Abstract
This paper reports a series of heterodivalent linked macrocyclic β-sheets 6 that are not only far more active against Aβ aggregation than their monovalent components 1a and 1b but also are dramatically more active than their homodivalent counterparts 4 and 5. The macrocyclic β-sheet components 1a and 1b comprise pentapeptides derived from the N- and C-terminal regions of Aβ and molecular template and turn units that enforce a β-sheet structure and block aggregation. Thioflavin T fluorescence assays show that heterodivalent linked macrocyclic β-sheets 6 delay Aβ1-40 aggregation six-to-eightfold at equimolar concentrations and substantially delay aggregation at sub-stoichiometric concentrations, while homodivalent linked macrocyclic β-sheets 4 and 5 and monovalent macrocyclic β-sheets 1a and 1b only exhibit more modest effects at equimolar or greater concentrations. A model to explain these observations is proposed, in which the inhibitors bind to and stabilize the early β-structured Aβ oligomers and thus delay aggregation. In this model, heterodivalent linked macrocyclic β-sheets 6 bind to the β-structured oligomers more strongly, because N-terminal-derived component 1a can bind to the N-terminal-based core of the β-structured oligomers, while the C-terminal-derived component 1b can achieve additional interactions with the C-terminal region of Aβ. The enhanced activity of the heterodivalent compounds suggests that polyvalent inhibitors that can target multiple regions of amyloidogenic peptides and proteins are better than those that only target a single region.
Introduction
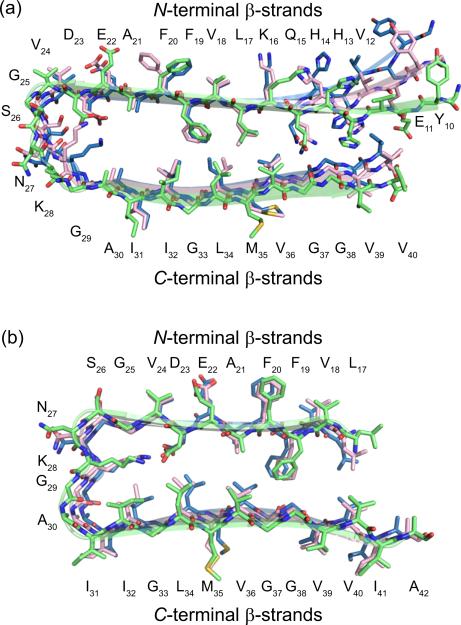
Amyloid-β (Aβ) fibrils associated with Alzheimer's disease contain layered β-sheet structures involving β-strands from both the N- and C-terminal regions of Aβ peptides.1 NMR-based structural models of Aβ fibrils show that Aβ peptides self-assemble into parallel β-sheets that fold into U-shaped superstructures (Figure 1).2 The two parallel β-sheets of the U-shaped superstructure are layered in an antiparallel fashion. Similar fibril structures also occur in human islet amyloid polypeptide associated with type II diabetes and likely occur more widely in amyloids.3
Figure 1.
NMR-based structural models of Aβ fibrils. (a) Model of Aβ1-40 fibrils. (b) Model of Aβ1-42 fibrils.
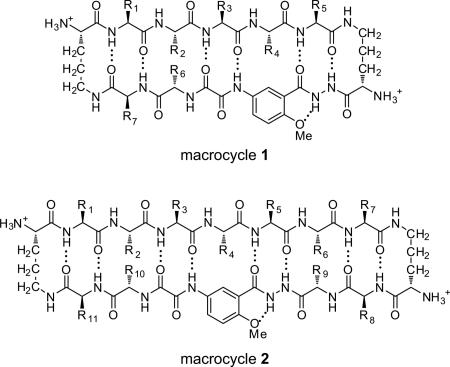
Macrocyclic β-sheets containing turn and template units provide useful chemical tools with which to understand and control amyloid aggregation.4 Our laboratory has introduced 42-and 54-membered ring macrocycles 1 and 2 that can fold into β-sheet structures and display preorganized β-strands. Macrocycle 1 incorporates a pentapeptide β-strand into the upper strand, while macrocycle 2 incorporates a heptapeptide β-strand. When these macrocycles display amyloidogenic β-strands, they are able to inhibit or suppress amyloid aggregation through β-sheet interactions. We have demonstrated that macrocycles 1 containing pentapeptide VQIVY can inhibit aggregation of the tau-derived peptide Ac-VQIVYK-NH2 (AcPHF6) associated with Alzheimer's disease5 and that macrocycles 2 containing amyloidogenic heptapeptide sequences can inhibit aggregation of Aβ, β2-microglobulin, and α-synuclein and can detoxify Aβ aggregates.4c,4d We have also demonstrated that the activity of macrocyclic β-sheets against Aβ aggregation can be dramatically enhanced through expansion from macrocycle 1 to macrocycle 2.4d
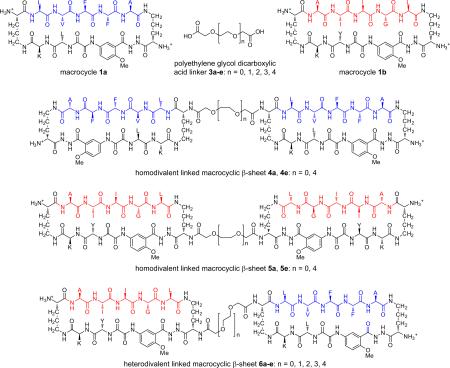
Polyvalency is a powerful means for designing ligands that bind more strongly to targets.6 We have previously shown that macrocycle 1 can readily be linked to form divalent macrocyclic β-sheet structures that display two β-sheet domains.4a Here, we ask whether this divalency can lead to better inhibitors against Aβ aggregation. To address this question, we designed divalent linked macrocyclic β-sheets by connecting two macrocycles 1 through polyethylene glycol dicarboxylic acid (PEG diacid) linkers 3.7 We also ask whether targeting two different hydrophobic regions of Aβ with these divalent linked macrocyclic β-sheets would lead to better activity than targeting a single hydrophobic region. To address this question, we designed homodivalent linked macrocyclic β-sheets 4 and 5 and heterodivalent linked macrocyclic β-sheets 6. Homodivalent linked macrocyclic β-sheets 4 contain two copies of macrocycle 1a containing Aβ17-21(R1–R5 = LVFFA) linked through PEG diacid linkers, while homodivalent linked macrocyclic β-sheets 5 contain two copies of macrocycle 1b containing Aβ30-34(R1–R5 = AIIGL) linked through PEG diacid linkers. Heterodivalent linked macrocyclic β-sheets 6 contain one copy of macrocycle 1a and one copy of macrocycle 1b linked through PEG diacid linkers.
Our studies show that divalent linked macrocyclic β-sheets generally exhibit enhanced activity against Aβ aggregation and that heterodivalent linked macrocyclic β-sheets 6 are unexpectedly more active than homodivalent linked macrocyclic β-sheets 4 and 5.
Results
Syntheses of Divalent Linked Macrocyclic β-Sheets 4-6
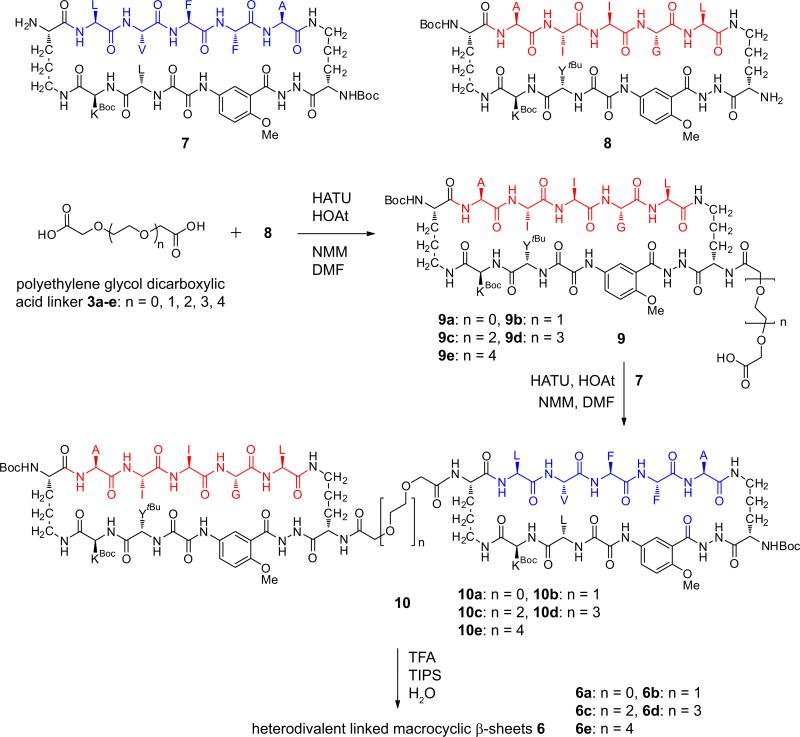
Divalent β-sheets 4-6 were synthesized by coupling PEG diacid linkers 3 with macrocycles 7 and 8, which each contain a single free amino group in one of the δ-linked ornithine turn units (Scheme 1). Homodivalent β-sheets 4 and 5 were synthesized by coupling macrocycles 7 or 8 with 0.45 molar equivalents of the appropriate PEG diacid linkers 3 (Scheme S1). Heterodivalent β-sheets 6a-e were synthesized by first coupling macrocycle 8 with a tenfold excess of PEG diacid linkers 3a-e to give monoacids 9a-e, and then coupling the monoacids with macrocycle 7 (Scheme 1).
Scheme 1.
Inhibition of Aβ Aggregation by Divalent Linked Macrocyclic β-Sheets
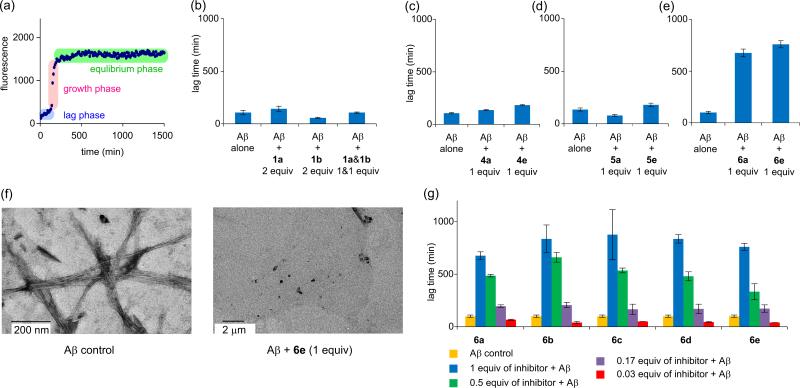
We used thioflavin T (ThT) fluorescence assays to investigate the effects of divalent β-sheets 4-6 and monovalent homologues 1a and 1b on Aβ1-40 aggregation.8 The time-course of Aβ aggregation generally demonstrates a sigmoidal curve, containing a lag phase, a growth phase, and an equilibrium phase (Figure 2a). The duration of the lag phase is widely used as a diagnostic indicator of inhibition of Aβ aggregation. We thus used this lag time to evaluate the activity of 1a, 1b, and 4-6 against Aβ1-40 aggregation.
Figure 2.
ThT fluorescence assays. (a) Fibrillation kinetics of Aβ1-40 monitored by a ThT fluorescence assay. This plot displays three phases of Aβ1-40 aggregation: the lag phase, the growth phase, and the equilibrium phase. (b) Lag time of Aβ1-40 aggregation with and without macrocycles 1a and 1b. (c) Lag time of Aβ1-40 aggregation with and without homodivalent β-sheets 4a and 4e. (d) Lag time of Aβ1-40 aggregation with and without homodivalent β-sheets 5a and 5e. (e) Lag time of Aβ1-40 aggregation with and without heterodivalent β-sheets 6a and 6e. (f) TEM image of Aβ1-40 (15 μM) after incubation for 6.5 h without (left) and with (right) heterodivalent β-sheet 6e (1 equivalent). (g) Lag time of Aβ1-40 aggregation with heterodivalent β-sheets 6a-e at 0.03, 0.17, 0.5, and 1 molar equivalents. All ThT assays were carried out on 15 μM Aβ1-40 in HEPES buffer at 31°C.
ThT fluorescence assays show that macrocycle 1a containing sequence Aβ slightly delays Aβ1-40 aggregation, macrocycle 1b containing sequence Aβ30-34 accelerates Aβ1-40 aggregation, and a mixture of 1 molar equivalent of macrocycle 1a and 1 molar equivalent of macrocycle 1b does not significantly change the lag time (Figure 2b). Macrocycle 1a delays Aβ1-40 aggregation by 30% at 2 equivalents, increasing the lag time from 107 minutes to 143 minutes, while macrocycle 1b accelerates Aβ1-40 aggregation by 50%, reducing the lag time from 107 minutes to 57 minutes. A mixture of 1 equivalent of macrocycle 1a and 1 equivalent of macrocycle 1b exhibits a lag time of 106 minutes, which is within statistical variation of that of Aβ1-40 alone. These results are consistent with trends that we have observed in the effects of macrocycles 2 against Aβ aggregation and also support that the central hydrophobic sequence Aβ17-21 plays an important role in Aβ aggregation and in the activity of macrocycles 1 and 2 against Aβ1-40 aggregation.4d,9
ThT fluorescence assays show that heterodivalent β-sheets are not only far more active than their monovalent components but also are dramatically more active against Aβ1-40 aggregation than their homodivalent counterparts. Heterodivalent β-sheets 6a and 6e dramatically delay Aβ1-40 aggregation by 570% and 660% respectively at 1 equivalent (15 μM), while homodivalent β-sheets 4a, 4e, and 5e slightly delay aggregation by 30–70% and homodivalent β-sheet 5a accelerates aggregation by 40% (Figures 2c-e). Transmission electron microscopy (TEM) studies of samples taken from the ThT assays indicate that Aβ1-40 forms fibrils in the absence of heterodivalent β-sheet 6e and does not form fibrils in the presence of heterodivalent β-sheet 6e during the delayed lag time (Figure 2f).
It is interesting that there is no significant difference in lag time between heterodivalent β-sheet 6a, which has a short linker (n = 0), and heterodivalent β-sheet 6e, which has a longer linker (n = 4). To investigate the effect of the linker length, we synthesized additional heterodivalent β-sheets 6b-d, which have linkers of intermediate length (n = 1, 2, and 3). ThT fluorescence assays show that heterodivalent β-sheets 6a-e delay Aβ1-40 aggregation by 570–770% at 1 equivalent (Figure 2g). These results indicate that the size of the PEG-based diacid linkers does not substantially affect the activity of heterodivalent β-sheets 6. ThT fluorescence assays also show that heterodivalent β-sheets 6 inhibit Aβ1-40 aggregation at sub-stochiometric concentrations in a dose-dependent manner. Heterodivalent β-sheets 6a-e delay Aβ1-40 aggregation at 0.17–1.0 equivalents (2.5–15 μM) by 70–770% (Figure 2g). Surprisingly, heterodivalent β-sheets 6a-e all nucleate Aβ1-40 aggregation at 0.03 equivalents (0.5 μM), accelerating Aβ1-40 aggregation by 30–60%. These results indicate that both the activity and the role of the heterodivalent β-sheets in Aβ1-40 aggregation depend on their concentrations.10
Discussion
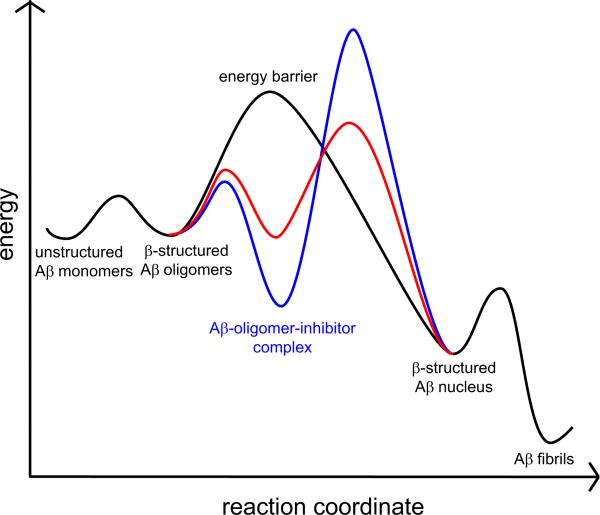
It is surprising that the heterodivalent linked β-sheets show enhanced inhibitory activity, given that only one of their components inhibits Aβ1-40 aggregation and the other accelerates aggregation. A model based on both nucleation-dependent polymerization and that which we have previously proposed may explain this enhanced inhibition.4d,11 In this model, Aβ1-40 aggregates to form early β-structured oligomers, which proceed to form a β-structured nucleus, and finally polymerize to form cross-β fibrils. Inhibitors bind to and stabilize the early β-structured oligomers and thus delay aggregation, while accelerators create a new, lower energy pathway for aggregation. Figure 3 provides a reaction free-energy diagram for the native, inhibited, and accelerated Aβ1-40 aggregation with black, blue, and red curves. Better inhibitors bind to the early β-structured oligomers more strongly and thus better delay the formation of the β-structured nucleus.12
Figure 3.
Effect of inhibitors and accelerators on the energetics of Aβ aggregation. The black curve corresponds to a pathway in which Aβ1-40 aggregates without inhibitors, while the blue and red curves corresponds to pathways in which Aβ1-40 aggregates with inhibitors and accelerators respectively.
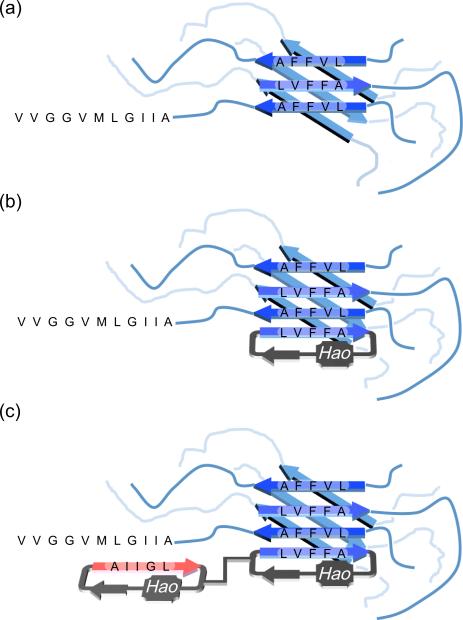
The hydrophobic N-terminal Aβ17-21 (LVFFA) region forms the core of the β-structured oligomers, in which hydrogen bonding and hydrophobic interactions create a multilayered β-sheet structure (Figure 4a). Similar multilayered β-sheet structures are also observed in macrocycle 1a and the amyloid-like fibrils formed by peptide fragment Aβ16-21 (KLVFFA).4b,13 Macrocycle 1a containing the N-terminal LVFFA pentapeptide complements and binds to the oligomers through similar types of interactions and thus inhibits aggregation (Figure 4b). Macrocycle 1b containing the C-terminal AIIGL pentapeptide better complements the C-terminal region of Aβ1-40 and facilitates the transition of Aβ1-40 to the U-shaped superstructure associated with fibrils. In the U-shaped superstructure, the C-terminal region also forms β-sheet structure and is packed against the N-terminal region. By facilitating the formation of the U-shaped superstructure, macrocycle 1b accelerates aggregation.
Figure 4.
Model for enhanced activity of heterodivalent β-sheets 6 against Aβ1-40 aggregation. (a) Aβ oligomer. (b) Aβ-oligomer-1a complex. (c) Aβ-oligomer-6 complex.
The modest effect of homodivalent linkage in 4 and 5 suggests that the β-structured oligomers do not present multiple exposed β-sheet edges in sufficient proximity to be bridged by short PEG linkers. Heterodivalent linked macrocyclic β-sheets 6 bind to the β-structured oligomers more strongly, because the LFVVA-containing macrocycle can bind to the core of the β-structured oligomers while the AIIGL-containing macrocycle can achieve additional interactions with the C-terminal region of Aβ1-40 (Figure 4c). This working model may provide a framework for the design of even more effective inhibitors that target both the N- and C-terminal regions of Aβ.
Conclusion
The heterodivalent design of linked macrocyclic β-sheets 6 enhances their activity against Aβ aggregation. The enhanced activity suggests that polyvalent inhibitors that can target multiple regions of Aβ are better than ones that only target a single region. The strategy described herein may be able to be applied to design of inhibitors against aggregation of other amyloid proteins.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (1R01GM097562 and R01AG033069) for grant support, Dr. Mihaela Necula for helpful guidance and assistance in preliminary experiments, Mr. Ming-Je Sung for assistance in TEM experiments, and Dr. Robert Tycko for providing the coordinates for Aβ1-40 used in Figure 1.
Footnotes
Supporting Information
Details of synthesis of divalent linked macrocyclic β-sheets 4, 5, and 6; thioflavin T fluorescence assays of Aβ1-40 with 1a, 1b, and 4-6; TEM; ESIMS and HPLC data of 4, 5, 6, and 9. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.a Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. J. Biol. Chem. 2009;284:4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jakob-Roetne R, Jacobsen H. Angew. Chem., Int. Ed. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]; c Fändrich M, Schmidt M, Grigorieff N. Trends Biochem. Sci. 2011;36:338–345. doi: 10.1016/j.tibs.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tycko R. Annu. Rev. Phys. Chem. 2011;62:279–99. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Lührs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Döbeli H, Schubert D, Riek R. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Petkova AT, Yau W-M, Tycko R. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Luca S, Yau W-M, Leapman R, Tycko R. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hebda JA, Miranker AD. Annu. Rev. Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]; c Wiltzius JJW, Sievers SA, Sawaya MR, Eisenberg D. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Middleton CT, Marek P, Cao P, Chiu C-C, Singh S, Woys AM, de Pablo JJ, Raleigh DP, Zanni MT. Nature. Chem. 2012;4:355–360. doi: 10.1038/nchem.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Woods RJ, Brower JO, Castellanos E, Hashemzadeh M, Khakshoor O, Russu WA, Nowick JS. J. Am. Chem. Soc. 2007;129:2548–2558. doi: 10.1021/ja0667965. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu C, Sawaya MR, Cheng P-N, Zheng J, Nowick JS, Eisenberg D. J. Am. Chem. Soc. 2011;133:6736–6744. doi: 10.1021/ja200222n. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zheng J, Liu C, Sawaya MR, Vadla B, Khan S, Woods RJ, Eisenberg D, Goux WJ, Nowick JS. J. Am. Chem. Soc. 2011;133:3144–3157. doi: 10.1021/ja110545h. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cheng P-N, Liu L, Zhao M, Eisenberg D, Nowick JS. Nature. Chem. 2012 doi: 10.1038/nchem.1433. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Inouye H, Sharma D, Goux WJ, Kirschner DA. Biophys. J. 2006;90:1774–1789. doi: 10.1529/biophysj.105.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Mammen M, Choi S-K, Whitesides GM. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; b Kim Y-S, Lee J-H, Ryu J, Kim D-J. Curr. Pharm. Des. 2009;15:637–658. doi: 10.2174/138161209787315648. [DOI] [PubMed] [Google Scholar]
- 7.a Zutshi R, Shultz MD, Ulysse L, Lutgring R, Bishop P, Schweitzer B, Vogel K, Franciskovich J, Wilson M, Chmielewski J. Synlett. 1998:1040–1044. [Google Scholar]; b Wittmann V, Takayama S, Gong KW, Weitz-Schmidt G, Wong C-H. J. Org. Chem. 1998;63:5137–5143. [Google Scholar]
- 8.LeVine H. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 9.a Tjernberg LO, Näslund J, Lindqvist F, Johansson J, Karlström AR, Thyberg J, Terenius L, Nordstedt CJ. Biol. Chem. 1996;271:8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]; b Sciarretta KL, Gordon DJ, Meredith SC. Peptide-based inhibitors of amyloid assembly. Methods Enzymol. 2006;413:273–312. doi: 10.1016/S0076-6879(06)13015-3. [DOI] [PubMed] [Google Scholar]; c Williams AD, Shivaprasad S, Wetzel R. J. Mol. Biol. 2006;357:1283–1294. doi: 10.1016/j.jmb.2006.01.041. [DOI] [PubMed] [Google Scholar]; d Estrada LD, Soto C. Curr. Top. Med. Chem. 2007;7:115–126. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]; e Miller Y, Ma B, Nussinov R. Chem. Rev. 2010;110:4820–4838. doi: 10.1021/cr900377t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The acceleration of Aβ1-40 aggregation at low concentrations of heterodivalent β-sheets 6a-e suggests that 6a-e may accelerate aggregation in monomeric form and inhibit aggregation in oligomeric form.
- 11.Finder VH, Glockshuber R. Neurodegener. Dis. 2007;4:13–27. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 12.An alternative model for the inhibition involves binding of the heterodivalent inhibitors to the N- and C-terminal β-sheet regions of small Aβ fibrils and thus the prevention of their elongation by a capping mechanism. The observation that even inhibitors with very short linkers (e.g., 6a, n = 0) block aggregation does not appear to be consistent with this alternative model, because the separation of the N- and C-terminal β-sheet regions of the Aβ fibrils is larger than the linker (reference 2b).
- 13.Colletier J-P, Laganowsky A, Landau M, Zhao M, Soriaga AB, Goldschmidt L, Flot D, Cascio D, Sawaya MR, Eisenberg D. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16938–16943. doi: 10.1073/pnas.1112600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.