Abstract
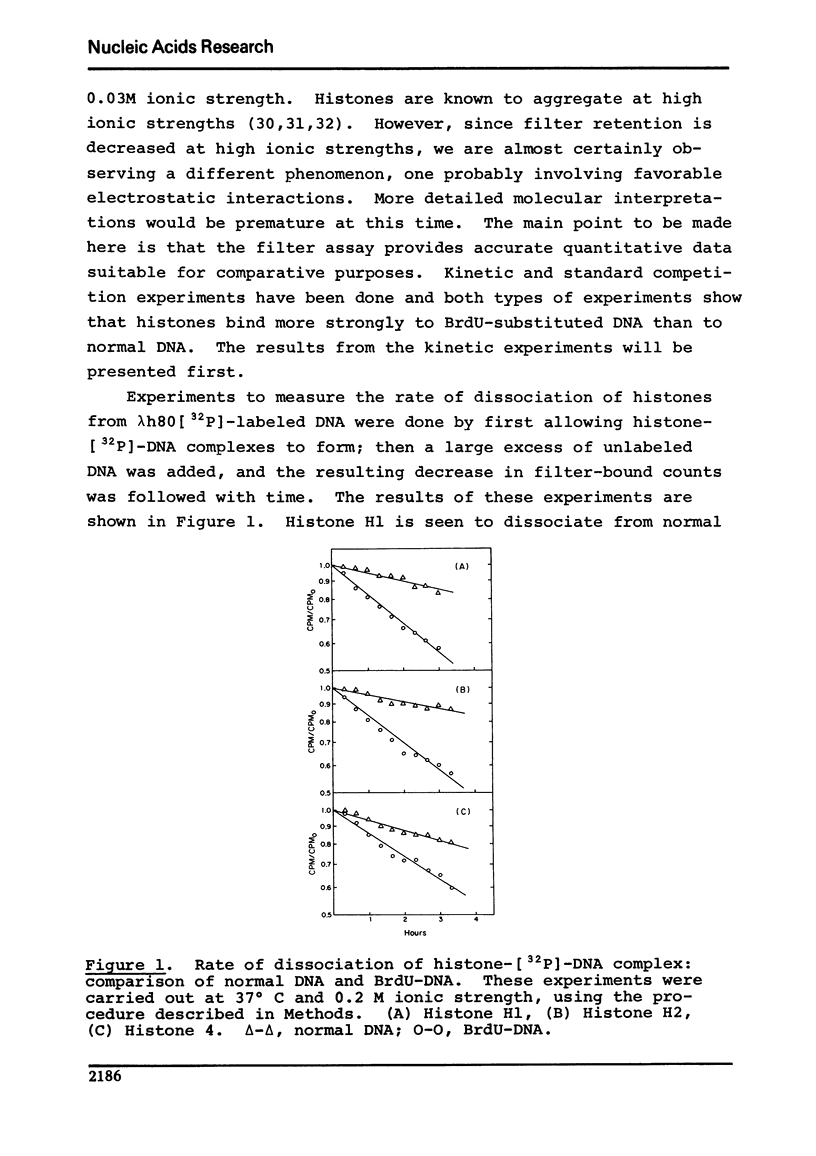
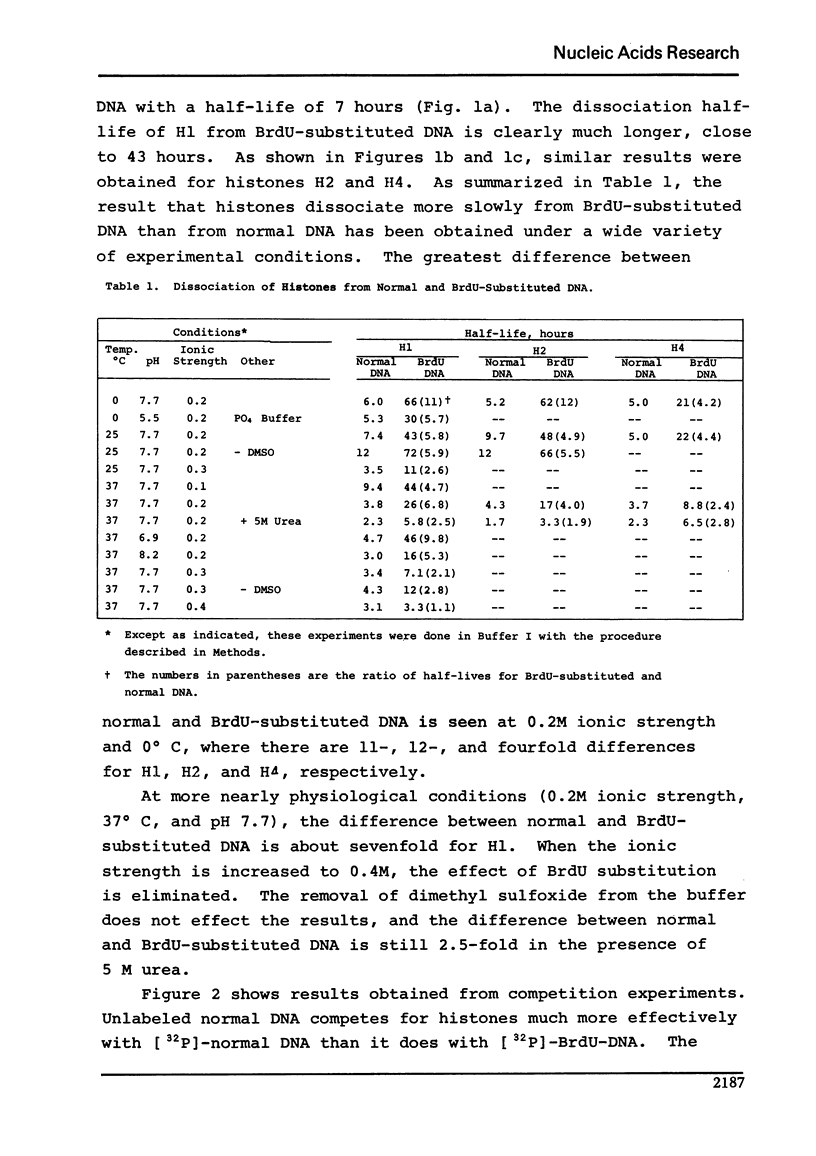
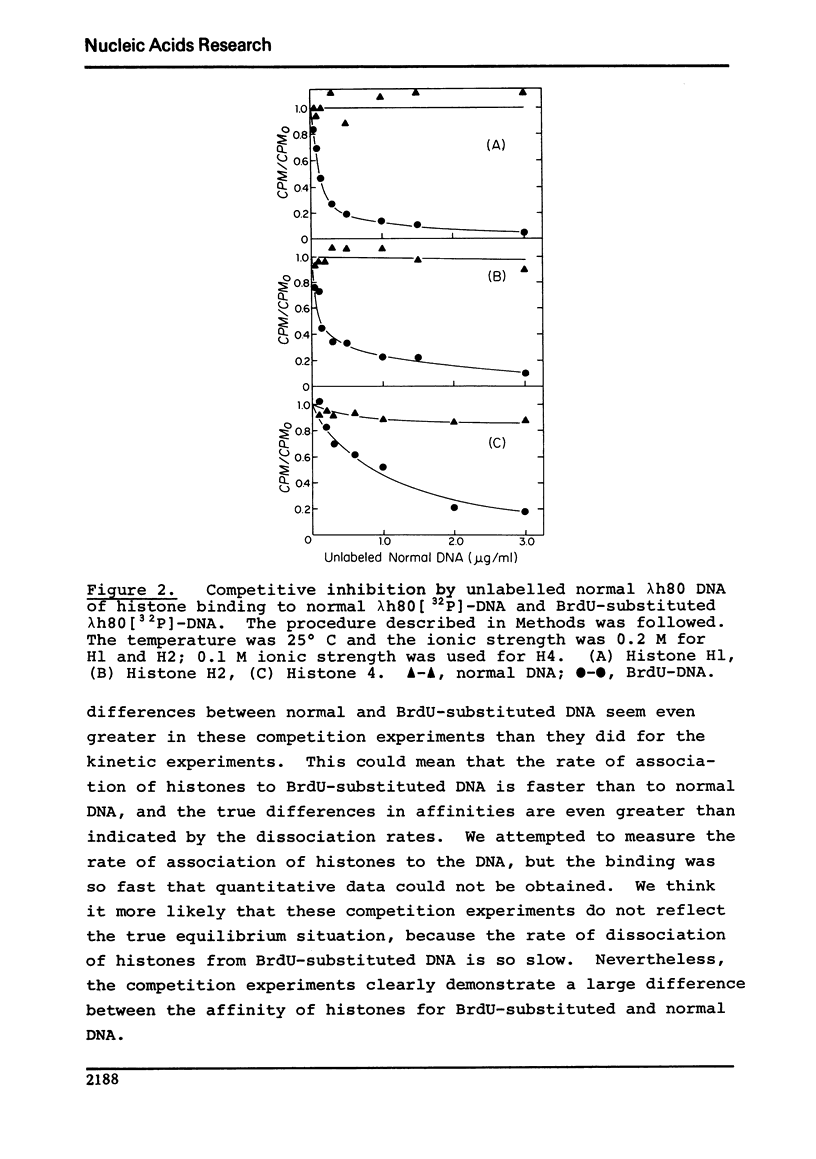
Using a membrane filter assay, we have obtained results from both kinetic and competition experiments indicating that histones bind more strongly to bromodeoxyuridine-substituted DNA than to normal DNA. At 37 degrees C in our standard buffer of 0.2 M ionic strength, the rate of dissociation of histones H1, H2, and h4 from BrdU-substituted DNA is respectively 7, 4, and 2 times slower than it is from normal DNA. Competition experiments show an even greater difference between BrdU-substituted and normal DNA with respect to histone binding. The tighter binding of histones to BrdU-substituted DNA is of interest because of the known effects of BrdU on eukaryotic chromosome condensation and staining, virus induction, and the inhibition of differentiation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- David J., Gordon J. S., Rutter W. J. Increased thermal stability of chromatin containing 5-bromodeoxyuridine-substituted DNA. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2808–2812. doi: 10.1073/pnas.71.7.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle J. H., Peacocke A. R. The molecular weights and association of the histones of chicken erythrocytes. FEBS Lett. 1971 Oct 15;18(1):138–140. doi: 10.1016/0014-5793(71)80429-5. [DOI] [PubMed] [Google Scholar]
- Ewars P. A., Sooter K. V. Ultracentrifuge tudies of histone fractions from calf thymus deoxyribonucleoprotein. Biochem J. 1969 Sep;114(2):227–235. doi: 10.1042/bj1140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M., Bonner J. On the similarity of plant and animal histones. Biochemistry. 1966 Aug;5(8):2563–2570. doi: 10.1021/bi00872a012. [DOI] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Bekhoe I. Effects of 5-bromo-2'-deoxyuridine and dimethyl sulfoxide on properties and structure of chromatin. J Mol Biol. 1974 Oct 15;89(1):137–162. doi: 10.1016/0022-2836(74)90167-3. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to operator analogues: comparison of poly(d(A-T)), poly(d(A-BrU)), and poly(d(A-U)). Biochem Biophys Res Commun. 1971 Dec 17;45(6):1542–1547. doi: 10.1016/0006-291x(71)90195-1. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. The binding of lac repressor and the catabolite gene activator protein to halogen-substituted analogues of poly[d(A-T)]. Biochim Biophys Acta. 1976 May 3;432(2):185–191. doi: 10.1016/0005-2787(76)90160-x. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Crozier T., Kluge N., Melderis H., Dube S. Action of 5-bromodeoxyuridine on the induction of haemoglobin synthesis in mouse leukaemia cells resistant to 5-BUdR. Nat New Biol. 1973 Jun 13;243(128):203–205. doi: 10.1038/newbio243203a0. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Histone 3. V. The amino acid sequence of pea embryo histone 3. J Biol Chem. 1973 Oct 10;248(19):6834–6840. [PubMed] [Google Scholar]
- Renz M. Preferential and cooperative binding of histone I to chromosomal mammalian DNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):733–736. doi: 10.1073/pnas.72.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- STOCKDALE F., OKAZAKI K., NAMEROFF M., HOLTZER H. 5-BROMODEOXYURIDINE: EFFECT ON MYOGENESIS IN VITRO. Science. 1964 Oct 23;146(3643):533–535. doi: 10.1126/science.146.3643.533. [DOI] [PubMed] [Google Scholar]
- Seecof R. L., Dewhurst S. A. A 5-bromodeoxyuridine-sensitive interval during drosophila myogenesis. Differentiation. 1976 Mar 16;6(1):27–32. doi: 10.1111/j.1432-0436.1976.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Seale R. L. Characterization of chromatin extensively substituted with 5-bromodeoxyuridine. Biochemistry. 1974 Oct 22;13(22):4609–4616. doi: 10.1021/bi00719a022. [DOI] [PubMed] [Google Scholar]
- Stellwagen R. H., Tomkins G. M. Preferential inhibition by 5-bromodeoxyuridine of the synthesis of tyrosine aminotransferase in hepatoma cell cultures. J Mol Biol. 1971 Feb 28;56(1):167–182. doi: 10.1016/0022-2836(71)90092-1. [DOI] [PubMed] [Google Scholar]
- Vogel T., Singer M. F. Interaction of f1 histone with superhelical DNA. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2597–2600. doi: 10.1073/pnas.72.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett R. R., Li H. J., Isenberg I. Salt effects on histone IV conformation. Biochemistry. 1972 Aug 1;11(16):2952–2957. doi: 10.1021/bi00766a005. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Anderson M. The action of 5-bromodeoxyuridine on differentiation. Dev Biol. 1972 Jun;28(2):443–447. doi: 10.1016/0012-1606(72)90026-7. [DOI] [PubMed] [Google Scholar]
- Zakharov A. F., Baranovskaya L. I., Ibraimov A. I., Benjusch V. A., Demintseva V. S., Oblapenko N. G. Differential spiralization along mammalian mitotic chromosomes. II. 5-bromodeoxyuridine and 5-bromodeoxycytidine-revealed differentiation in human chromosomes. Chromosoma. 1974 Jan 29;44(4):343–359. doi: 10.1007/BF00284894. [DOI] [PubMed] [Google Scholar]
- Zakharov A. F., Egolina N. A. Differential spiralization along mammalian mitotic chromosomes. I. BUdR-revealed differentiation in Chinese hamster chromosomes. Chromosoma. 1972;38(4):341–365. doi: 10.1007/BF00320156. [DOI] [PubMed] [Google Scholar]



