Abstract
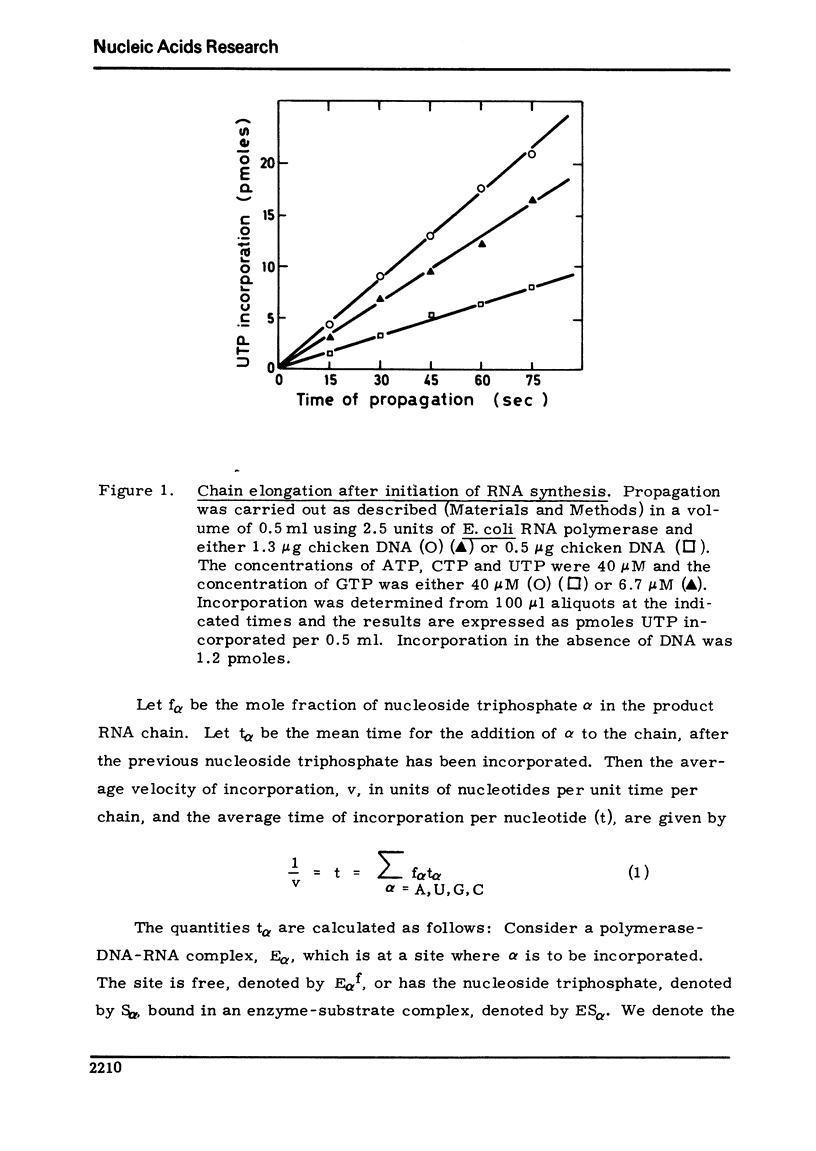
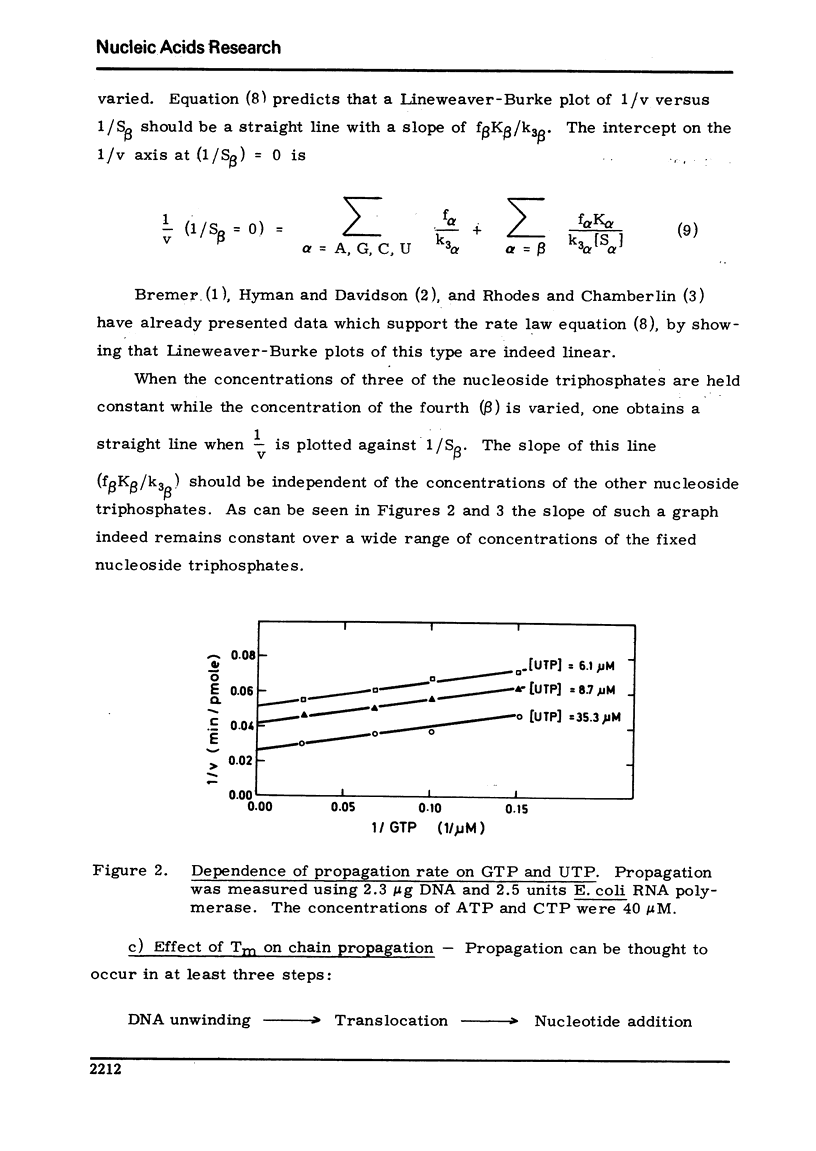
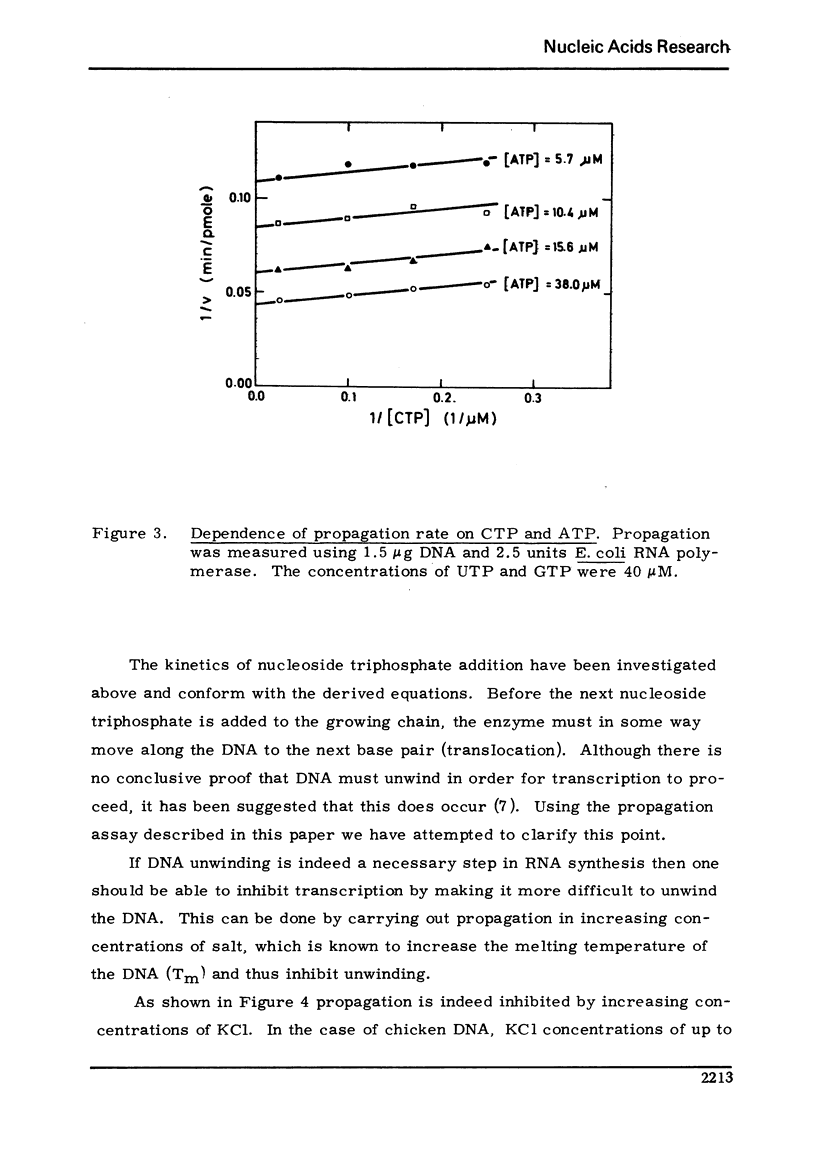
Using an assay specific for chain elongation of E. coli RNA polymerase the kinetics of this propagation reaction have been studied. The kinetic behaviour is consistent woth the mathematical model formulated for this multisubstrate enzyme. The effect of increasing salt concentration on the kinetics of the reaction indicated that DNA unwinding is probably a necessary step in the propagation step, although this may not be the rate limiting step under all conditions.
Full text
PDF


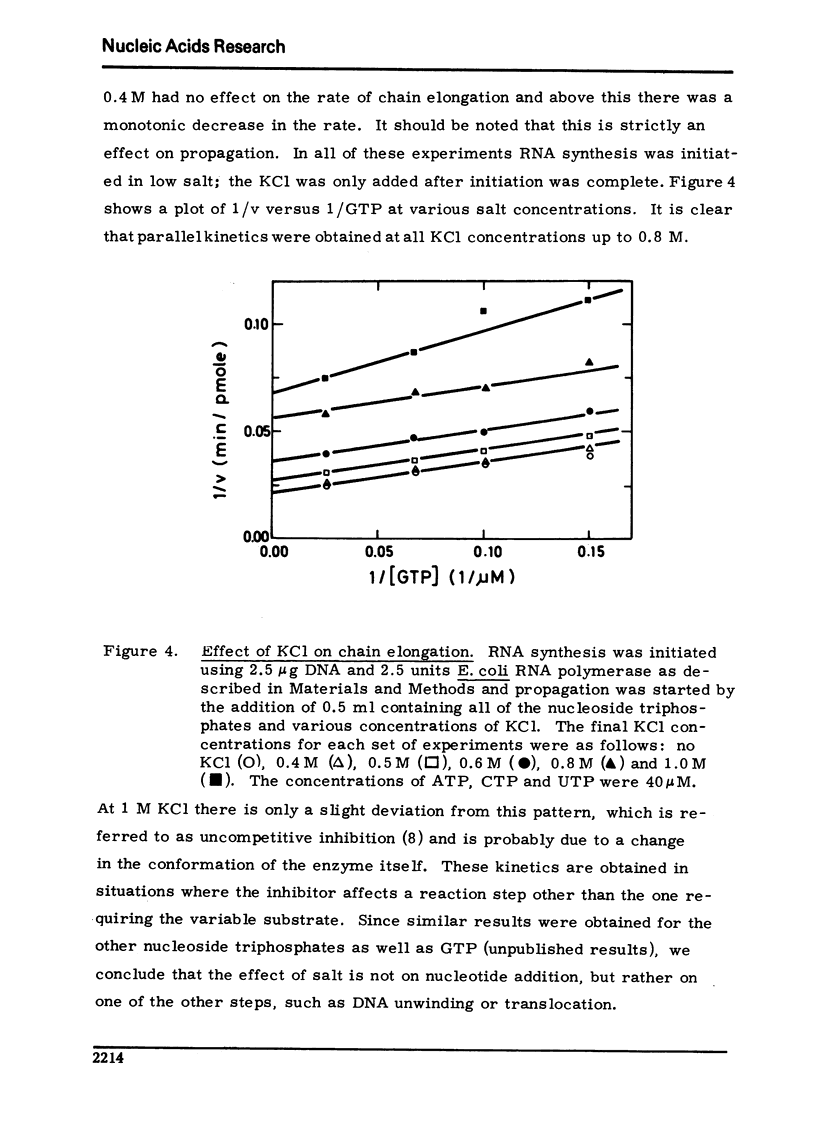

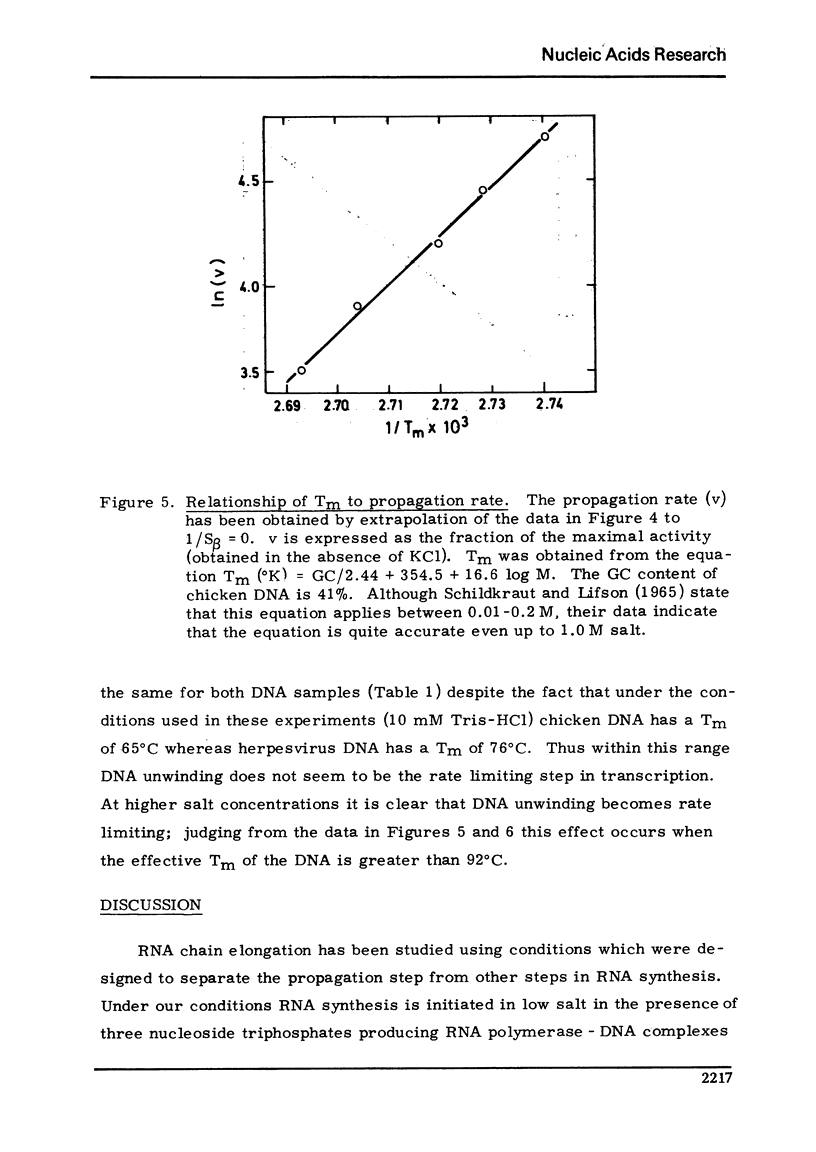
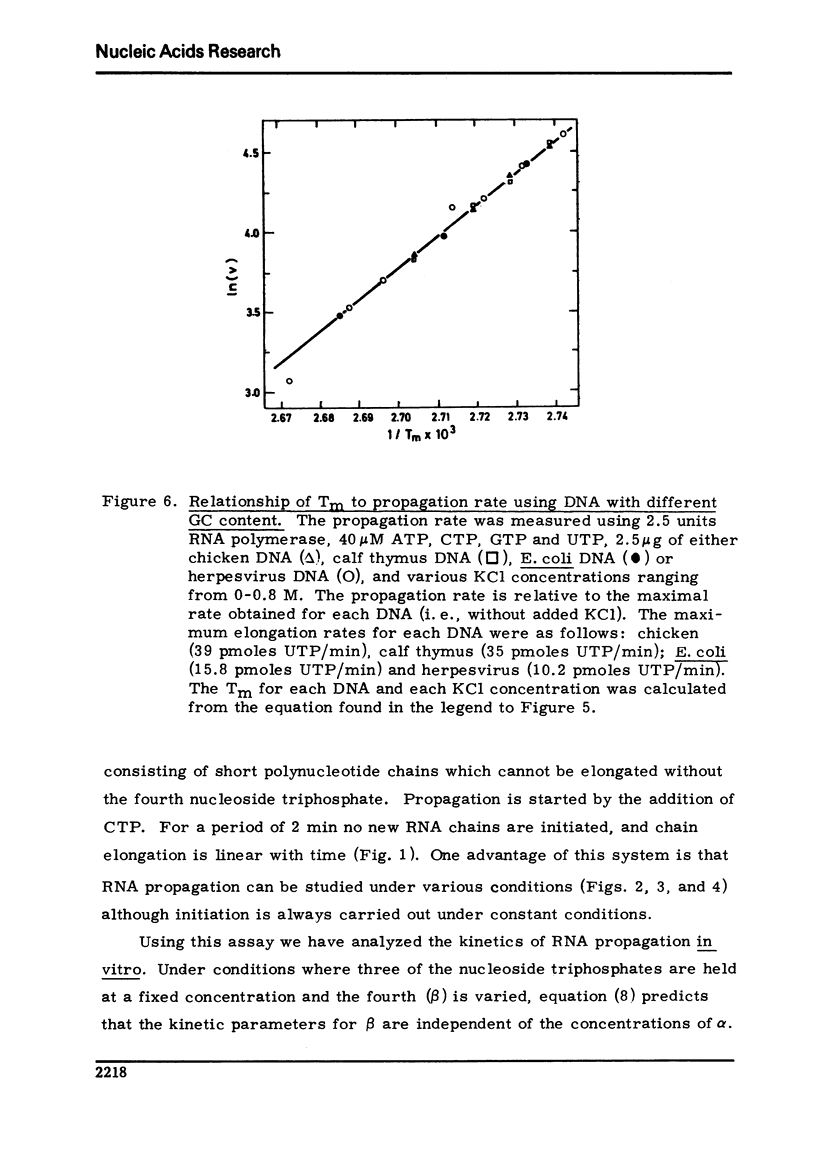



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bick M. D., Lee C. S., Thomas C. A., Jr Local destabilization of DNA during transcription. J Mol Biol. 1972 Oct 28;71(1):1–9. doi: 10.1016/0022-2836(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Bremer H. Chain growth rate and length of enzymatically synthesized RNA molecules. Mol Gen Genet. 1967;99(4):362–371. doi: 10.1007/BF00330911. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Boyer H. W. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):116–124. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- Florentiev V. L., Ivanov V. I. RNA polymerase: two-step mechanism with overlapping steps. Nature. 1970 Nov 7;228(5271):519–522. doi: 10.1038/228519a0. [DOI] [PubMed] [Google Scholar]
- Hyman R. W., Davidson N. Kinetics of the in vitro inhibition of transcription by actinomycin. J Mol Biol. 1970 Jun 14;50(2):421–438. doi: 10.1016/0022-2836(70)90202-0. [DOI] [PubMed] [Google Scholar]
- Kosaganov Iu N., Zarudnaia M. I., Lazurkin Iu S., Frank-Kamenetskii M. D., Beabealashvilli R. S., Savochkina L. P. Local unwinding of DNA during RNA synthesis in vitro. Nat New Biol. 1971 Jun 16;231(24):212–214. doi: 10.1038/newbio231212a0. [DOI] [PubMed] [Google Scholar]
- Maitra U., Barash F. DNA-dependent synthesis of RNA by Escherichia coli RNA polymerase: release and reinitiation of RNA chains from DNA templates. Proc Natl Acad Sci U S A. 1969 Oct;64(2):779–786. doi: 10.1073/pnas.64.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Chamberlin M. J. Ribonucleic acid chain elongation by Escherichia coli ribonucleic acid polymerase. I. Isolation of ternary complexes and the kinetics of elongation. J Biol Chem. 1974 Oct 25;249(20):6675–6683. [PubMed] [Google Scholar]
- Riley P. A. A suggested mechanism for DNA transcription. Nature. 1970 Nov 7;228(5271):522–525. doi: 10.1038/228522a0. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]



