the cytosolic fraction of the muscle homogenate without protease inhibitor, and 10µl of substrate (1mM) were combined in a 96-well fluorescent microplate. Caspase activity was accessed at wavelength of 400nm for excitation and 505nm for emission. The microplate was incubated for 2-hours at 37°C and caspase activity was determined by subtracting the time 2-hour reading from the initial reading. Caspase activity is expressed as the relative fluorescent units normalized to the protein concentration of each muscle sample (RFU / mg protein).
Western Immunoblots
The protein content of CuZnSOD, apoptosis peptidase activating factor 1 (Apaf-1), apoptosis inducing factor (AIF) and cytochrome C were measured in the cytosolic (mitochondrial free) protein fraction. MnSOD, Bax (Bcl-2-associated X protein), Bcl-2 (B-cell leukemia/lymphoma-2) protein abundance was measured in isolated mitochondrial proteins. Thirty µg of protein was loaded into each well of a 4–12% gradient polyacrylamide gel (Novex, Invitrogen) and separated by routine SDS-polyacrylamide gel electrophoresis (PAGE) for 1.5 hours at 20°C followed by transfer to a nitrocellulose membrane. All membranes were blocked in 5% non-fat milk protein (NFM) for 1-hour at room temperature. The membranes were incubated overnight at 4°C, with primary antibodies directed against MnSOD (#sc-11407, Santa Cruz Biotechnology, Santa Cruz, CA), MnSOD (#A300449A, Bethyl Lab Inc., Montgomery, TX), AIF (#sc-13116, Santa Cruz), Apaf-1 (#3018, BioVision, Mountain View, CA), cytochrome c (#sc-13561, Santa Cruz), Bcl-2 (#2876, Cell Signaling Technology, Boston, MA), or Bax (#2772, Cell Signaling). The antibodies were diluted in Tris-buffered saline with 0.05% Tween-20 (TBS-T) and 0.002% sodium azide. The membranes were washed in TBS-T followed by incubation in the appropriate dilutions of secondary antibodies (diluted in 5% NFM in TBS-T) conjugated to horseradish peroxidase. The signals were developed using a chemi-luminescent substrate (ECL Advanced, Amersham Bioscience) and visualized by exposing the membranes to X-ray films (BioMax MS-1; Eastman Kodak). Digital records were obtained from each blot and the protein bands of interest were quantified using 1-D analysis software (Eastman Kodak, USA). The protein bands were quantified as optical density (OD) × band area and expressed in arbitrary units, normalized to GAPDH. The molecular sizes of the immunodetected proteins were verified by using pre-stained standard (LC5925, Invitrogen Life Technologies, Bethesda, MD).
Results
Xanthine Oxidase activity
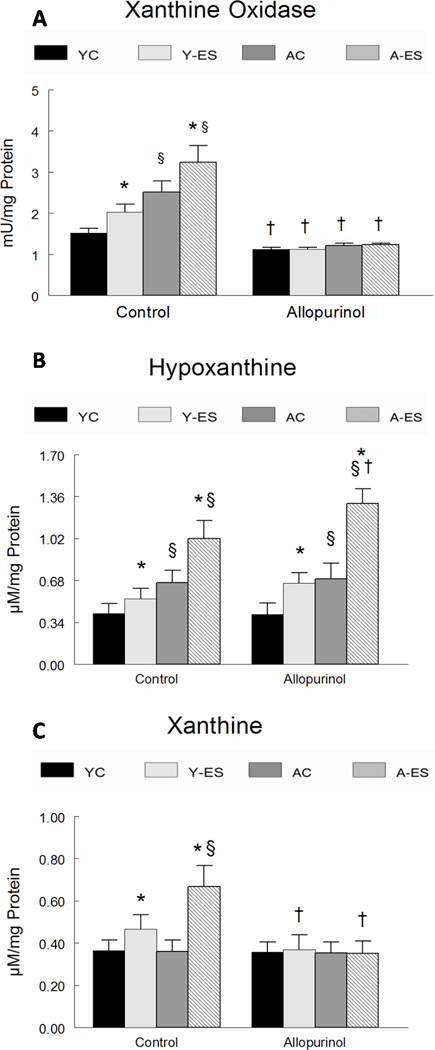
Allopurinol blocked in situ electrically stimulated isometric contraction-induced and aging-induced increases in muscle xanthine oxidase activity. Xanthine oxidase activity was 65% greater (P<0.05) in control gastrocnemius muscles of aged mice that received the sham surgeries but no allopurinol, as compared to young adult mice (Figure 1A). Xanthine oxidase activity increased in muscles from young adult (33%, P<0.05) and aged animals (28%, P<0.05) after in situ electrically stimulated isometric contractions, compared to the contra-lateral control muscles. Allopurinol administration completely blunted both the aging-induced and in situ electrically evoked increases in muscle xanthine oxidase activity. Allopurinol reduced xanthine oxidase levels of aged muscles so that there were no differences between any of the allopurinol treated muscles (Figure 1A).
Figure 1. Allopurinol attenuated the increase in xanthine oxidase activity, hypoxanthine and xanthine associated with electrically evoked contractions.
(A) Xanthine oxidase activity was determined fluorometrically. Data are expressed as mU of activity per mg of total protein in gastrocnemius muscle homogenate. (B) Hypoxanthine and (C) xanthine levels were determined by a fluorometric assay. Data are expressed as µM concentration per mg of total protein in the gastrocnemius muscle homogenate. The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. * significant difference (P<0.05) between electrically stimulated muscles from contra-lateral control muscles; § significant effect of aging within the sham surgery or allopurinol treatment groups (P<0.05). † significant effect (P<0.05) of allopurinol treatment.
Hypoxanthine and Xanthine
Hypoxanthine, which is a product of purine degradation, and a substrate for xanthine oxidase, was elevated by both aging and in situ electrically stimulated isometric contractions in gastrocnemius muscles, but it was not affected by allopurinol. The hypoxanthine concentration was greater in muscles of aged animals (62%, P<0.05) than muscles of young adult animals (0.41 ± 0.07 µM/mg protein in young vs. 0.667 ± 0.09 µM/mg protein in aged, P<0.05) (Figure 1B). While in situ electrically stimulated isometric contractions increased hypoxanthine concentrations in both the young adult (31%, P<0.05) and aged (54%, P<0.05) muscles, allopurinol did not change hypoxanthine concentrations in either control muscles or in muscles after electrically stimulated isometric contractions in young or aged animals (Figure 1B).
Xanthine oxidase catalyzes the reaction of hypoxanthine and oxygen producing xanthine and superoxide. Xanthine abundance was similar in control muscles from young and aged mice. Electrically evoked isometric contractions increased xanthine oxidase in muscles of young adult and aged mice, but this electrically evoked increase was fully blunted by allopurinol treatment. In situ electrically stimulated isometric contractions elevated xanthine levels in the gastrocnemius muscle from young adult animals by 28% (0.364 ±0.05 µM/mg protein in control muscles vs. 0.465 ± 0.07 µM/mg protein in muscles after electrically evoked contractions, P<0.05) and by 86% in aged muscles (0.359 ± 0.06 µM/mg protein control muscles vs. 0.668 ± 0.1 µM/mg protein in muscles after electrically evoked contractions, P<0.05). Allopurinol prevented any increase in xanthine associated with in situ electrically stimulated isometric contractions (Figure 1C).
Hydrogen peroxide (H2O2)
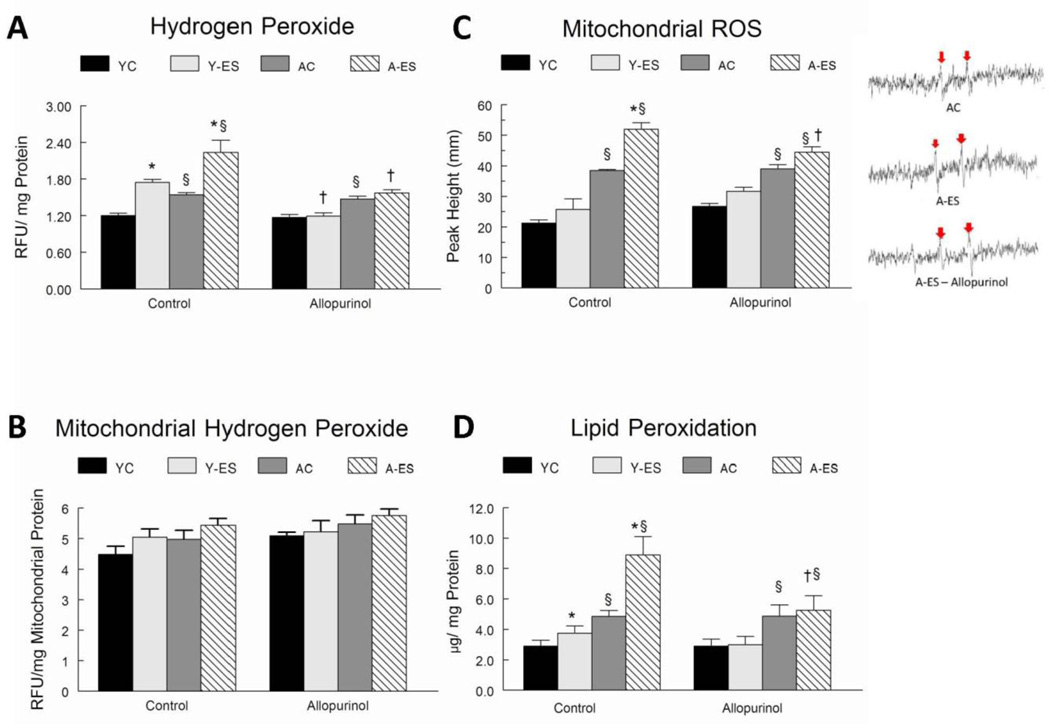
H2O2 was measured as an indicator of oxidant production in muscle samples. Whole muscle homogenate levels of H2O2 were elevated in gastrocnemius muscles after in situ electrically stimulated isometric contractions in both young adult (24%, P<0.05) and aged (44%, P<0.05) animals as compared to the age-matched control limb. H2O2 was higher in both control muscles (21%, P<0.05) and after electrically evoked contractions (39%, P<0.05) in muscles of aged animals as compared to muscles from young adult animals. Allopurinol attenuated the increase in H2O2 associated with in situ electrically stimulated isometric contractions in both age groups (Figure 2A).
Figure 2. Inhibition of xanthine oxidase activity attenuated the increase in hydrogen peroxide (H2O2) concentration, lipid peroxidation and mitochondrial ROS radical production in response to in situ electrically stimulated contractions of aged mice.
(A) The H2O2 concentrations were determined a fluorometrically in total muscle homogenates. Data are expressed as Relative Fluorescent Units (RFU) per mg of total protein in gastrocnemius muscle homogenate. (B) The H2O2 concentrations were determined a fluorometrically in isolated mitochondrial homogenates. Data are expressed as Relative Fluorescent Units (RFU) per mg of total mitochondrial protein. (C) Mitochondria were isolated from control and electrically stimulated gastrocnemius muscles of young adult and old mice that received either placebo or allopurinol treatments. Electron paramagnetic resonance (EPR) spectroscopy was performed to index ROS radical generation. Isolated mitochondrial were incubated with 5,5-dimethyl-1-pyrroline-N-oxide in the presence or absence of excess complex I respiratory substrates glutamate and malate, incubated for 3 min at 37°C. Radical ROS levels were measured at room temperature with instrument settings of 1000 mW, modulation amplitude 1.0 G, receiver gain 1.00 × 104, conversion time 40.960 ms, time constant 40.960 ms and sweep time 41.943 ms, microwave frequency 9.752 GHz, Microwave power 126.90 mW. A representative spectra is shown and the red arrows indicate ROS radical spikes. (D) Data represent combined malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) and are normalized to the total protein concentration in the gastrocnemius muscle homogenate. The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. * significant difference (P<0.05) of in situ electrically stimulated muscle from contra-lateral control muscle; § significant effect of aging within the sham surgery or allopurinol treatment groups (P<0.05). † significant difference (P<0.05) of the allopurinol treatment.
Mitochondrial ROS production
Two approaches were used to determine if allopurinol affected ROS production in mitochondria. In the first method, H2O2 was measured in isolated mitochondria. H2O2 was similar in isolated mitochondria from control and electrically stimulated gastrocnemius muscles of young adult and aged mice. Furthermore, there was no decrease in H2O2 levels measured in mitochondria of animals that were treated with allopurinol (Figure 2B). However, H2O2 diffuses readily from mitochondria, and therefore electron paramagnetic resonance spectroscopy (EPR) was used as a second approach, to measure superoxide and hydrogen radical oxidant production (i.e., ROS) in isolated mitochondria. EPR showed increased ROS radical production in mitochondria from electrically-stimulated vs. control muscles in aged mice, and greater ROS radical production in mitochondria that were isolated from muscles of aged vs. young adult animals. Allopurinol treatment significantly blunted the production of ROS radicals in isolated mitochondria from electrically stimulated muscles of old mice, but it did not change ROS radical production in mitochondria from electrically stimulated muscles of young adult mice (Figure 2C).
Lipid Peroxidation
The levels of malondialdehyde (MDA) and 4-hydroxyalkenals (HAE), both products of lipid peroxidation, were 56% (P<0.05) greater in control gastrocnemius muscles of aged vs. young adult mice (4.8 ± 0.64 µM/mg protein in aged mice vs. 3.06 ± 0.64 µM/mg protein in young animals; P<0.05). Lipid peroxidation was elevated by 29% (3.95 ± 0.74 µM/mg protein vs. 3.06 ± 0.64 µM/mg protein; P<0.05) within the muscles of young adult muscles after in situ electrically stimulated isometric contractions and by 92% (9.2 ± 1.76 µM/mg protein vs. 4.8 ± 0.64 µM/mg protein, P<0.05) in the aged electrically stimulated muscles. Allopurinol did not change in MDA + HAE levels that were associated with electrically evoked contractions in the young adult (3.22 ± 0.61 µM/mg control muscles vs. 3.27 ± 0.78 µM/mg in muscles after electrically evoked contractions, P>0.05) or aged muscles (5.17 ± 1.09 µM/mg proteins in control muscles vs. 5.68 ± 1.2 µM/mg proteins in muscles after electrically evoked contractions, P>0.05). Allopurinol did not depress lipid peroxidation in control muscles of aged mice (4.80 ± 0.64 µM/mg protein after sham surgery vs. 5.17 ± 1.09 µM/mg protein after allopurinol treatment, P>0.05) (Figure 2D).
Glutathione Levels
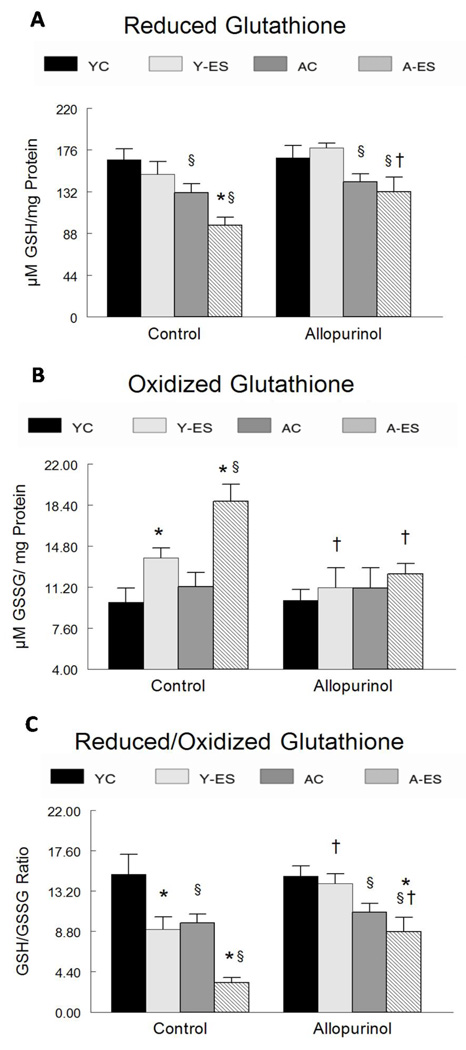
Reduced glutathione (GSH) is a major tissue antioxidant that provides reducing equivalents for the reduction of hydrogen peroxide to water. In a reaction catalyzed by glutathione peroxidase, two GSH molecules form a disulfide bond resulting in the oxidized form of glutathione (GSSG). The ratio of reduced to oxidized glutathione (GSH/GSSG) is used as an indicator of oxidative stress. GSH was decreased in gastrocnemius muscles with aging and in muscles of old mice after electrically evoked contractions, but partially rescued by allopurinol treatment. As shown in Figure 3A, the concentration of GSH was ~21%, (P<0.05) lower in muscles of aged as compared with young mice. Although it approached significance (p=0.066), the GSSG concentration was not statistically different in muscles obtained from young or aged mice (Figure 3B). The ratio of reduced to oxidized glutathione (GSH/GSSG) was 35% lower (P<0.05) in muscles of aged vs. young adult animals (Figure 3C). The GSH/GSSG ratio was reduced in gastrocnemius muscles after electrically evoked contractions in both young animals (15.0 ± 2.2 in control muscles vs. 9.0 ± 1.4 in muscles after electrically evoked contractions, P<0.05) and in aged animals (9.7 ± 0.98 in control muscles vs. 3.2 ± 0.54 in muscles after electrically evoked contractions, P<0.05) (Figure 3C). Allopurinol treatment prevented the decrease in the GSH/GSSG ratio in muscles from young mice after electrically evoked contractions, and partially attenuated the decrease in the GSH/GSSG ratio from control muscles from aged mice after electrically evoked contractions. These data suggest that aging increased oxidative stress and consequently lowered the GSH/GSSG ratio in these muscles, thus reducing the ability of the gastrocnemius muscle to tolerate increased oxidative production resulting from electrically evoked contractions.
Figure 3. Concentration of reduced glutathione (GSH), oxidized glutathione (GSSG) and the GSH/GSSG ratio.
(A) The concentration of reduced glutathione is expressed as µM GSH normalized to total protein concentration (mg) in the gastrocnemius homogenate. (B) The concentration of oxidized glutathione is expressed as µM GSSG normalized to the total protein concentration (mg) of the gastrocnemius homogenate. (C) Data are depicted as the ratio of GSH to GSSG normalized to total protein content. Lower ratios are an indication of increased oxidative stress. The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. * significant difference (P<0.05) from muscles after repeated in situ electrically stimulated isometric contractions as compared to the from contra-lateral control muscle; § significant difference (P<0.05) within either the sham surgery or allopurinol treatment groups due to aging. † significant difference (P<0.05) due to the allopurinol treatment.
Glutathione Peroxidase (GPx)
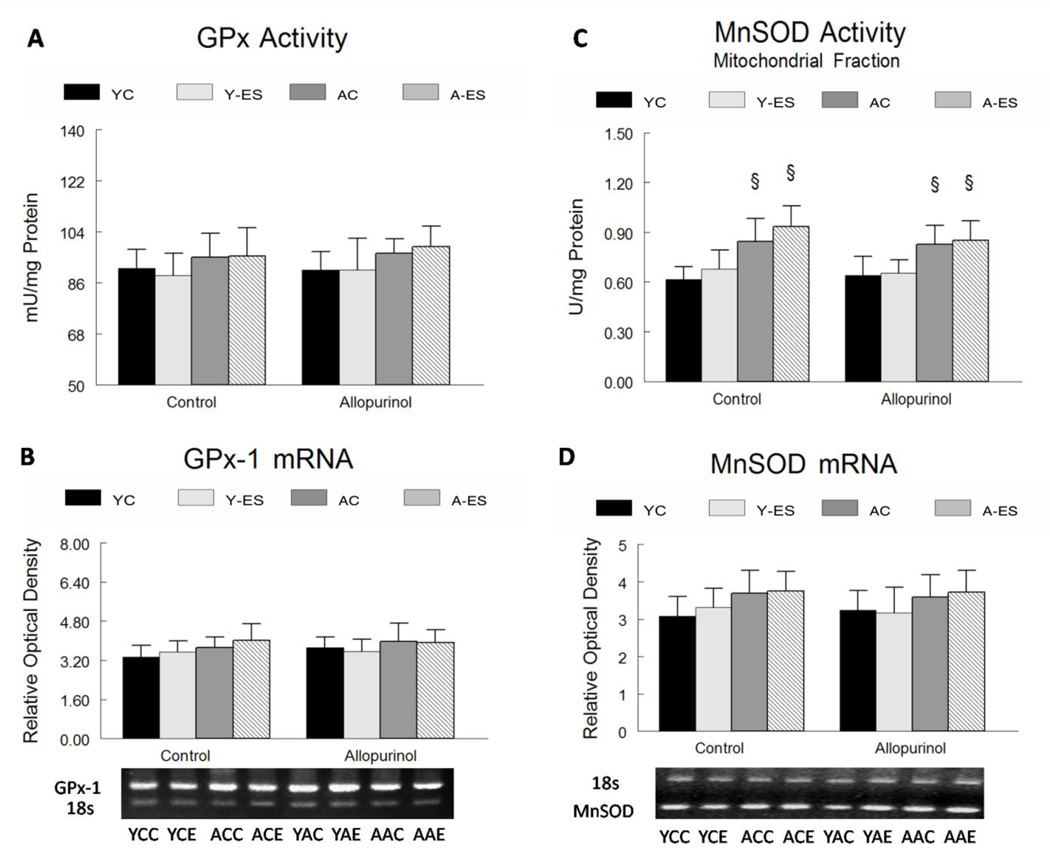
Neither aging, in situ electrically stimulated isometric contractions, nor xanthine oxidase inhibition produced any significant changes in glutathione peroxidase enzyme activity or GPx-1 mRNA levels in mouse gastrocnemius muscles (Figure 4A and Figure 4B).
Figure 4. Glutathione peroxidase (GPx) manganese superoxide dismutase (MnSOD) activity and mRNA regulation with in situ electrically stimulated isometric contractions and allopurinol treatment.
The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. § significant difference (P<0.05) within the sham surgery or allopurinol treatment groups due to aging. (A) Total GPx activity is expressed as mU of GPx per ml of homogenate normalized to mg of to total protein concentration in the gastrocnemius homogenate. (B) GPx-1 mRNA expression was determined from the total muscle homogenate by RT-PCR. The data are expressed as optical density (OD) × band area normalized to 18s rRNA, and expressed in relative optical density. The inserts show representative gels for GPx-1 mRNA and 18s rRNA in young and aged (control and electrically stimulated) gastrocnemius muscle. (C) MnSOD activity was determined in the mitochondrial fraction that was obtained from the gastrocnemius muscle. MnSOD activity is expressed as mU of MnSOD per ml of homogenate normalized to mg of protein in the sample. (D) MnSOD mRNA expression was determined from the muscle by RT-PCR. The data are expressed as optical density (OD) × band area, normalized to 18s rRNA and expressed as relative optical density. The inserts show representative gels for MnSOD mRNA and 18s rRNA in young and aged (control and after in situ electrically stimulated isometric contractions) gastrocnemius muscle.
YCC= Young, Control surgery, Control non-electrically stimulated; YCE= Young, Control surgery, Electrically stimulated; YAC=Young, Allopurinol, Control non-electrically stimulated; YAE= Young, Allopurinol, Electrically stimulated; ACC= Aged, Control surgery, Control non-electrically stimulated; ACE= Aged, Control surgery, Electrically evoked contractions; AAC= Aged, Allopurinol, Control non-electrically stimulated; AAE Aged, Allopurinol, Electrically stimulated
Manganese Superoxide Dismutase (MnSOD)
MnSOD activity was greater (37%, P<0.05) in the gastrocnemius muscles from aged animals compared to young adults (Figure 4C). Neither in situ electrically stimulated isometric contractions nor allopurinol had any significant affect on MnSOD activity in either age group. Aging, in situ electrically stimulated isometric contractions and xanthine oxidase inhibition all failed to affect muscle levels of MnSOD mRNA (Figure 4D).
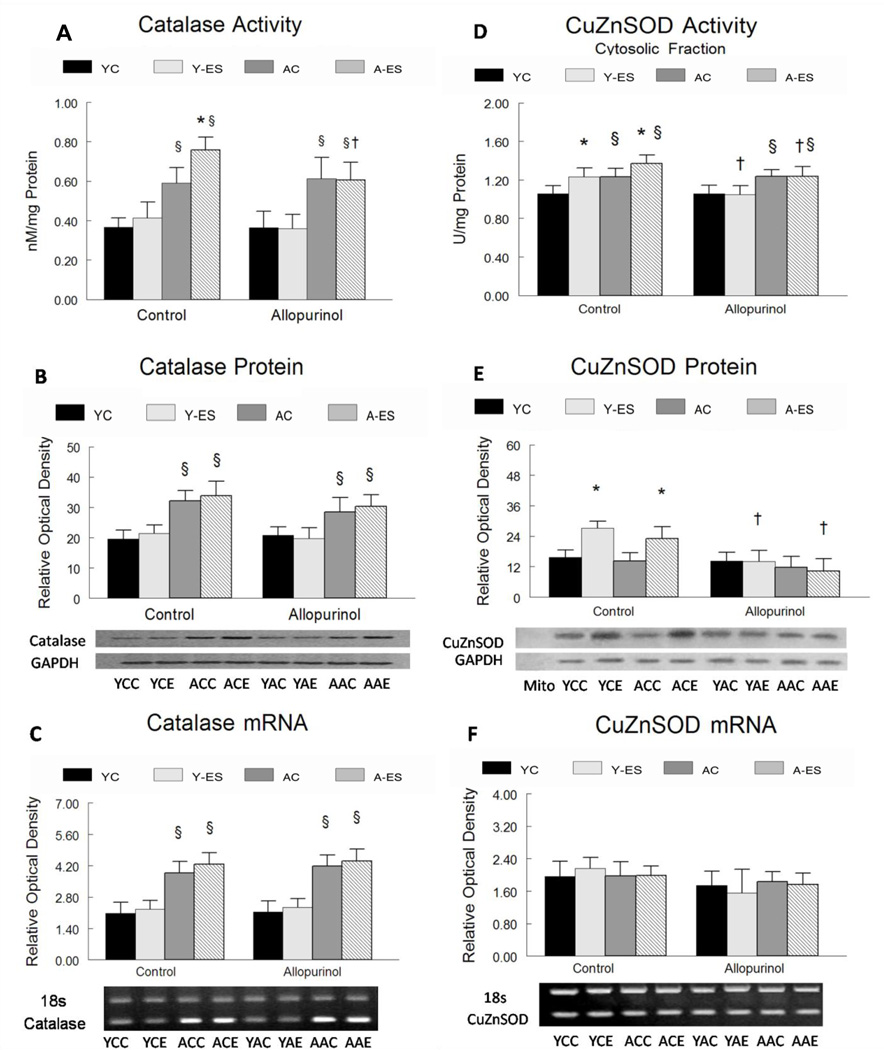
Catalase
Allopurinol blunted the increase in catalase activity that occurred in muscles after electrically evoked contractions, but it did not change the aging-associated increase in muscle catalase activity. The enzymatic activity of catalase was greater (66%, P<0.05) in the gastrocnemius muscles from aged animals compared to young adult animals (Figure 5A) whereas catalase mRNA was ~180% greater (P<0.05) in muscles from old vs. young adult mice (Figure 5C). Neither catalase protein levels, nor mRNA content, were affected by allopurinol. In situ electrically stimulated isometric contractions increased the catalase activity by ~20% (P<0.05) in the muscles from aged animal, but it had no affect on catalase activity in muscles from the young adult animals. However, in aged gastrocnemius muscles, electrically evoked contractions did not elicit a significant increase in catalase protein abundance or mRNA content. The increase in catalase activity associated with electrically evoked contractions in the aged gastrocnemius muscles was completely attenuated by allopurinol administration (0.612 ± 0.11 nM/min/mg in control muscles vs. 0.606 ± 0.1 nM/min/mg in muscles after electrically evoked contractions, P>0.05). In situ electrically stimulated isometric contractions did not alter catalase protein abundance, or mRNA content in the gastrocnemius muscle from young adult or aged animals (Figure 5 B and Figure 5C).
Figure 5. Catalase and copper zinc superoxide dismutase (CuZnSOD) activity, protein expression and mRNA regulation with in situ electrically stimulated isometric contractions and allopurinol treatment.
The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. * significant difference (P<0.05) of electrically stimulated muscles from contra-lateral control muscles; § significant difference (P<0.05) within the sham surgery or allopurinol treatment groups due to aging. † significant difference (P<0.05) due to the allopurinol treatment. (A) Total catalase activity is expressed as nM of activity per min normalized to mg of total protein in the gastrocnemius homogenate. (B) Catalase protein expression was determined in the mitochondrial free cytosolic fraction by western immunoblotting. The data are expressed as optical density (OD) × band area, normalized to GAPDH and expressed in relative optical density. The inserts show representative blots for catalase and GAPDH content young and aged gastrocnemius muscle. (C) Catalase mRNA expression was determined by RT-PCR from the total muscle homogenate. The data are expressed as optical density (OD) × band area, normalized to 18s rRNA and expressed in relative optical density. The inserts show representative gels for catalase mRNA and 18s rRNA in gastrocnemius muscles from young and aged (control muscles and in muscles that received in situ electrically stimulated isometric contractions). (D) CuZnSOD activity was determined in the mitochondrial free cytosolic fraction of gastrocnemius muscles. CuZnSOD activity is expressed as mU of CuZnSOD per ml of homogenate normalized to mg of protein in homogenate. (E) CuZnSOD protein abundance was determined in the mitochondrial free cytosolic fraction by western immunoblotting. The data are expressed as optical density (OD) × band area, normalized to GAPDH and expressed in relative optical density. The inserts show representative blots for CuZnSOD and GAPDH content in gastrocnemius muscles. (F) CuZnSOD mRNA expression was determined by RT-PCR from total muscle homogenates. The data are expressed as optical density (OD) × band area, normalized to 18s rRNA and expressed in relative optical density. The inserts show representative gels for CuZnSOD mRNA and 18s rRNA from gastrocnemius muscles after control conditions and in muscles that received in situ electrically stimulated isometric contractions in young and aged mice.
YCC= Young, Control surgery, Control non-electrically stimulated; YCE= Young, Control surgery, Electrically stimulated; YAC=Young, Allopurinol, Control non-electrically stimulated; YAE= Young, Allopurinol, Electrically stimulated; ACC= Aged, Control surgery, Control non-electrically stimulated; ACE= Aged, Control surgery, Electrically stimulated; AAC= Aged, Allopurinol, Control non-electrically stimulated; AAE Aged, Allopurinol, Electrically stimulated
Copper-Zinc Superoxide Dismutase (CuZnSOD)
Allopurinol blunted the increase in CuZnSOD activity from muscles after electrically evoked contractions but not aging. CuZnSOD activity was greater (11%, P<0.05) in control gastrocnemius muscles of aged animals as compared with young animals (1.06 ± 0.08 U/mg young vs. 1.23 ± 0.08 U/mg aged, P<0.05, P<0.05) (Figure 5D). Allopurinol treatment did not affect CuZnSOD activity in control muscles. In situ electrically stimulated isometric contractions increased CuZnSOD activity in muscles of young adult (16%, P<0.05) and aged (11%, P<0.05) animals. Electrically evoked contractions also increased CuZnSOD protein abundance by 73% (P<0.05) in muscles from young animals and by 62% (P<0.05) in muscles from aged mice (Figure 5E). Allopurinol suppressed the increase in CuZnSOD activity and protein content from gastrocnemius muscles after electrically evoked contractions in both young and aged mice. CuZnSOD mRNA levels were not altered by age, electrically evoked contractions or allopurinol (Figure 5F).
Pro-apoptotic mitochondrial signaling proteins
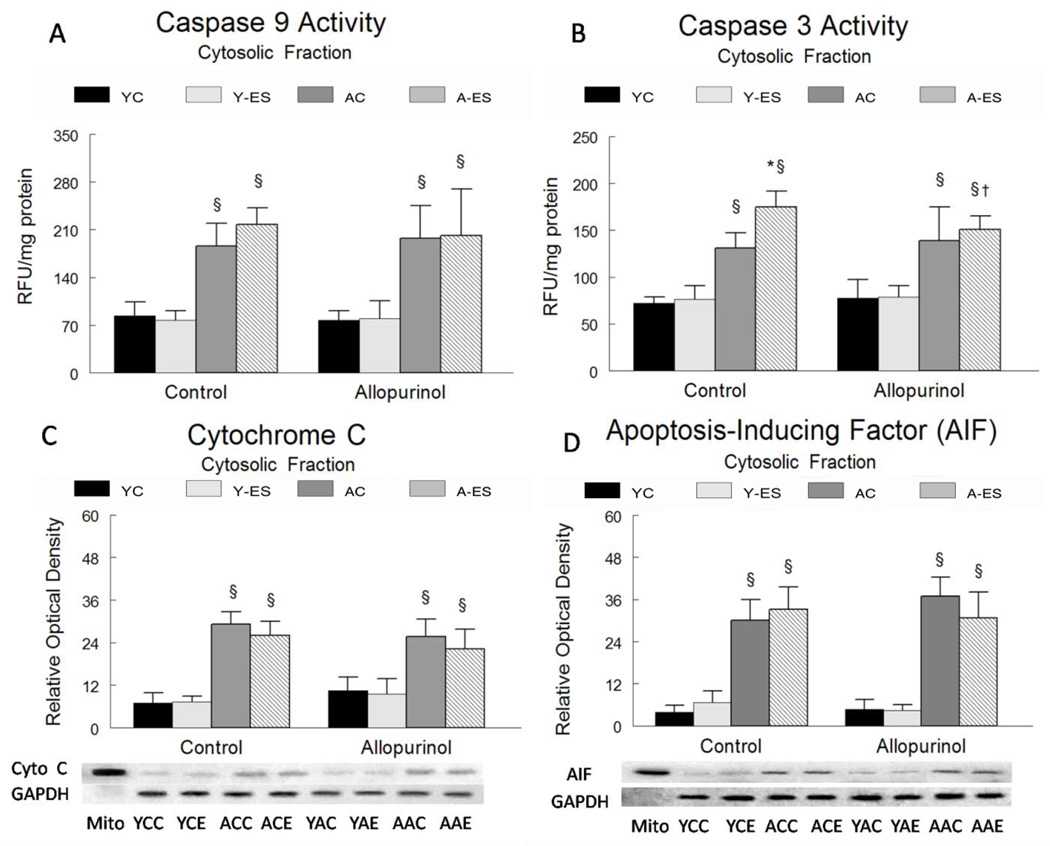
Cytosolic levels of gastrocnemius caspase 9 and caspase 3 activities were elevated with aging, but they were not affected by electrically evoked contractions or allopurinol treatment. Muscles from aged animals had greater levels of caspase 9 activity (122%, P<0.05) and caspase 3 activity (85%, P<0.05), as compared to young adult mice. Although electrically stimulated contractions did not increase caspase 9 activity, caspase 3 activity was 30% greater (P<0.05) in muscles of aged mice after electrically evoked contractions as compared to control muscles. Allopurinol suppressed the increase in caspase 3 activity that was induced by electrically evoked contractions. Neither electrically stimulated contractions nor allopurinol altered apoptotic signaling in the muscles of young adult animals (Figure 6A, Figure 6B).
Figure 6. Aging increases apoptotic signaling in skeletal muscle.
The normalized data for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles are presented as mean ± SEM. * significant difference (P<0.05) from muscles that received in situ electrically stimulated isometric contractions muscle and contra-lateral control muscle; § significant difference (P<0.05) within the sham surgery or allopurinol treatment groups due to aging. † significant difference (P<0.05) due to the allopurinol treatment. Caspase 9 (A) and caspase 3 (B) levels were determined in the mitochondrial free cytosolic fraction of the gastrocnemius muscle homogenate by a fluorometric assay. Data are expressed as Relative Fluorescent Units (RFU) per mg of protein in the homogenate. (C) Cytosolic cytochrome c protein expression was determined in the mitochondrial free fraction of the gastrocnemius homogenate by western immunoblotting. The data are expressed as optical density (OD) × band area. The data are normalized to GAPDH and expressed in relative optical density. The inserts show representative blots for cytochrome c and GAPDH in gastrocnemius muscles from young and aged mice (control and repetitive electrically stimulated contractions). (D) Cytosolic AIF protein expression was determined in the mitochondrial free fraction of the gastrocnemius homogenate by western immunoblotting. The data are expressed as optical density (OD) × band area, normalized to GAPDH and expressed in relative optical density. The inserts show representative blots for AIF and GAPDH in young and aged (control and repetitive loading) gastrocnemius muscle.
YCC= Young, Control surgery, Control non-electrically stimulated; YCE= Young, Control surgery, Electrically stimulated; YAC=Young, Allopurinol, Control non-electrically stimulated; YAE= Young, Allopurinol, Electrically stimulated; ACC= Aged, Control surgery, Control non-electrically stimulated; ACE= Aged, Control surgery, Electrically stimulated; AAC= Aged, Allopurinol, Control non-electrically stimulated; AAE Aged, Allopurinol, Electrically stimulated
Mitochondrial proteins cytochrome c and AIF are released from the mitochondria to the cytosol in response to pro-apoptotic stimuli. Cytochrome c and AIF are apoptotic intermediates that when present in the cytosol, suggest that mitochondria permeability has increased as a result of an increase in the formation of mitochondrial pore openings. Both cytochrome c (~237%) (Figure 6C) and AIF (~725%) (Figure 6D) were higher in the cytosolic fractions of gastrocnemius muscles from aged animals compared to muscles from young adult mice. Neither electrically evoked contractions nor allopurinol treatment altered cytochrome c or AIF accumulation in the cytosol of muscles of young or aged mice.
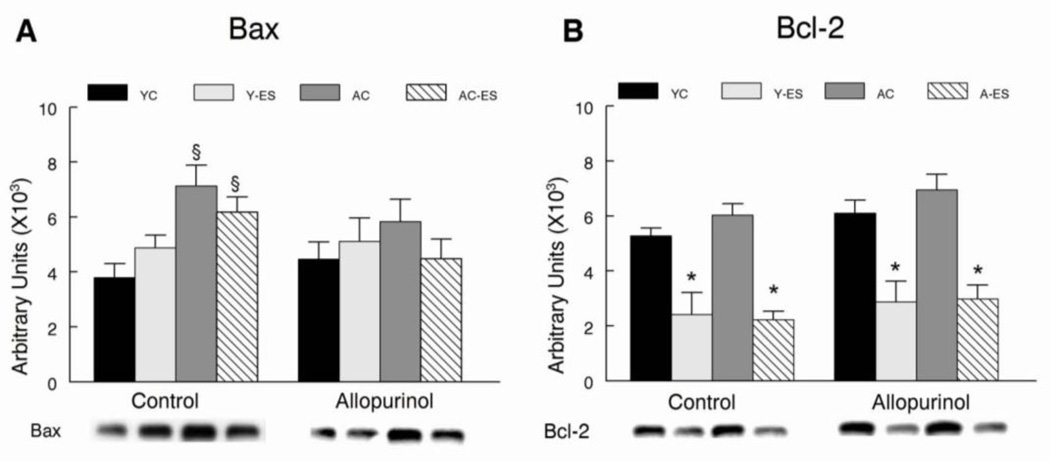
Bax and Bcl-2 protein abundance was estimated by western blot analyses from isolated mitochondria that were obtained from control and electrically stimulated gastrocnemius muscles. Bax protein abundance was greater in control mitochondria from aged animals than in young adult animals, but allopurinol removed any differences in mitochondrial Bax from young and aged animals. Electrically-evoked contractile activity did not alter Bax abundance in mitochondria homogenates from either young adult or aged animals. Bcl-2 protein abundance was similar in mitochondria from control muscles of young adult and aged mice. However, repeated electrically-evoked isometric contractions reduced Bcl-2 levels similarly in muscle mitochondrial homogenates from both young adult and aged animals.
Muscle Functional Measurements
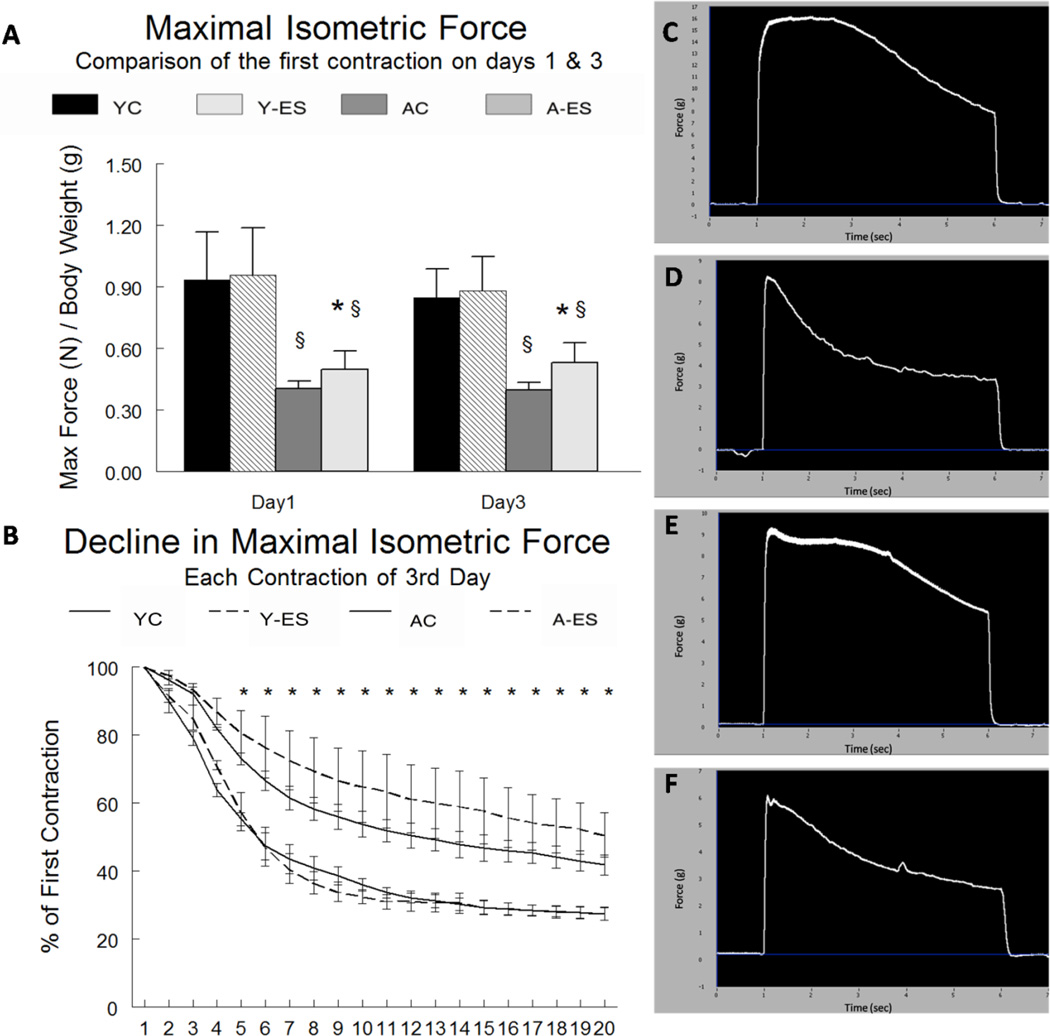
Maximal isometric plantar flexor muscle force was measured as an indicator of muscle function. The gastrocnemius muscle provides the greatest contribution to plantar flexion. The maximal isometric force recorded for the third day (which was the first contraction of that day) was normalized to the animal’s body weight. Maximal isometric force was depressed with aging, but improved with allopurinol treatment. Maximal isometric force per gram of body weight was lower (−36.6%, P<0.05) in the plantar flexor muscles of aged animals as compared to the young adult animals. Maximal isometric force in the plantar flexors from aged mice was greater (35%, P<0.05) in animals provided allopurinol as compared to animals given the sham surgery, but allopurinol had no effect on force production in the young adult animals. Maximal isometric force did not differ between the first and third session of electrically evoked contractions in control or allopurinol groups for either young adult or aged mice (Figure 8A).
Figure 8. Maximal isometric forces from the plantar flexor muscles.
(A) Data are presented for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles as mean ± SEM. of the maximal isometric force (N) recorded on the third day of in situ electrically stimulated contractions by the left plantar flexor muscle group normalized to the body weight in grams (g BW) of the animal. § significant effect of aging within the sham surgery or allopurinol treatment groups (P<0.05). (B) Data are expressed as the mean ± SEM of the relative difference between the maximal isometric forces produced on the each contraction to the maximal isometric force on the first contraction. All force measurements were normalized to body weight (g). * indicates a significant difference (P<0.05) in both the aged sham surgery and allopurinol treatment animals versus the young adult sham surgery and allopurinol treatment animals. (C–F) Representative force × time curves from the 3rd session of electrically stimulated muscle contractions in the allopurinol treated young adult and aged animals. (C) First contraction of the 3rd day in a young adult animal. (D) Twentieth contraction of the 3rd day in a young adult animal. (E) First contraction of the 3rd day in an aged animal. (F) Twentieth contraction of the 3rd day in an aged animal.
The rate of fatigue for the plantar flexors was assessed by calculating the net loss of force throughout the session of electrically evoked contractions relative to the first contraction in the session. Fatigue resistance was greater in muscles from the aged animals, but allopurinol treatment had no effect on isometric muscle fatigue in either young adult or aged animals (Figure 8B).
Discussion
The main findings from this study are that in skeletal muscle: 1) Acute in situ electrically stimulated isometric contractions and aging increased xanthine oxidase, hypoxanthine, xanthine, and markers of oxidative stress and damage. 2) Reducing xanthine oxidase via allopurinol suppressed the in situ electrically stimulated isometric contraction-associated elevations in H2O2, and lipid peroxidation, prevented the loss of GSH that was induced by electrically evoked contractions, and prevented the increase of catalase and CuZnSOD activities, but had no effect on GPx and MnSOD activity or mRNA in electrically activated muscles of aged animals. 3) Allopurinol suppressed the in situ electrically stimulated isometric contraction-induced increase in caspase 3 activity in muscle homogenates and Bax protein abundance in isolated mitochondria of muscles from aged animals, but it did not reduce other markers of apoptotic signaling associated with aging or electrically evoked contractions. Increased pro-oxidant production and damage have been shown to be associated with exercise [17, 18, 20, 23, 24, 34, 36, 40–42] and aging [12, 34, 36, 43–46]. Our data are consistent with these findings, because we found that both in situ electrically stimulated isometric contractions and aging lead to increases in skeletal muscle H2O2 concentrations, lipid peroxidation, xanthine oxidase activity and a decrease in the GSH/GSSG ratio and greater mitochondrial ROS production. Nevertheless, maximal electrically-evoked muscle contractions did not result in any obvious structural damage or inflammation to the fibers in the gastrocnemius muscle (Supplemental Figure 1). H2O2 is highly permeable to membranes and therefore it can exit the mitochondria to the cytosol readily. Therefore, it was not surprising that H2O2 abundance in mitochondria fractions was not altered by electrical contractions or allopurinol (Figure 2B). Nevertheless, our data clearly show that H2O2 abundance is elevated in muscle homogenates (Figure 2A) and ROS radicals are increased in isolated muscle mitochondria (Figure 2C) after electrically-evoked muscle contractions in aged as compared to young adult mice. Allopurinol suppresses mitochondrial ROS production in electrically contracting muscles (Figure 2A) and mitochondria (Figure 2C) from aged mice. The suppression of H2O2 that was measured in muscle homogenates after allopurinol treament in electrically stimulated muscle of young adult mice (Figure 2A) was likely from non-mitochondrial ROS sources, because ROS radical production was unaffected by allopurinol treatment in mitochondria of young adult mice after evoked contractions (Figure 2C), yet muscle homogenates for H2O2 (Figure 2A) and lipid peroxidation (Figure 2D) were suppressed in muscles from young animals. In contrast, it is likely that both mitchondria and non-mitochondrial sources of ROS were suppressed after electrically evoked contractions in aged mice that received the allopurinol treatement.
Although allopurinol appeared to have a direct effect on mitochondria of aged mice, the mechanism for this is not known. One possibility is that this observation might represent an indirect effect on mitochondrial ROS production, by reducing oxidants (e.g., xanthine oxidase) in the mitochondrial niche and the cell’s cytoplasm, which in turn, reduces oxidation of mitochondrial proteins and reduces mitochondrial impairment and ROS production. Total muscle ROS accumulation could also be suppressed by lowering xanthine oxidase without changing other ROS sourses, because exercise-induced accumulation of ROS can originate from other cellular sources including NADPH oxidases located within the sarcoplasmic reticulum, transverse tubules, and the fiber sarcolemma (reviewed in [47]). It is also possible that allopurinol may have had a greater effect in the fast contracting gastrocnemius as compared to the responses that might have been found if a slow contracting muscle had been examined. This is feasible, because mitochondria from fast type II muscle fibers, such as in the gastrocnemius muscles that was investigated in the current study, have higher levels of ROS production than mitochondria from slow type I muscle fibers [48]. However, additional work is required to investigate this possibility.
Although either electrically-evoked or voluntary isometric exercise that exceeds ~60% of maximal force production produces muscle ischemia [49], and ischemia can produce xanthine oxidase, we cannot rule out the possibility that ischemia-induced xanthine oxidase could differ between the repeated electrically evoked isometric muscle model used in this study, and models that would use voluntary exercise.
Non-damaging isometric muscle contractions have been shown to elevate superoxide production in the extracellular space of the gastrocnemius muscle in mice [33]. We would anticipate that the additional superoxide from the isometric contractions in the current study would be quickly converted to H2O2 by SOD, and this conversion could account for most of the observed increase H2O2 content. H2O2, has the potential to induce widespread oxidant damage simply because it easily crosses cellular membranes.
Xanthine oxidase is a source of oxidative stress in muscles after electrically stimulated isometric contractions
Xanthine oxidase produces superoxide in the cytosol of contracting rodent skeletal muscles [41]. In addition, xanthine oxidase has been shown to be present in endothelial cells from human skeletal muscle [50] and despite its location, it is relevant to muscle function, because it affects the responses of human muscle to exercise [51–53]. The present data suggest that muscle xanthine oxidase activity increased with aging, and it is elevated with in situ electrically stimulated isometric contractions in muscles of both aged and young adult mice. The inhibition of xanthine oxidase via allopurinol reduced the indices of oxidative stress associated with electrically evoked contractions (H2O2 concentration, lipid peroxidation and the GSH/GSSG ratio). These data are consistent with the idea that xanthine oxidase makes an important contribution to oxidant production during exhaustive exercise [1, 24, 33, 34, 41]. Furthermore, increases in post-exercise concentrations of hypoxanthine are accurate predictors of muscle energy exhaustion during exercise [54]. It is likely that the additional hypoxanthine was converted to xanthine and superoxide via xanthine oxidase. Although we did not measure superoxide formation in the cytosol, recent data show that superoxide is produced and released into the extracellular space via after isometric muscle [33].
In aged mice, hypoxanthine, which is upstream from xanthine oxidase, was increased in muscles of mice after electrically evoked contractions, when xanthine oxidase activity was inhibited by allopurinol (Figure 1). In contrast, xanthine, H2O2 and lipid peroxidation, were all blunted by allopurinol in muscles of old mice after electrically evoked contractions. The increased hypoxanthine likely occurred as a result of reducing the conversion of hypoxanthine to xanthine, when xanthine oxidase was inhibited by allopurinol. Acute inhibition of xanthine oxidase by allopurinol reduced the indices of oxidative stress in muscles that were elevated by electrically evoked contractions but not in control muscles of aged mice.
To our knowledge, this is the first study to show that hypoxanthine concentration was greater in muscles of aged vs. young adult mice; yet, the concentration of xanthine was similar in muscles of young and aged animals. It is possible that xanthine oxidase in muscles of aged mice was less efficient at converting hypoxanthine to xanthine and superoxide, and this resulted in the accumulation of hypoxanthine. However, additional investigations are needed to determine if this is the case. Another possibility is that that xanthine oxidase was not limiting, but rather, xanthine was converted to uric acid at a rate similar to xanthine conversation, which yielded no net change in xanthine levels in muscles of aged animals. When xanthine oxidase activity was inhibited in muscles from aged control animals, there was no change in either hypoxanthine, or xanthine concentrations.
The impact of allopurinol on endogenous antioxidant enzymes in aging in muscles after electrically evoked contractions
In general, mRNA content for antioxidant enzymes was not altered by allopurinol or aging. These data suggest that the observed differences in protein content and activity for antioxidant enzymes arise from post-transcriptional and/or post-translational modifications [34, 36]. In contrast with other antioxidant enzymes, catalase mRNA was greater in muscles of old vs. young animals (Figure 5C). The increased catalase mRNA, activity and protein content may be an attempt to counterbalance the depletion of glutathione levels that has been observed within aging [13].
It is well known that strenuous muscle contraction promotes GSH oxidation and reduces the GSH/GSSH ratio [55]. Aging also decreases the GSH/GSSG ratio in skeletal muscle and this decrease is exacerbated by increased muscle activity [56]. Our data are consistent with these findings and show that aging increased oxidative stress and lowered the GSH/GSSG ratio in muscles from aged animals. This presumably lowered the potential for the gastrocnemius muscles to tolerate the increased oxidative production that occurred in response to the repetitive electrically evoked contractions. In the current study, allopurinol treatment prevented the decrease in the GSH/GSSG ratio in muscles from young mice after electrically evoked contractions, and it partially offset the decrease in the GSH/GSSG ratio that was observed in muscles from non-supplemented aged mice after electrically evoked contractions.
The affects of electrically evoked contractions and xanthine oxidase inhibition on the endogenous antioxidant enzymes
Oxidant sensitive transcription factors such as nuclear factor kappaB (NF-κB) have been shown to up-regulate antioxidant gene expression in response to chronic exercise [41, 57]. However the attenuation of oxidant production via inhibition of xanthine oxidase has been shown to prevent NF-κB activation and the subsequent up-regulation of MnSOD transcriptional activity after exhaustive aerobic treadmill running [41]. In contrast, we did not find evidence for transcriptional regulation of MnSOD, GPx, catalase, CuZnSOD or MnSOD in skeletal muscle after three days of electrically evoked contractions. Nevertheless, the activities of the cytosolic localized antioxidants CuZnSOD and catalase, were greater in both young adult and aged gastrocnemius muscles, in response to in situ electrically stimulated isometric contractions. This might be in part, a result of the need to buffer cytosolic oxidants arising from anaerobic metabolic pathways, as compared aerobic types of exhaustive exercise [41, 57] which, would be expected to have a greater need to buffer mitochondria associated antioxidants arising from oxidative metabolism. In contrast to the lack of increase in antioxidant enzymes resulting from an acute series of muscle contractions over three days as in the current study, it is likely that the antioxidant enzyme adaptations and especially mitochondrial antioxidant enzymes (e.g., MnSOD) would have increased in response to chronic loading types of exercise.
It has been postulated that low levels of oxidative stress can promote beneficial adaptive responses including an improved antioxidant defense capacity, because preventing oxidative stress associated with exercise prevents these positive adaptations [41]. For example, in the current study, inhibition of xanthine oxidase-induced oxidative stress by allopurinol blunted the increase in the cytosolic protein content and activity of CuZnSOD in response to in situ electrically stimulated isometric contractions. Inhibition of xanthine oxidase by allopurinol in aged animals also attenuated the increase in catalase activity associated with electrically evoked contractions. Together, these data suggest that inhibition of xanthine oxidase reduces the need for an increase in antioxidant enzymes in response to electrically evoked contractions.
Allopurinol reduces apoptotic signaling in aged muscles
The current data are consistent with previous findings, showing that aging is associated with increases in apoptotic signaling in skeletal muscle [39]. Mitochondrial proteins, cytochrome c and apoptosis-inducing factor (AIF) and Bax protein abundance were elevated in the cytosolic fraction of the muscle homogenates. Downstream from mitochondrial-released cytochrome c, both the activity of the initiator caspase 9, and the executioner caspase 3, were elevated in muscles of aged as compared to young adult animals.
Aging has been associated with a depolarization of the mitochondrial membrane, decreased mitochondrial respiratory activity and decreased antioxidant enzyme activity [13]. These decreases lead to a detrimental release and accumulation of oxidants within the cells [13]. While most oxidants originate from the mitochondria in aging muscles [12, 58], our data show that xanthine oxidase also contributes to oxidant production in aging and muscles after electrically evoked contractions.
Increased cellular stress can activate redox sensitive pathways that initiate mitochondria apoptotic signaling [39, 59, 60]. Aged skeletal muscle has elevated basal oxidant production, and exhaustive aerobic exercise further increases oxidant production [12] and apoptotic signaling [61]. In this investigation, we show decreased mitochondrial Bcl-2 protein abundance and increased muscle levels of caspase 3 activity, without significant increases in caspase 9 activity or cytosolic cytochrome c in gastrocnemius muscle of aged mice after electrically evoked contractions as compared to control muscles. These findings are consistent with other observations in gastrocnemius muscle in aged rats, where exhaustive exercise increased caspase 3 activity without changes in caspase 9 activity [61]. One possibility is that the increased caspase 3 activity in the aged gastrocnemius muscle may be triggered via the extrinsic apoptotic pathway rather than through the mitochondria.
Allopurinol decreased mitochondrial Bax protein abundance and blunted the increase in caspase 3 activity in gastrocnemius muscle from aged animals after electrically evoked contractions. Although this suggests that xanthine oxidase activity has a role in regulating apoptotic signaling, this is likely not through mitochondria signaling pathways, because allopurinol had no effect on suppressing the elevated levels of cytochrome c, AIF, caspase 9 and caspase 3 associated with aging. Further research is needed to determine the upstream mechanisms that regulate the increase in caspase 3 activity after electrically evoked contractions to determine if allopurinol blunts extrinsic apoptotic signaling in response to aging and muscle contractions.
Allopurinol affects maximal isometric force in aged animal
Loss of muscle force with aging is typically greater than the loss in muscle mass per se. This occurs as a result of both a decrease in muscle mass and altered myosin cross-bridge function, which is due at least in part to oxidative modifications of myosin and actin protein structures (Reviewed in [62]). An important novel finding in this study is that allopurinol administration increased plantar flexor maximal isometric force by 35% (P<0.05), without having an effect on the force production in muscles from young adult animals. Our data in young adult animals differs from recent data from Gomez-Cabrera and colleagues [33] who reported a loss of in vitro maximal force production in extensor digitorum longus and soleus muscles from young (3 mo of age) mice that were incubated with oxypurinol (the active metabolite of allopurinol). These differences may have been the result of different experimental approaches (in vitro vs. in vivo), the compound used to suppress xanthine oxidase (allopurinol vs. oxypurinol), and/or the muscle that was studied. Nevertheless, the data in the current study suggest that suppressing xanthine oxidase has the potential to improve maximal force production in aged muscles. Our in vivo data are not inconsistent with the idea that ROS generation during loading is important in various signaling pathways in muscle that lead to improved muscle force production [63]. However, excessive ROS production can also decrease force production (Reviewed in [64]), and the higher basal ROS levels in aged muscles coupled with the contraction-induced increases in ROS may have contributed to force reduction in the current study.
Recent data have shown that xanthine oxidase inhibits ryanodine receptor and/or dihydropyridine receptor function [65, 66]. This results in dysregulation of calcium release from sarcoplasmic reticulum stores and lowers force production, while reducing or removing xanthine oxidase improves muscle function. Although speculative, the calcium leakage from the sarcoplasmic reticulum as a result of altered s-nitrosylation of the ryanodine receptor by xanthine oxidase [65] could also result in elevated intracellular calcium, which in turn could elevate xanthine oxidase levels and contribute to greater calcium dysregulation and loss of force. Our data are consistent with these data and support a model where, buffering of xanthine oxidase-induced oxidant stress in muscle of old animals may have lowered ROS levels to acceptable levels, so that force production was improved by the allopurinol treatment as a result of improved calcium handling.
Muscle fatigue during repetitive isometric contractions
In the current study, we found that relative fatigue over each session of evoked contractions was less in muscles of aged as compared to young adult animals. This observation is consistent with the literature which suggests that the rate of fatigue decreases with aging [67]. Several possibilities have been proposed to explain this finding, including recent observations that the metabolic cost of producing muscle contractions is decreased in aging [68]. Alternatively, the decreased fatigue may be due to the well known shifting of fiber types towards a greater percentage of type I fibers [69]. Although allopurinol administration did increase maximal isometric force in the aged animals, it did not significantly influence the rate at which force decline in either age group. This is consistent with a recent study that found no improvement in fatigability of hind limb muscles of young mice in response to an in vitro protocol of repeated electrical stimulation, where xanthine oxidase was reduced by oxypurinol [33].
Conclusion
The data in this study indicate that xanthine oxidase derived oxidant production has a wide range of effects on skeletal muscle physiology and function in aged mice. In this study, we sought to determine if xanthine oxidase played an important role in oxidant stress-induced regulation of aging after isometric contractions. We did not anticipate that the relatively short duration of xanthine oxidase inhibition used in the current experimental protocol would be adequate to relieve the chronic basal elevations in oxidative stress that is associated with advanced aging. The novel findings in this study show that compared to control conditions, suppression of xanthine oxidase activity by allopurinol reduced xanthine oxidase activity, H2O2 levels, lipid peroxidation and caspase-3 activity and reduced Bax accumulation in mitochondria of electrically stimulated muscles. In addition, allopurinol prevented the electrically evoked muscle contraction associated loss of GSH, prevented the increase of catalase and CuZnSOD activities, and increased maximal isometric force in the plantar flexor muscles of aged mice after electrically evoked maximal repetitive isometric contractions.
The suppression of antioxidant enzymes by allopurinol in aged muscles after electrically evoked contractions might be viewed as a negative adaptation. However, another perspective is that antioxidant inhibition of xanthine oxidase activity reduced oxidative stress in aged muscles and removed the need for short-term adaptation of the endogenous antioxidant enzymes catalase and CuZnSOD to repetitive isometric contractions. Acute reduction of xanthine oxidase levels in aging muscles by allopurinol, reduced caspase 3 activity and Bax accumulation in mitochondria, but it did not affect other indicators of mitochondria-associated apoptotic signaling. Additional studies are required to determine if long-term inhibition of xanthine oxidase will have an important role in reducing apoptotic signaling in extrinsic pathways. Finally, xanthine oxidase inhibition improved maximal isometric force in the plantar flexor muscles from the aged mice. From a clinical perspective, it is important to determine if allopurinol will provide an effective strategy for reducing oxidant stress and improving loss of muscle function with aging in exercising humans.
Supplementary Material
10 µm thick frozen sections were stained with hematoxylin and eosin. Nuclei are stained blue, and fibers are stained pink. (A) Control, non-contracted muscle. (B). Gastrocnemius muscle section after 3 consecutive days of maximal electrically evoked isometric contractions. There was no obvious increase in inflammation or muscle structural damage in the repetitively contracted muscles as compared to intra-animal control muscles.
Figure 7. Allopurinol blunts the increase in mitochondrial Bax that is elevated by electrically-evoked contractions in skeletal muscle from aged mice.
(A) Bax protein abundance was estimated by western blot analyses in homogenates from isolated mitochondria. These data suggest that Bax is greater in mitochondria from aged animals, but allopurinol removed any differences in mitochondrial Bax from young and aged animals. Electrically-evoked activity did not alter Bax abundance in mitochondria homogenates from young adult mice buit it blunted the increase in mitochondrial Bax in muscles from aged animals. (B) Bcl-2 protein abundance was estimated in homogenates from gastrocnemius muscles of control muscles and muscles after three days of electrically evoked contractions. Bcl-2 levels were similar in control muscles from young adult and aged mice. Repeated electrically-evoked isometric contractions reduced Bcl-2 levels similarly in muscle homogenates from young adult and aged animals. Data are presented for young control (YC), young electrically stimulated (Y-ES), aged control (AC) and aged electrically stimulated (A-ES) muscles, as mean ± SEM. The inserts show representative blots of mitochondrial Bax and Bcl-2. Ponceau S staining of the membranes confirmed equal loading and electrotransfer of the proteins from the gels to the membranes.
Acknowledgements
This study was supported by funding from the National Institutes of Health: National Institute on Aging Grant R01AG021530.
Abbreviations
- AIF
apoptosis inducing factor
- Apaf-1
apoptosis peptidase activating factor 1
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell leukemia/lymphoma-2
- BHT
butylated hydroxytoluene
- CuZnSOD
Copper-Zinc Superoxide Dismutase
- GPx
Glutathione Peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HAE
4-hydroxyalkenals
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
- MnSOD
Mitochondrial Manganese Superoxide Dismutase
- NF-κB
nuclear factor kappaB
- NFM
non-fat milk protein
- RFU
relative fluorescent units
- TBS-T
Tris-buffered saline with 0.05% Tween-20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hellsten Y, Ahlborg G, Jensen-Urstad M, Sjodin B. Indication of in vivo xanthine oxidase activity in human skeletal muscle during exercise. Acta Physiol Scand. 1988;134:159–160. doi: 10.1111/j.1748-1716.1988.tb08475.x. [DOI] [PubMed] [Google Scholar]
- 2.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Ji LL, Gomez-Cabrera MC, Vina J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl Physiol Nutr Metab. 2007;32:930–935. doi: 10.1139/H07-098. [DOI] [PubMed] [Google Scholar]
- 6.Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.DeMartino GN, Ordway GA. Ubiquitin-proteasome pathway of intracellular protein degradation: implications for muscle atrophy during unloading. Exerc Sport Sci Rev. 1998;26:219–252. [PubMed] [Google Scholar]
- 8.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806–C812. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani G, Madeddu C, Maccio A, Gramignano G, Lusso MR, Massa E, et al. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol Biomarkers Prev. 2004;13:1651–1659. [PubMed] [Google Scholar]
- 10.Kagan VE, Tyurina YY, Bayir H, Chu CT, Kapralov AA, Vlasova II, et al. The "pro-apoptotic genies" get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem Biol Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565:309–323. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 13.Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev. 2007;128:286–292. doi: 10.1016/j.mad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid MB. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 18.Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Cur Med Chem. 2001;8:829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 19.Corte ED, Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972;126:739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino T, Nishino T. The conversion from the dehydrogenase type to the oxidase type of rat liver xanthine dehydrogenase by modification of cysteine residues with fluorodinitrobenzene. J Biol Chem. 1997;272:29859–29864. doi: 10.1074/jbc.272.47.29859. [DOI] [PubMed] [Google Scholar]
- 21.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, et al. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 22.Ashraf M, Samra ZQ. Subcellular-Distribution of Xanthine-Oxidase During Cardiac Ischemia and Reperfusion - An Immunocytochemical Study. J Submicr Cyt Path. 1993;25:193–201. [PubMed] [Google Scholar]
- 23.Duarte JA, Appell HJ, Carvalho F, Bastos ML, Soares JM. Endothelium-derived oxidative stress may contribute to exercise-induced muscle damage. Int J Sports Med. 1993;14:440–443. doi: 10.1055/s-2007-1021207. [DOI] [PubMed] [Google Scholar]
- 24.Vina J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardo FV, et al. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev S, Davies KJA. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 26.McCord JM, Roy RS, Schaffer SW. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv Myocardiol. 1985;5:183–189. [PubMed] [Google Scholar]
- 27.Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254:G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- 28.McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol. 2005;25:1623–1628. doi: 10.1161/01.ATV.0000170827.16296.6e. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2009 doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoutsen B, de Jong JW. Age-dependent increase in xanthine oxidoreductase differs in various heart cell types. Circ Res. 1987;61:604–607. doi: 10.1161/01.res.61.4.604. [DOI] [PubMed] [Google Scholar]
- 32.Aranda R, Domenech E, Rus AD, Real JT, Sastre J, Vina J, et al. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res. 2007;41:1195–1200. doi: 10.1080/10715760701481461. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Cabrera MC, Close GL, Kayani AC, McArdle A, Vina J, Jackson MJ. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R2–R8. doi: 10.1152/ajpregu.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan MJ, Jackson JR, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, et al. Aging-Dependent Regulation of Antioxidant Enzymes and Redox Status in Chronically Loaded Rat Dorsiflexor Muscles. J Gerontol A Biol Sci Med Sci. 2008;63:1015–1026. doi: 10.1093/gerona/63.10.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Dabkowski ER, Williamson CL, Bukowski VC, Chapman RS, Leonard SS, Peer CJ, et al. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol. 2009;296:H359–H369. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- 40.Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc. 2000;32:1576–1581. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. Journal of Applied Physiology. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 43.Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–622. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 45.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol. 1990;258:C429–C435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]
- 46.Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, et al. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854:102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- 47.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011 doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290:C844–C851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 49.Bonde-Petersen F, Robertson CH., Jr Blood flow in "red" and "'white" calf muscles in cats during isometric and isotonic exercise. Acta Physiol Scand. 1981;112:243–251. doi: 10.1111/j.1748-1716.1981.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 50.Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol. 1997;498(Pt 1):239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Cabrera MC, Martinez A, Santangelo G, Pallardo FV, Sastre J, Vina J. Oxidative stress in marathon runners: interest of antioxidant supplementation. Br J Nutr. 2006;96(Suppl 1):S31–S33. doi: 10.1079/bjn20061696. [DOI] [PubMed] [Google Scholar]
- 52.Delample D, Durand F, Severac A, Belghith M, Mas E, Michel F, et al. Implication of xanthine oxidase in muscle oxidative stress in COPD patients. Free Radic Res. 2008;42:807–814. doi: 10.1080/10715760802429039. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Cabrera MC, Pallardo FV, Sastre J, Vina J, Garcia-del-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. JAMA. 2003;289:2503–2504. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- 54.Bianchi GP, Grossi G, Bargossi AM, Fiorella PL, Marchesini G. Can oxypurines plasma levels classify the type of physical exercise? J Sports Med Phys Fitness. 1999;39:123–127. [PubMed] [Google Scholar]
- 55.Sen CK, Marin E, Kretzschmar M, Hanninen O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol. 1992;73:1265–1272. doi: 10.1152/jappl.1992.73.4.1265. [DOI] [PubMed] [Google Scholar]
- 56.Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, et al. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp Gerontol. 2010;45:882–895. doi: 10.1016/j.exger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 58.Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, et al. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 59.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sciences. 2009;84:468–481. doi: 10.1016/j.lfs.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocturk S, Kayatekin BM, Resmi H, Acikgoz O, Kaynak C, Ozer E. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol. 2008;102:515–524. doi: 10.1007/s00421-007-0612-7. [DOI] [PubMed] [Google Scholar]
- 62.Prochniewicz E, Thompson LV, Thomas DD. Age-related decline in actomyosin structure and function. Exp Gerontol. 2007;42:931–938. doi: 10.1016/j.exger.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid MB, Durham WJ. Generation of reactive oxygen and nitrogen species in contracting skeletal muscle: potential impact on aging. Ann N Y Acad Sci. 2002;959:108–116. doi: 10.1111/j.1749-6632.2002.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 64.Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawler JM, Kim JH, Kwak HB, Barnes WS. Redox modulation of diaphragm contractility: Interaction between DHPR and RyR channels. Free Radic Biol Med. 2010;49:1969–1977. doi: 10.1016/j.freeradbiomed.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA. Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R729–R739. doi: 10.1152/ajpregu.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
10 µm thick frozen sections were stained with hematoxylin and eosin. Nuclei are stained blue, and fibers are stained pink. (A) Control, non-contracted muscle. (B). Gastrocnemius muscle section after 3 consecutive days of maximal electrically evoked isometric contractions. There was no obvious increase in inflammation or muscle structural damage in the repetitively contracted muscles as compared to intra-animal control muscles.