Introduction
Therapeutic advances have significantly improved the prognosis of cancer patients [1]. This has generated new expectations especially for women of adolescent and reproductive age for whom an increased hope of recovery from disease implies a prospect of parenthood [2]. Unfortunately, radio- and chemotherapies have major effects on ovarian function, often leading to premature ovarian failure. Over the last several years, different strategies have been developed to preserve female germ cells and, with them, reproductive function. Before a cancer treatment is started, parts of ovarian cortex can be explanted, cryopreserved and re-implanted orthotopically after clinical remission. With this approach, ovarian and reproductive function can be restored, at least transiently, as demonstrated by the birth of naturally conceived children [3]. Alternatively, following controlled ovarian stimulation, mature oocytes can be retrieved, cryopreserved and used at later stages to achieve a pregnancy with embryos generated in vitro [4]. Retrieval and storage of immature or in vitro matured oocytes may offer an additional opportunity for female germ cell preservation in cancer patients [5]. In fact, germinal vesicle (GV)-stage oocytes can be collected from antral follicles in the absence of gonadotropin administration and cryopreserved before or after in vitro maturation (IVM). Therefore, IVM may be preferable in cases in which tumour estrogen-sensitivity and/or urgency to start therapy conflict with the implementation of a controlled ovarian stimulation treatment. In this report, we describe the recovery of immature oocytes from antral follicles during a laparotomic conservative surgery for ovarian cancer. These oocytes were matured in vitro, cryopreserved by vitrification at the mature stage and subsequently warmed to pursue a pregnancy in an IVF cycle. This experience offers the proof of principle that opportunistic retrieval of immature oocytes during surgery is a realistic possibility to preserve female reproductive potential.
Case report
In July 2010, a 38-year-old woman with a previous history of infertility (one spontaneous abortion in 2005, three intrauterine inseminations in 2008, and one ICSI cycle in April 2010) underwent laparotomic surgery for ovarian adenocarcinoma. The tumour displayed moderate differentiation (G2) and was staged as IIC according to the FIGO classification [6]. In the course of intervention, the extent and severity of lesions required contextual right annexectomy and excision of an intact cyst from the left ovary.
In consideration of the clinical condition and desire of parenthood by the patient, and with approval of the local IRB, immature oocytes were collected during the surgical procedure from medium-sized antral follicles visible on the surface of the left ovary unaffected by the cyst. Oocyte collection was considered since the intervention was scheduled on day 9 of a menstrual cycle. Three cumulus cell-oocyte complexes (COCs) were recovered by aspiration from 4 to 10 mm follicles with a 1 ml syringe connected to a 26 gauge needle. COCs appeared with an intact GV-stage oocyte enclosed in an uninterrupted vestment of cumulus cells. Within 30 min after collection, COCs were transported in HEPES-buffered medium to the IVF laboratory for IVM.
IVM was carried out using a single well petri dish containing 0.5 ml of IVM medium (Origio, Måløv, Denmark) supplemented with recombinant FSH 0.075 IU/ml (Merck Serono, Rome, Italy) and HCG 0.10 IU/ml (Merck Serono, Rome, Italy) [7]. After 30 h of culture, COCs were treated with 40 IU/ml cumulase solution (Origio, Måløv, Denmark) to remove cumulus cells. Two oocytes displayed an extruded first polar body and an overall normal morphology. Therefore, they were considered suitable for immediate cryopreservation by vitrification. A third oocyte was found at the GV breakdown (GVBD) stage. After a supplementary culture period of 18 h, this oocyte also emitted the first polar body and was cryopreserved.
Vitrification and successive warming was carried out as described elsewhere [8] using ethylene glycol, PROH and sucrose as cryoprotectants and cryoleaf as a carrier (Origio, Måløv, Denmark).
During a period of time of 5 months after surgical intervention, the patient was subjected to chemotherapy involving six cycles of carbotaxol treatment. Subsequently, in October 2011, she was admitted for assisted reproduction treatment with her own cryopreserved in vitro matured oocytes. At that time, on day 3 of a menstrual cycle, serum FSH and AMH levels were 13.0 mIU/ml and 1.14 ng/ml, respectively, suggestive of a reduced ovarian reserve. Two of the three cryopreserved oocytes survived after warming. Before cryopreservation, the two survived oocytes had achieved meiotic maturation by 30 and 48 h of culture. Two hours after warming, oocytes were inseminated by ICSI with spermatozoa prepared from a semen sample of the patient’s partner. Normal fertilization, evidenced by the presence of two pronuclei and two polar bodies, was observed at 18 h after microinjection in the oocyte previously matured for a period of 30 h, while the other oocyte did not show signs of fertilization or activation, even after protracted observation. The single fertilized egg was cultured in a microdrops under oil of ISM1 medium (Origio, Måløv, Denmark). By 48 h post-insemination, the ensuing embryo reached the 2-cell stage with less than 5 % of cellular fragmentation. At that time point, embryo transfer was carried out using a soft catheter (Gynetics, Lommel, Belgium).
For endometrial preparation, the patient received oestradiol supplementation, 4 mg a day (Progynova, Schering-Plough, Milano, Italy), starting from the second day of the cycle up to an endometrial thickness of 8 mm. Afterwards, the estradiol dose was increased to 6 mg a day, with the addition of 600 mg progesterone (Prometrium, Rottapharm, Monza, Italy). Both steroid supplementations were continued until the time of serum β-HCG quantitative analysis which resulted negative at 13 days after embryo transfer.
Discussion
Ovarian tissue and mature oocyte cryopreservation are fundamental strategies by which the reproductive potential of women suffering from premature ovarian failure, especially after radio- or chemotherapy, may be preserved. To date, several children have been born from women who conceived in vivo or in vitro after re-implantation of ovarian tissue [3]. Giving rise to very numerous births in IVF patients, oocyte cryopreservation has proven itself to be a productive approach [9], although its capability in cases of fertility preservation remains to be documented. However, a single methodology is not applicable to the diverse clinical and personal conditions of women in need of fertility preservation. For example, ovarian tissue cryopreservation is unlikely to be a suitable solution for women with relatively poor ovarian reserve. On another hand, requiring ovarian stimulation with gonadotropins, oocyte cryopreservation is not appropriate for pre-pubertal girls.
Oocyte IVM is emerging as a possible supplementary approach to fertility preservation. Originally, it was developed as a milder treatment for infertility. In fact, immature oocytes can be retrieved in the absence of or after minimal ovarian stimulation [7]. In a fertility preservation context, this makes IVM particularly appropriate in cases in which the type of cancer and/or schedule of radio- chemotherapy discourage the application of a conventional ovarian stimulation regimen [5].
Another advantage of oocyte IVM resides in the fact that it can be implemented as a supplementary measure during ovarian cortex cryopreservation. In fact, explanted ovarian tissue may include antral follicles representing a source of GV-stage oocytes that may be collected in vitro prior to cryopreservation [10]. Alternatively, immature oocytes can be recovered in situ from antral follicles localized on the ovarian surface at the time of a surgical procedure. Huang et al. [11] reported a case of cryopreservation of oocytes recovered during surgery, but their experience did not involve warming and use of cryopreserved oocytes.
This case report documents maturation in vitro, cryopreservation and use for fertility preservation of oocytes recovered during surgery for ovarian cancer treatment. The collection of immature oocytes did not have implications for the patient. In fact, laparotomy was independently dictated by the pathology and the conservative nature of the intervention, while oocytes were obtained without previous gonadotropin stimulation.
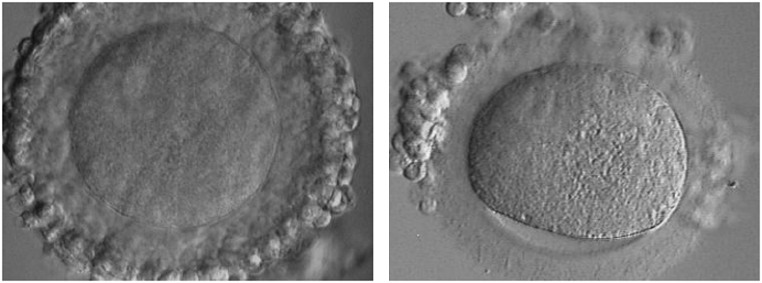
It is still debated whether GV oocytes should be cryopreserved either at the immature stage or after maturation, once meiotic resumption and first polar body extrusion have occurred. In previous IVM cases, we have observed that the cumulus cell vestment is often largely disrupted after vitrification at the GV stage (Fig. 1). Because cumulus cells are critical for successful in vitro maturation, we chose to cryopreserve oocytes after in vitro maturation, at a stage when interaction between oocyte and somatic cells is no longer needed. The choice to cryopreserve at the mature stage was also based on the fact that a few pregnancies have been achieved from oocytes matured in vitro and vitrified at the metaphase II stage [12]. Cao and Chian [13] have also reported that cryopreservation at the mature stage after IVM is a more valuable option.
Fig. 1.
Representative images of a human immature COC before (left) and after (right) vitrification. The cumulus mass vestment appears largely disrupted by the process of cryopreservation
The single 2-cell embryo transferred during an oocyte cryopreservation cycle did not evolve into a viable pregnancy. Various factors may explain this outcome, such as exiguity of the retrieved material, inadequacy of current in vitro maturation systems, or the patient clinical history. Notwithstanding, starting from only three retrieved immature oocytes, a developing embryo was replaced giving the patient a tangible opportunity for establishing a pregnancy from a source that otherwise would have been lost. Future refinement of strategy and techniques could lead opportunistic recovery of immature oocytes to a condition of clinical applicability in female fertility preservation.
Footnotes
Capsule
During laparotomy for ovarian cancer, three immature oocytes were retrieved from antral follicles. Oocyte were matured, cryopreserved and subsequently used in an IVF cycle in which one embryo was transferred.
References
- 1.Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 2.Wallace WHB. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117:2301–2310. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 4.Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27:495–499. doi: 10.1007/s10815-010-9434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang JYJ, Chian RC, Gilbert L, Fleiszer D, Holzer H, Dermitas E, et al. Retrieval of immature oocytes from unstimulated ovaries followed by in vitro maturation and vitrification: a novel strategy of fertility preservation for breast cancer patients. AJS. 2010;200:177–183. doi: 10.1016/j.amjsurg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Beahrs O, Henson D, Hutter R, Myers M, editors. AJCC Cancer Staging Manual. 3rd edn. American Joint Committee on Cancer. 3rd ed. Philadelphia; 1998.
- 7.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19:343–351. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 8.Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19:171–180. doi: 10.1016/S1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 9.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Huang JYJ, Tulandi T, Holzer H, Tan SL, Chian R-C. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 11.Huang JYJ, Buckett WM, Gilbert L, Tan SL, Chian R-C. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol Oncol. 2007;105(2):542–544. doi: 10.1016/j.ygyno.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Chian R-C, Gilbert L, Huang JYJ, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91:372–376. doi: 10.1016/j.fertnstert.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y-X, Chian R-C. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27(6):456–464. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]