Abstract
Background
There is good evidence to show that varicocele repair can improve conventional sperm parameters, as well as, sperm DNA integrity, in infertile men with a clinical varicocele.
Objective
To examine the effect of varicocelectomy on sperm quality, specifically, sperm nuclear chromatin integrity and sperm mitochondrial DNA (mtDNA) copy number.
Design, Setting, and Participants
A prospective study done between March 2007 and January 2008. We evaluated a consecutive series of infertile men (n = 14) presenting to Ovo clinic with one year or more history of infertility, a clinically palpable varicocele and poor motility (<25 % rapid progressive and <50 % progressive).
Surgical Procedure
Microsurgical sub-inguinal varicocelectomy.
Outcome Measurements and Statistical Analysis
Conventional sperm parameters, sperm mtDNA copy number (by real time PCR) and sperm chromatin structure assay (SCSA) parameters (%DFI,% HDS) before and 4 months after microsurgical varicocelectomy.
Results and Limitations
Sperm concentration and SCSA parameters (%DFI and %HDS) improved significantly after surgery (P < 0.05). Sperm mitochondrial DNA copy number decreased significantly after surgery (27 ± 30 to 9 ± 6 copies per sperm, respectively, P = 0.032). There was a significant negative correlation between mitochondrial DNA copy number and sperm motility (r = − 0.71, P = 0.002).
Conclusion
These findings support the concept that correction of a varicocele can improve spermatogenesis and sperm function, as mitochondrial DNA copy number has been suggested to reflect the efficiency of spermatogenesis and has been inversely related to sperm motility.
Keywords: Sperm DNA, mitochondrial DNA, sperm motility, male infertility, varicocele
Introduction
Sperm nuclear DNA damage has emerged as an objective diagnostic tool in the evaluation of male factor infertility [1, 2]. More recently, sperm mitochondrial DNA (mtDNA) integrity and copy number have also been studied in the context of male infertility [3, 4]. During spermiogenesis, sperm cytoplasm is shed and there is a sharp reduction in the number of sperm mitochondria and in mtDNA content (to ten-fold less of its initial value) such that mature mammalian spermatozoa contain only approximately 22–75 mitochondria situated along the sperm midpiece [5–7]. Human oocytes contain an average of 200,000 mtDNA copies and oocyte mtDNA copy number is proportional to oocyte quality [8]. In contrast, sperm mtDNA copy number is inversely related to male fertility [4]. Paternal transmission of mitochondrial DNA defects is exceedingly rare because of the low numbers of mitochondria in sperm (compared to oocytes) and the fact that sperm mitochondria are degraded in the oocyte cytoplasm [9–11].
Mitochondria generate ATP (cellular energy) by oxidative phosphorylation [12–14]. ATP is required for sperm motility and hyperactivation, suggesting that sperm mitochondrial function may be important for flagellar propulsion and sperm fertilizing capacity [12–15]. Nonetheless, the role of mitochondria and oxidative phosphorylation in sperm function and motility has been debated. On the one hand, studies have shown that sperm motility is significantly reduced when spermatozoa are incubated with mitochondrial-specific inhibitors such as, rotenone, potassium cyanide and oligomycin [16]. Moreover, sperm mitochondrial mutations have been associated with oligoasthenozoospermia and isolated asthenozoospermia [16–18]. On the other hand, another important source of sperm ATP is derived from glycolytic enzymes along the sperm tail fibrous sheath [19], thus, undermining the importance of mitochondrial function in sperm motility. Moreover, the fact that a low (not high) number of mtDNA copies per mature sperm is associated with good sperm quality does not support the idea that mitochondrial function is related to sperm motility [4, 15, 17, 20]. In fact, a high number of sperm mitochondria (and mtDNA copies) has been associated with defective sperm function [4, 21]. It has been suggested that an increased number of mitochondria may be responsible for oxidative stress (and ensuing sperm dysfunction) through the excessive generation of reactive oxygen species – ROS, produced as a byproduct of oxidative phosphorylation [22–25].
It is reported that varicocele repair results in improved semen quality in 60 to 80 % of infertile men [26, 27]. However, the true effect of adult varicocelectomy on male fertility remains controversial largely because of the paucity of randomized and controlled trials [28–30]. As well, using the improvement in conventional semen parameters as an outcome measure after varicocelectomy is limited by virtue of the high degree of biological variability of these parameters. An improvement in sperm DNA integrity after varicocele repair would provide more credibility as to the therapeutic effect of varicocelectomy because compared to standard semen parameters, measures of sperm DNA damage exhibit a lower degree of biologic variability and may be better predictors of male fertility potential [31–35]. Additionally, a reduction in the mtDNA copy number after varicocele repair would provide another biological mechanism for the improved sperm function (motility) after varicocele repair.
As such, the purpose of this study was to objectively evaluate the impact of varicocelectomy on sperm mtDNA copy number and nuclear DNA integrity in infertile men with a clinical varicocele and poor sperm motility.
Material and methods
Patient population
We conducted a prospective study of couples presenting for infertility evaluation at the OVO fertility clinic in Montreal, Canada, between March 2007 and January 2008. Men presenting to our clinic with one year or more history of infertility, a clinically palpable varicocele and poor motility (<25 % rapid progressive and <50 % progressive) were recruited. Baseline testicular volumes (estimated with an orchidometer) and serum FSH, LH and testosterone levels were obtained. Men with azoospermia, severe oligozoospermia (<5 million sperm per ml), complete asthenozoospermia or evidence of genital tract infection were excluded. Couples in whom the wife had tubal obstruction or ovulatory failure were not included. Fertile sperm donors with normozoospermia were used as controls.
The infertile men recruited for the study were asked to submit two semen samples (at 1 to 2 months before surgery and at 4 months after varicocelectomy) for evaluation of standard sperm parameters, sperm chromatin structure assay (SCSA) parameters (%DFI - DNA fragmentation index and % HDS—high DNA stainability, an index of chromatin compaction) and sperm mitochondrial DNA (mtDNA) copy number (assessed by real time PCR). All of the men underwent microsurgical varicocelectomy (from September 2007 to June 2008) and all of the operations were performed by the same surgeon (AZ), as previously described [36].
The study was approved by the ethics review board at McGill University and all men signed an informed consent prior to participating. Patient information for this study remained confidential and within the institution.
Semen handling
Samples were obtained by masturbation after 3–5 days of sexual abstinence. After liquefaction of semen, standard semen parameters (volume, concentration, motility) were obtained using a computer-assisted semen analyzer—CASA (Sperm Vision HR, Penetrating Innovations LLC, Verona WI, USA). All of the semen samples had motile sperm and none had detectable numbers of somatic cells or round cells (<1 somatic or round cell per 100 spermatozoa and < 1 million round cells per ml) (WHO 1999).
Following liquefaction, a 100–500 μL aliquot of semen (containing approximately 10 million spermatozoa) was collected from the original sample and frozen at −70°C for later DNA extraction and evaluation of sperm mitochondrial DNA (mtDNA) copy number. Two additional aliquots of semen (containing approximately 2 million spermatozoa) were collected from the original sample and frozen at −70°C for later evaluation of sperm DNA fragmentation index (% DFI) and high DNA stainability (% HDS).
Sperm DNA fragmentation index (DFI) and high DNA stainability (HDS)
Sperm DNA damage was assessed by the sperm chromatin structure assay (SCSA) and the results were expressed as sperm %DFI (an index of DNA damage) and sperm %HDS (a measure of nuclear chromatin compaction) as previously described [32, 37]. The SCSA was performed with a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA). The WinList software (Verity Softwarehouse Inc., Topsham, ME, USA) was used to generate the cytogram and histogram plots and, the %DFI and %HDS values.
Mitochondrial DNA copy number assessment by real-time PCR
MtDNA copy number was analyzed using an absolute quantification method [38, 39]. Briefly, 2 separate master mixes containing 300 nM each of primers for amplification of CO II-Cytochrome C oxidase 2 (mtDNA) and calicin (reference single copy nuclear gene) were prepared. Primer sequences were as follows:
CO II F: CCCCACATTAGGCTTAAAAACAGAT
CO II R: TATACCCCCGGTCGTGTAGCGGT
Calicin F: CTGGTCGCTACATCTACATCTC
Calicin R: CAGGTCAGGCAACTTGGTC
1 ng/μl of template DNA was mixed with Fast SYBR Master Mix (Applied Biosystems) and aliquoted in duplicates into a 96-well plate (20 μl/well). Real-time PCR was done using AB 7500 Fast Series (Applied Biosystems ABI 7500 Fast System, Life Technologies Corp, Carlsbad, California, USA). A thermal run was started with an initial denaturation step at 95°C for 30 s. This was followed by 40 cycles at 95°C for 3 s and 61°C for 30 sec, followed by a melt curve analysis. Standard curves for absolute quantification of both the CO II and calicin genes were generated for each run using a recommended new procedure (Chan et al., 2011 personal observation) [40]. Testing of paired samples (pre- and post-surgery) was always carried out on the same run.
The cycling threshold or CT values were calculated for each sample by the SDS 2.1 software [38, 39]. Mean quantities of both the mtDNA and calicin from each sample were calculated relative to the external standards. Each sample was processed in duplicates and in two independent runs. Mitochondrial DNA copy number per sperm was quantified by dividing the mean quantity of COII by that of calicin, the single copy nuclear gene [40]. The inter-run variability (coefficient of variation) of mtDNA copy number assessment was 21.6 % (95 % CI: 16.9, 26.3).
Data analysis
Results are expressed as means ± one SD. Differences between the pre- and 4-month post-varicocelectomy parameters were estimated by Wilcoxon signed-ranks test. The calculations of correlation coefficients between parameters (variables) were done using a nonparametric procedure, the Spearman rank-order correlation. All hypothesis testing was two-sided with a probability value of 0.05 deemed as significant. Analyses were conducted with the Sigma Stat program (SPSS, Chicago, IL).
Results
We evaluated 14 men who met the inclusion criteria. All men had provided pre- and post-varicocelectomy semen samples. Mean (±SD) left and right testicular volumes were 14 ± 2 and 15 ± 4 ml, respectively. The mean (±SD) serum FSH, LH and total testosterone levels were 6 ± 4 IU/L, 4 ± 1 IU/L and 13 ± 5 nmol/L, respectively. At baseline, the 14 men had a mean (± SD) sperm concentration of 39 ± 37 million per ml (median: 36.6, range: 9–130 million per ml), total motility of 30 ± 13 (range 3–46 %), %DFI of 22 ± 12 (range 4–38 %) and %HDS of 11 ± 6 (range 5–24 %). The mean mitochondrial DNA copy number in infertile men with varicocele (27 ± 30, range: 6–66 copies) was significantly higher than that of fertile donors (5 ± 3, range: 2–9 copies) (P = 0.046).
Sperm DNA integrity improved significantly at 4 months after surgery (n = 14; %DFI decreased from 22.4 ± 11.9 (range 4–38 %) before surgery to 12.6 ± 6.0 (range 3–23 %) at 4 months after surgery: see Table 1). Similarly, sperm chromatin compaction also improved significantly at 4 months after surgery (n = 14; %HDS decreased from 11 ± 6 % (range 5–24 %) before surgery to 6 ± 4 % (range 3–23 %) at 4 months after surgery: see Table 1). Sperm mitochondrial DNA copy number decreased significantly after surgery (from 27 ± 30 (range 6–66 copies) before surgery to 9 ± 6 (range 2–20 copies), P = 0.032). Sperm concentration and total motility improved after surgery, although, the improvement in motility was not statistically significant (Table 1).
Table 1.
Conventional sperm parameters, mitochondrial DNA copy number (mtDNA copy), sperm DNA fragmentation index (sperm % DFI) and sperm high DNA stainability (sperm %HDS) before, and, 4 months after microsurgical varicocelectomy (n = 14)
| Parameter | Pre-Op | Post-Op 4-months | P-value |
|---|---|---|---|
| Sperm concentration (×106/mL) | 39 ± 37 | 93 ± 100 | 0.018a |
| Total sperm motility (%) | 30 ± 13 | 42 ± 22 | 0.09a |
| Sperm mtDNA copy | 27 ± 30 | 9 ± 6 | 0.032a |
| Sperm % DFI | 22.4 ± 11.9 | 12.6 ± 6.0 | 0.002a |
| Sperm % HDS | 11.2 ± 5.7 | 6.4 ± 3.9 | 0.003a |
Values are means ± SD;
aWilcoxon signed-ranks test; NS = not significant (at P < 0.05)
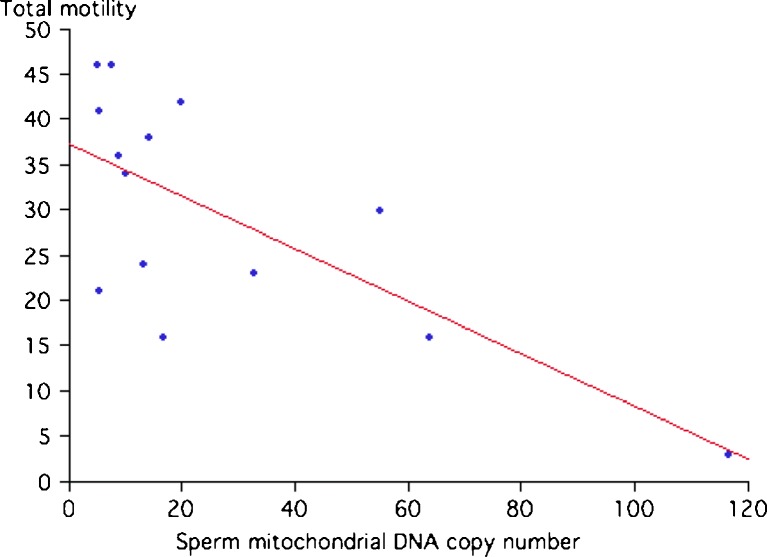
We observed a significant inverse correlation (by univariate analysis) between sperm motility and sperm mtDNA copy number (r = − 0.71, p < 0.004) (see Fig. 1). In contrast, weak and non-significant relationships were found between mitochondrial DNA copy number, and, sperm concentration (r = −0.37), %HDS (r = 0.42) and %DFI (r = 0.42).
Fig. 1.
Correlation between sperm mitochondrial DNA copy number and total motility (%) (R-squared = 0.5180, P = 0.002)
Discussion
In the present study, we have found that the average number of mitochondrial DNA (mtDNA) copies per spermatozoa was significantly higher in infertile men with a clinical varicocele and poor sperm motility than in fertile donors (27 ± 30 vs. 5 ± 3). We also observed a significant inverse correlation between sperm motility and sperm mtDNA copy number (r = − 0.71, p < 0.004). Together, these data support the notion that a high mtDNA copy number is associated with poor sperm function and male infertility. These findings are in keeping with studies that have used real time PCR to evaluate mtDNA copy number [4, 41–43]. In contrast, studies that have used other techniques in the estimation of mtDNA copy number (e.g. southern blot, where cross-hybridization with mitochondrial pseudo genes can falsely raise the copy number) have reported wider ranges of values, with much higher numbers of mitochondria per spermatozoon [44, 45]. As such, the large differences in sperm mtDNA copy number reported in the literature are mainly due to differences in mtDNA quantification techniques.
We speculate that the small (in absolute terms) difference in sperm mtDNA copy number between fertile and infertile men (~20 copies [5]) is unlikely to be associated with a sperm functional difference. Rather, the relatively high copy number in infertile men should be viewed as a marker of sperm dysfunction. An elevated sperm mtDNA copy number is most likely indicative of impaired spermatogenesis and improper regulation of mtDNA replication in men who generally have poor quality sperm. The high mtDNA content in spermatozoa of men with poor semen quality may therefore be an indicator of defective spermiogenesis and may also reflect problems in energy metabolism in both testicular cells and mature male gametes [46]. Amaral et al. showed higher mtDNA content in a group of infertile patients with OAT compared to patients with normal sperm parameters. Interestingly, the expression of the mtDNA replication factors, POLG and TFAM, was inversely related to mtDNA copy number, and hence, to sperm quality [43]. Additionally, a number of investigators have suggested that an elevated mtDNA copy number occurs as a response to cellular stress or injury (e.g. oxidative stress) [22, 25]. The elevated mtDNA copy number may also be the result of a feedback process operating indirectly to compensate for the low respiratory chain activity, thus leading to increased production of abnormal mitochondrial DNA [4, 15, 41].
We have found that varicocelectomy is associated with a significant improvement in sperm concentration and, DNA and chromatin integrity, as previously reported [47–49]. We have also shown that varicocelectomy is associated with a significant reduction in sperm mtDNA copy number (from 27 to 9), a previously unreported observation. Although the small sample size (n = 14) and the lack of a control group (infertile men with varicocele who did not undergo surgery) limit our ability to draw major conclusions on the effects of varicocelectomy, the decrease in sperm %DFI is in line with prior studies [48, 50–53] and the absolute decrease in sperm mtDNA copy number is substantial. The reduction in %DFI at 4 months after surgery is further evidence in support of a favorable effect of varicocelectomy on sperm quality because unlike conventional sperm parameters (concentration, motility), measures of DNA damage exhibit a low degree of biological variability. The significant decrease in mtDNA copy number after varicocelectomy provides additional support in favor of varicocele repair for male infertility and sheds new light on the potential mechanisms responsible for the improved sperm function after varicocelectomy. The clinical significance of a 67 % reduction in sperm mtDNA copy number (from 27 to 9) is unclear in view of the limited clinical data on this parameter. However, the 61 % reduction in sperm %DFI (from 22.4 to 12.6 %) is potentially clinically significant based on the reported influence of sperm DNA fragmentation on reproductive outcomes [2, 32, 54–59].
We speculate that the decrease in sperm mtDNA copy number after varicocelectomy is the result of improved spermiogenesis, with enhanced shedding of residual cytoplasm (and retained mitochondria) and/or improved regulation of mtDNA replication [43, 60]. The improvement in spermiogenesis after varicocelectomy was originally reported by Johnsen and Agger [61]. These investigators obtained pre- and post-operative testicular biopsies and reported that histology scores improved after varicocelectomy. A number of investigators have shown that varicocele is associated with an increased level of seminal oxidative stress and that varicocele repair can lower the levels of oxidative stress and this could represent an additional mechanism by which mtDNA copy number could be reduced [50, 51, 62]. In the present study, varicocelectomy was also associated with a significant increase in mean sperm concentration and in the percentage of motile sperm, although the improvement in sperm motility did not reach statistical significance. Although we did not monitor the impact of varicocelectomy on sperm morphology, we do not feel that this additional parameter would alter the final conclusions, as the primary goal was to examine the effect of surgery on mitochondrial DNA copy number.
In summary, in this prospective study of infertile men with clinical varicocele and poor sperm motility, we have shown that microsurgical varicocelectomy is associated with an improvement in sperm nuclear DNA integrity and a reduction in mitochondrial DNA (mtDNA) copy number. Although the sample size is small, these preliminary findings are of importance because they are first to show that mitochondrial DNA copy number is reduced by varicocelectomy, hence, the data provide an additional mechanism for the reported beneficial effect of varicocele repair on male fertility potential, and, support the premise that varicocelectomy improves spermatogenesis and sperm function.
Footnotes
Capsule In a prospective study of infertile men with clinical varicocele and poor sperm motility, we have found that microsurgical varicocelectomy is associated with an improvement in sperm nuclear DNA integrity and a reduction in mitochondrial DNA copy number.
References
- 1.Lewis SE, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med. 2008;54(3):111–125. doi: 10.1080/19396360801957739. [DOI] [PubMed] [Google Scholar]
- 2.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl. 2009;30(3):219–229. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
- 3.Lewis SE. Importance of mitochondrial and nuclear sperm DNA in sperm quality assessment and assisted reproduction outcome. Hum Fertil (Camb) 2002;5(3):102–109. doi: 10.1080/1464727022000199012. [DOI] [PubMed] [Google Scholar]
- 4.Song GJ, Lewis V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil Steril. 2008;90(6):2238–2244. doi: 10.1016/j.fertnstert.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 5.Bahr GF, Engler WF. Considerations of volume, mass, DNA, and arrangement of mitochondria in the midpiece of bull spermatozoa. Exp Cell Res. 1970;60(3):338–340. doi: 10.1016/0014-4827(70)90526-4. [DOI] [PubMed] [Google Scholar]
- 6.St John JC, Sakkas D, Barratt CL. A role for mitochondrial DNA and sperm survival. J Androl. 2000;21(2):189–199. [PubMed] [Google Scholar]
- 7.Otani H, Tanaka O, Kasai K, Yoshioka T. Development of mitochondrial helical sheath in the middle piece of the mouse spermatid tail: regular dispositions and synchronized changes. Anat Rec. 1988;222(1):26–33. doi: 10.1002/ar.1092220106. [DOI] [PubMed] [Google Scholar]
- 8.Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 9.Cummins JM. Fertilization and elimination of the paternal mitochondrial genome. Hum Reprod. 2000;15(Suppl 2):92–101. doi: 10.1093/humrep/15.suppl_2.92. [DOI] [PubMed] [Google Scholar]
- 10.Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod. 2000;63(2):582–590. doi: 10.1095/biolreprod63.2.582. [DOI] [PubMed] [Google Scholar]
- 11.Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitin tag for sperm mitochondria. Nature. 1999;402(6760):371–372. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- 12.Rajender S, Rahul P, Mahdi AA. Mitochondria, spermatogenesis and male infertility. Mitochondrion 10(5):419–428. [DOI] [PubMed]
- 13.Cummins JM. Mitochondria in reproduction. Reprod Biomed Online. 2004;8(1):14–15. doi: 10.1016/S1472-6483(10)60493-2. [DOI] [PubMed] [Google Scholar]
- 14.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15(5):553–572. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- 15.Kao SH, Chao HT, Liu HW, Liao TL, Wei YH. Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil Steril. 2004;82(1):66–73. doi: 10.1016/j.fertnstert.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 16.St John JC, Jokhi RP, Barratt CL. The impact of mitochondrial genetics on male infertility. Int J Androl. 2005;28(2):65–73. doi: 10.1111/j.1365-2605.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 17.Lestienne P, Reynier P, Chretien MF, Penisson-Besnier I, Malthiery Y, Rohmer V. Oligoasthenospermia associated with multiple mitochondrial DNA rearrangements. Mol Hum Reprod. 1997;3(9):811–814. doi: 10.1093/molehr/3.9.811. [DOI] [PubMed] [Google Scholar]
- 18.Spiropoulos J, Turnbull DM, Chinnery PF. Can mitochondrial DNA mutations cause sperm dysfunction? Mol Hum Reprod. 2002;8(8):719–721. doi: 10.1093/molehr/8.8.719. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, Botvinick EL, Berns MW. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J Cell Physiol. 2008;217(3):745–751. doi: 10.1002/jcp.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folgero T, Bertheussen K, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Hum Reprod. 1993;8(11):1863–1868. doi: 10.1093/oxfordjournals.humrep.a137950. [DOI] [PubMed] [Google Scholar]
- 21.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 83(1):52–62. [DOI] [PMC free article] [PubMed]
- 22.Lee HC, Yin PH, Lu CY, Chi CW, Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000;348(Pt 2):425–432. doi: 10.1042/0264-6021:3480425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757(5–6):553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 25.Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, Wei YH. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37(12):1307–1317. doi: 10.1080/10715760310001621342. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, Marmar JL. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70(3):532–538. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger MH, Wilets IF, Nagler HM. Treatment outcome after varicocelectomy. A critical analysis. Urol Clin North Am. 1994;21(3):517–529. [PubMed] [Google Scholar]
- 28.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361(9372):1849–1852. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 29.Ficarra V, Cerruto MA, Liguori G, Mazzoni G, Minucci S, Tracia A, Gentile V. Treatment of varicocele in subfertile men: The Cochrane Review–a contrary opinion. Eur Urol. 2006;49(2):258–263. doi: 10.1016/j.eururo.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Evers JH, Collins J, Clarke J. Surgery or embolisation for varicoceles in subfertile men. Cochrane Database Syst Rev 2009;(1):CD000479. [DOI] [PubMed]
- 31.Evenson DP, Jost LK, Baer RK, Turner TW, Schrader SM. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol. 1991;5(2):115–125. doi: 10.1016/0890-6238(91)90039-I. [DOI] [PubMed] [Google Scholar]
- 32.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, Angelis P, Claussen OP. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14(4):1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 33.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73(1):43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 34.Zini A, Kamal K, Phang D, Willis J, Jarvi K. Biologic variability of sperm DNA denaturation in infertile men. Urology. 2001;58(2):258–261. doi: 10.1016/S0090-4295(01)01180-3. [DOI] [PubMed] [Google Scholar]
- 35.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345(19):1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148(6):1808–1811. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 37.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75(4):674–677. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Kadlubar FF, Chen JZ. DNA supercoiling suppresses real-time PCR: a new approach to the quantification of mitochondrial DNA damage and repair. Nucleic Acids Res. 2007;35(4):1377–1388. doi: 10.1093/nar/gkm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan SW, Chen JZ. Measuring mtDNA damage using a supercoiling-sensitive qPCR approach. Methods Mol Biol. 2009;554:183–197. doi: 10.1007/978-1-59745-521-3_12. [DOI] [PubMed] [Google Scholar]
- 40.Chan SW, Nguyen PN, Ayele D, Chevalier S, Aprikian A, Chen JZ. Mitochondrial DNA damage is sensitive to exogenous H(2)O(2) but independent of cellular ROS production in prostate cancer cells. Mutat Res. 2011;716(1–2):40–50. doi: 10.1016/j.mrfmmm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 41.May-Panloup P, Chretien MF, Savagner F, Vasseur C, Jean M, Malthiery Y, Reynier P. Increased sperm mitochondrial DNA content in male infertility. Hum Reprod. 2003;18(3):550–556. doi: 10.1093/humrep/deg096. [DOI] [PubMed] [Google Scholar]
- 42.Mourier T, Hansen AJ, Willerslev E, Arctander P. The Human Genome Project reveals a continuous transfer of large mitochondrial fragments to the nucleus. Mol Biol Evol. 2001;18(9):1833–1837. doi: 10.1093/oxfordjournals.molbev.a003971. [DOI] [PubMed] [Google Scholar]
- 43.Amaral A, Ramalho-Santos J, St John JC. The expression of polymerase gamma and mitochondrial transcription factor A and the regulation of mitochondrial DNA content in mature human sperm. Hum Reprod. 2007;22(6):1585–1596. doi: 10.1093/humrep/dem030. [DOI] [PubMed] [Google Scholar]
- 44.Hecht NB, Liem H. Mitochondrial DNA is synthesized during meiosis and spermiogenesis in the mouse. Exp Cell Res. 1984;154(1):293–298. doi: 10.1016/0014-4827(84)90688-8. [DOI] [PubMed] [Google Scholar]
- 45.Manfredi G, Thyagarajan D, Papadopoulou LC, Pallotti F, Schon EA. The fate of human sperm-derived mtDNA in somatic cells. Am J Hum Genet. 1997;61(4):953–960. doi: 10.1086/514887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St John JC, Bowles EJ, Amaral A. Sperm mitochondria and fertilisation. Soc Reprod Fertil Suppl. 2007;65:399–416. [PubMed] [Google Scholar]
- 47.Werthman P, Wixon R, Kasperson K, Evenson DP. Significant decrease in sperm deoxyribonucleic acid fragmentation after varicocelectomy. Fertil Steril. 2008;90(5):1800–1804. doi: 10.1016/j.fertnstert.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Zini A, Blumenfeld A, Libman J, Willis J. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod. 2005;20(4):1018–1021. doi: 10.1093/humrep/deh701. [DOI] [PubMed] [Google Scholar]
- 49.Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, Mostafa T. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril. 2011;95(5):1705–1708. doi: 10.1016/j.fertnstert.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Chen SS, Huang WJ, Chang LS, Wei YH. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179(2):639–642. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101(12):1547–1552. doi: 10.1111/j.1464-410X.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 52.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 183(1):270–274. [DOI] [PubMed]
- 53.Azadi L, Abbasi H, Deemeh MR, Tavalaee M, Arbabian M, Pilevarian AA, Nasr-Esfahani MH. Zaditen (Ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int J Androl [DOI] [PubMed]
- 54.Spano M, Kolstad H, Larsen SB, Cordelli E, Leter G, Giwercman A, Bonde JP. Flow cytometric sperm chromatin structure assay as an independent descriptor of human semen quality. Scand J Work Environ Health. 1999;25(Suppl 1):28–30. [PubMed] [Google Scholar]
- 55.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 56.Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, Levine RJ, Rylander L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case–control study. Int J Androl 2009. [DOI] [PubMed]
- 57.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 57(1–2):78–85. [DOI] [PubMed]
- 58.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, Cedenho AP. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90(5):1716–1722. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 59.La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Effects of varicocelectomy on sperm DNA fragmentation, mitochondrial function, chromatin condensation, and apoptosis. J Androl 2011. [DOI] [PubMed]
- 60.Zini A, Buckspan M, Jamal M, Jarvi K. Effect of varicocelectomy on the abnormal retention of residual cytoplasm by human spermatozoa. Hum Reprod. 1999;14(7):1791–1793. doi: 10.1093/humrep/14.7.1791. [DOI] [PubMed] [Google Scholar]
- 61.Johnsen SG, Agger P. Quantitative evaluation of testicular biopsies before and after operation for varicocele. Fertil Steril. 1978;29(1):58–63. doi: 10.1016/s0015-0282(16)43038-4. [DOI] [PubMed] [Google Scholar]
- 62.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ., Jr Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80(6):1431–1436. doi: 10.1016/S0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]