Abstract
Objective
To evaluate the predictive value of random serum anti-Müllerian hormone (AMH) in the assessment of ovarian response in patients with diminished ovarian reserve (DOR; diagnosed after the observation of elevated baseline levels of early follicular follicle-stimulating hormone [FSH]) who were undergoing intracytoplasmic sperm injection-embryo transfer (ICSI-ET) and to compare the random serum AMH and baseline FSH levels in these patients for the prediction of poor ovarian response.
Design
Retrospective study.
Setting
University hospital.
Patients
One hundred and thirty-nine patients who were undergoing ICSI-ET cycles with early follicular FSH level >9 IU/mL.
Intervention(s)
None.
Main Outcome Measure(s)
Poor ovarian response in ICSI-ET cycles.
Results
For the identification of women at risk of cycle cancellation, an AMH cut-off level ≤1.2 ng/mL had 97.3 % sensitivity, 31.3 % specificity, 33.9 % positive predictive value, and 96.9 % negative predictive value in the women with high baseline FSH levels. An AMH cut-off level ≥1 ng/mL had a sensitivity of 58.7 % and specificity of 95.1 % for prediction of retrieval of 4 or more oocytes. By using a serum AMH cutoff level of 1.5 ng/mL, the ongoing pregnancies were predicted with 83.3 % sensitivity and 82.5 % specificity and yielded a positive predictive value of 31.2 % and a negative predictive value 98.1 %.
Conclusion
Measurement of random serum AMH level is a useful tool in the prediction of ovarian response in patients with high serum early follicular FSH levels.
Keywords: Anti-Müllerian hormone, Follicle-stimulating hormone, Diminished ovarian reserve
Introduction
In assisted reproductive technology, the potential for prediction of ovarian response to controlled ovarian hyperstimulation (COH) is still completely unknown. Pretreatment assessment of ovarian response to COH will allow individualisation of treatment protocol and maximise the potential response [1]. Several parameters can be used to predict ovarian reserve, including age, antral follicle count, baseline serum follicle-stimulating hormone (FSH) level, ovarian volume, and inhibin B level [2–5].
In recent years, the anti-Müllerian hormone (AMH), which is a member of the transforming growth factor B family [6], has been shown to take the form of a dimeric glycoprotein. It has been demonstrated that serum AMH level strongly correlates with the number of antral follicles and is a useful parameter for the prediction of ovarian response and pregnancy in assisted reproduction techniques (ART) cycles [7–11]. Unlike FSH levels, serum AMH levels do not fluctuate over the course of the menstrual cycle. An absence of variation in serum AMH levels may be related to the continuous development of small follicles with a growth potential that is independent of cyclic changes [12, 13].
There is lack of uniformity in the definition of diminished ovarian reserve (DOR). A peak E2 level <300 to <500 pg/mL, fewer than 3 to 6 developed follicles, retrieval of fewer than 3 to 5 oocytes, an elevated baseline early follicular serum FSH level, at least 1 cancelled cycle, advanced patient age (≥40 years), and many others have been used to define DOR [14–17]. Advanced age, abnormal ovarian reserve test results, and DOR previously diagnosed using different parameters are currently the most frequently used criteria in the definition of DOR [18]. In clinical practice, measurement of early follicular serum FSH level is commonly used to predict DOR [19]. However, its predictive value in terms of cycle outcome is lower than for that AMH level [20].
The aim of this study was to evaluate random serum AMH levels as a predictor of ovarian response to the intracytoplasmic sperm injection (ICSI) cycle and the outcome of ART in women with high early follicular serum FSH levels. In this report, we also compare the use of AMH with that of FSH to determine the superior parameter in the assessment of ovarian reserve among women with DOR, as diagnosed using high baseline early follicular serum FSH levels in ICSI-embryo transfer (ICSI-ET) cycles.
Materials and methods
Serum AMH levels were analysed retrospectively using data gathered between February 2010 and June 2011 in the Fertility and Reproductive Endocrine Center of Ondokuz Mayıs University. Between this period, 435 patients included into ICSI-ET cycle. Among the 435 women, 270 women with recorded serum AMH levels were reached. In our clinic, serum AMH was not routinely measured in all patients, but almost always measured in patients with suspected poor ovarian response. Of these 270 women, 139 women with baseline FSH level >9 mIU/mL before the first ICSI-ET cycles were chosen to represent the study population. The study was approved by the institutional review board of Ondokuz Mayıs University.
The serum AMH levels were measured before beginning the treatment, regardless of the day of menstrual cycle on which the sample was taken. Because the serum AMH level was usually obtained before the first ICSI-ET cycle, a total of 139 patients who were undergoing their first cycle were included in the study. The baseline data, including age, body mass index, and cause and duration of infertility, were retrieved from the patients’ medical records. The baseline FSH levels were measured on day 2 or 3 of the cycle. Serum AMH levels, antral follicule count, the total amount of gonadotropins used in the ICSI-ET cycle, and the E2 level at the time of human chorionic gonadotropin (hCG) injection were all obtained from patients’ records. In addition, the numbers of retrieved oocytes, oocytes fertilised, metaphase II oocytes, and frozen embryos were recorded for each patient. All the women underwent controlled ovarian stimulation with recombinant FSH or HMG. To prevent premature ovulation, the gonadotrophin-releasing hormone agonist or antagonists were administered. The daily doses of gonadotropins were adjusted according to the E2 levels and/or the number and diameter of the growing follicles. hCG (6.500 IU; Ovitrelle, Serono Pharmaceuticals) was administered when at least 1 follicle had reached 17 mm in diameter. Ultrasound-guided oocyte retrieval was performed 36 hours after hCG injection.
Statistical analysis
Statistical analysis was undertaken using NCSS (Number Cruncher Statistical System) 2007&PASS (Power Analysis and Sample Size) 2008 Statistical Software (Utah, USA). The student’s (t) test was used to test the significance of the difference between the two independent means. Differences between groups of variables not conforming to normality were tested with Mann–Whitney test. A P-value, 0.05 was considered statistically significant. Continuous values are presented as mean + SD and as median; outcome parameters are presented as mean + SD with 95 % confidence intervals (CI). To evaluate significance of a test, we determined its sensitivity, specificity, +ve predictive value (PPV) and predictive value (NPV). Regression analysis was used to calculate the continuous relationship of retrieved oocytes, cycle cancellation and ongoing pregnancy to serum AMH and baseline FSH. Cut off levels for AMH and FSH Receiver operator curves (ROC) were used
Results
The mean age of all the 139 women was 36.2 ± 5.2 years, with a median value of 36 years. The mean AMH level was 1.1 ± 1.9 ng/mL, and the median was 0.38 ng/mL. The mean FSH level was 12.9 ± 5 mIU/mL (median, 11.12 mIU/mL). Baseline variables of patients were summarized in Table 1. Treatment was cancelled in 37 (26.6 %) patients before hCG administration owing to inadequate response to ovarian stimulation and in 27 (19.4 %) patients because of failed fertilisation after oocyte retrieval. Overall, 75 patients (54 %) had embryo transfer, of whom 41 had 1 embryo transferred and 34 had 2 embryos transferred. Nineteen pregnancies resulted, giving a conception rate of 13.7 % (19/139) per cycle and 25 % (19/75) per embryo transfer. Seven of these pregnancies ended with biochemical abortion. Overall, the ongoing pregnancy rate was 8.6 % (12/139) per cycle.
Table 1.
Baseline variables of patients
| Mean ± SD | Median | |
|---|---|---|
| Age (years) | 36,20 ± 5,20 | 36,00 |
| BMI (kg/m2) | 26,67 ± 4,15 | 26,80 |
| Duration of infertility (years) | 10,92 ± 5,63 | 10,00 |
| AHM (ng/mL) | 1,12 ± 1,94 | 0,38 |
| FSH (IU/mL) (early follicular) | 12,94 ± 5,24 | 11,12 |
| LH (IU/mL) (early follicular) | 8,33 ± 3,41 | 7,78 |
| E2 (pg/mL)(early follicular) | 46,56 ± 24,70 | 43,11 |
| Antral follicle counts | 7,36 ± 6,14 | 5,00 |
| The total amount of gonadotropins used per cycle (IU) | 2314,38 ± 1451,13 | 2025,00 |
| Duration of stimulation (days) | 9,87 ± 1,94 | 10,00 |
| Preovulatory follicle counts | 5,52 ± 1,29 | 3,00 |
| E2 at the time of HCG injection (pg/mL) | 812,26 ± 695,57 | 670,00 |
| Endometrial thickness at the time of HCG injection (mm) | 7,77 ± 2,53 | 7,60 |
| The numbers of retrieved oocytes | 3,97 ± 4,93 | 3,00 |
| The numbers of metaphase II oocytes | 2,91 ± 3,81 | 2,00 |
| The numbers of fertilized oocytes | 2,21 ± 2,94 | 1,00 |
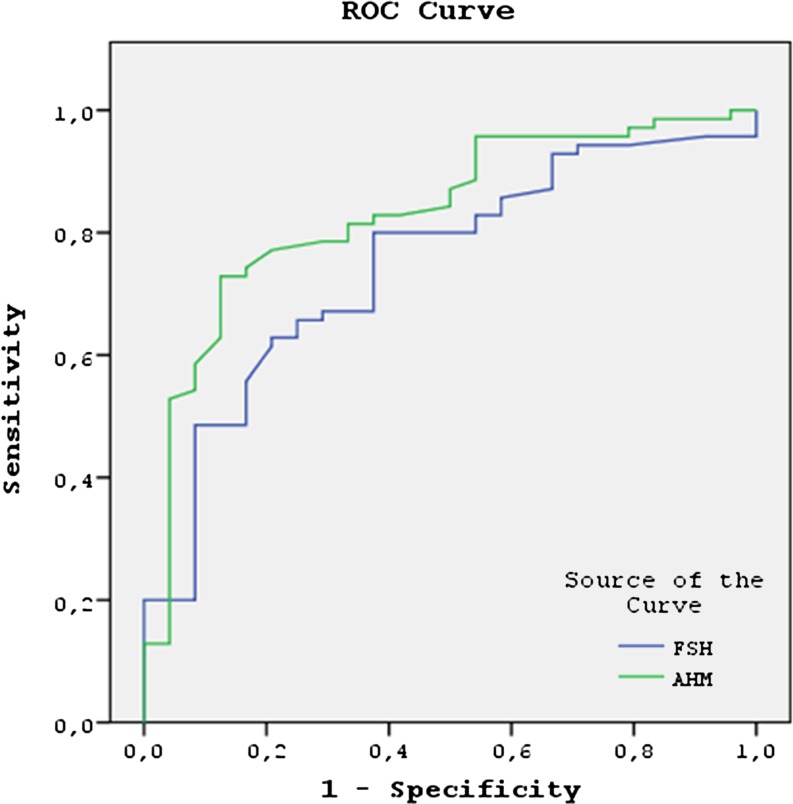
As mentioned earlier, the cycle was cancelled in 37 patients owing to an inadequate response to ovarian stimulation. The difference in AMH levels observed between the cancelled and ongoing cycles was statistically significant (0.4 ± 0.7 versus 1.37 ± 2.1, p < 0.001). For the identification of women at risk of cycle cancellation, an AMH cut-off level ≤1.2 ng/mL had 97.3 % sensitivity, 31.3 % specificity, 33.9 % positive predictive value, and 96.9 % negative predictive value in the women with high baseline FSH levels. The difference in FSH levels observed when comparing the cancelled and ongoing cycles was also statistically significant (15.1 ± 6.6 versus 12.1 ± 4.3, p = 0.002). An FSH cut-off level ≥12.7 mIU/mL had a sensitivity of 59.4 %, specificity of 80.4 %, and positive predictive value of 55 %, with a negative predictive value of 83.1 % for prediction of cycle cancellation. For prediction of cycle cancellation, the area under the curve (AUC) for FSH was similar (0.720; 95 % confidence interval [CI], 0.632–0.807; p < 0.001) to for AMH (0.685; 95 % CI, 0.588–0.782; p < 0.001) (Fig. 1).
Fig. 1.
For prediction of cycle cancellation, the area under the curve (AUC) between FSH (0.720; 95 % confidence interval [CI], 0.632–0.807; p < 0.001) and AMH (0.685; 95 % CI, 0.588–0.782; p < 0.001) was similar
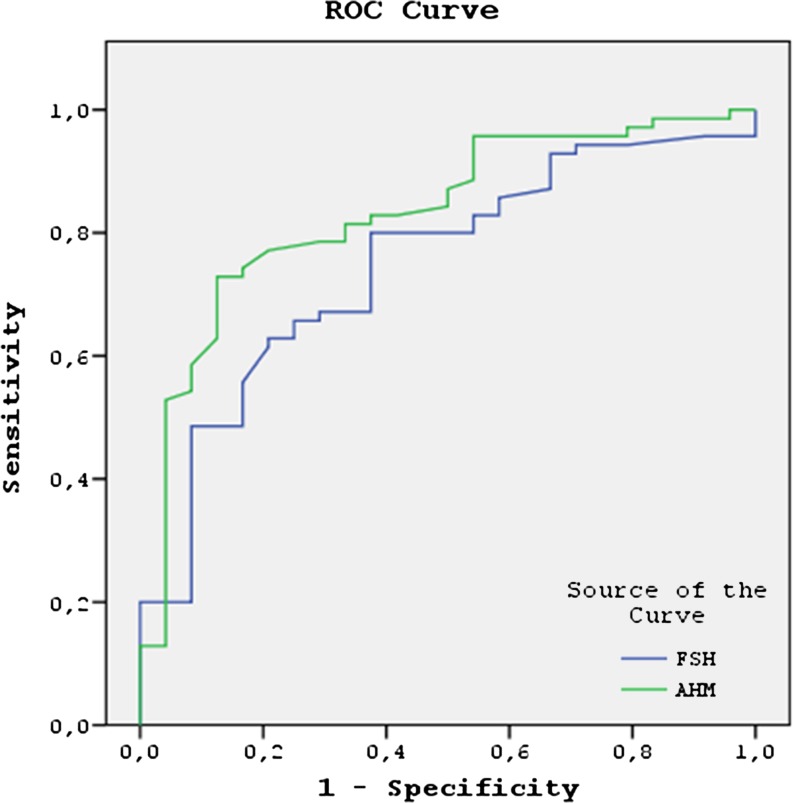
Similarly, the serum AMH levels were significantly higher and the serum FSH levels were significantly lower in the women in whom 4 or more oocytes were retrieved (p < 0.01 for both). Women with cancelled cycles were also included in those who had less than four oocytes retrieved. An AMH cut-off level ≥1 ng/mL has a sensitivity of 58.7 % and specificity of 95.1 % for prediction of retrieval of 4 or more oocytes in women with high baseline serum FSH levels. The positive and negative predictive values for retrieval of 4 or more oocytes at this cut-off level were 87.1 % and 80.6 %, respectively. The use of an FSH cut-off value of 11.5 mIU/mL predicted the retrieval of 4 or more oocytes with a sensitivity of 85.5 % and a specificity of 61.9 %, yielding a positive predictive value of 59.4 % and a negative predictive value of 86.6 %. The ROC analysis demonstrated that AMH (AUC, 0.822; 95 % CI, 0.752–0.893) was similar to FSH (AUC, 0.809; 95 % CI, 0.737–0.882; p < 0.001) in predicting the retrieval of 4 or more oocytes in the women with elevated baseline early follicular FSH levels (Fig. 2).
Fig. 2.
The ROC analysis demonstrated that AMH (AUC, 0.822; 95 % CI, 0.752–0.893) was similar to FSH (AUC, 0.809; 95 % CI, 0.737–0.882; p < 0.001) in predicting the retrieval of 4 or more oocytes in the women with elevated baseline early follicular FSH levels
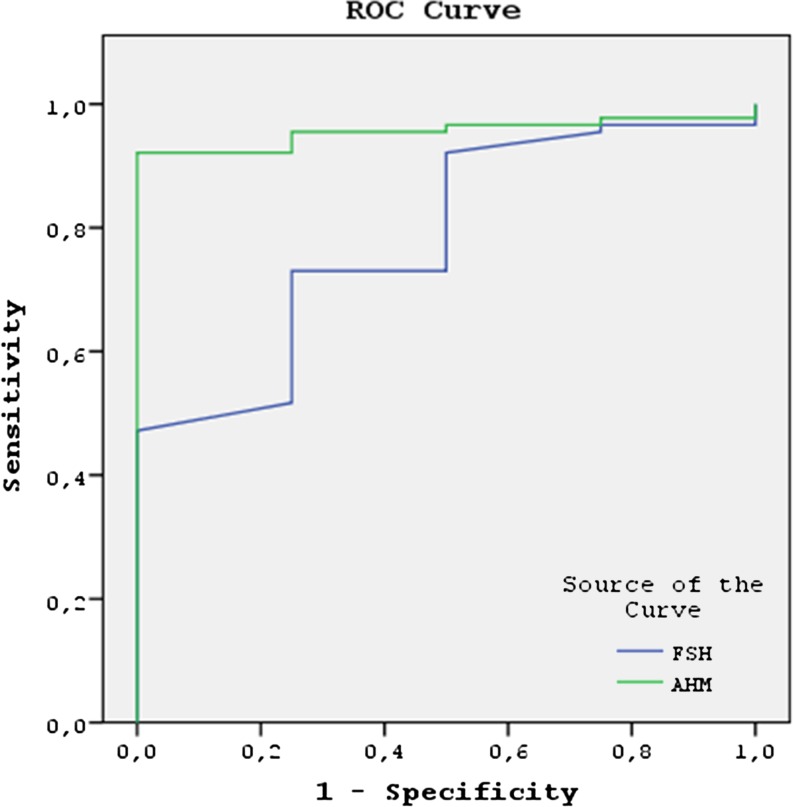
The subjects with ongoing pregnancy were shown to have significantly higher serum AMH and lower FSH levels than those who were not pregnant. While the median serum AMH level of the patients in whom an ongoing pregnancy was achieved was 2.8 ng/mL, it was found to be 0.3 ng/mL in those patients where an ongoing pregnancy was not achieved (p = 0.001). By using a serum AMH cut-off level of 1.5 ng/mL, the ongoing pregnancies were predicted with 83.3 % sensitivity and 82.5 % specificity and yielded a positive predictive value of 31.2 % and a negative predictive value 98.1 %. The median FSH levels of the women with ongoing pregnancy and those without ongoing pregnancy were 9.5 and 11.2 mIU/mL, respectively (p = 0.004). Considering a serum FSH level of 10.25 mIU/mL as the cut-off, the sensitivity and specificity for prediction of an ongoing pregnancy were 83.3 % and 65.0 %, respectively. With this cut-off level for FSH, the positive predictive value was 18.5 % and the negative predictive value was 97.6 %. The AUC for AMH was 0.888 (95 % CI, 0.815–0.960; p = 0.001), a result which was slightly superior to the AUC for FSH (AUC, 0.753; 95 % CI, 0.641–0.865; p = 0.004) in predicting the ongoing pregnancy. in the patients with DOR (Fig. 3).
Fig. 3.
For achieving pregnancy, the AUC for AMH was 0.888 (95 % CI, 0.815–0.960; p = 0.001), a result which was slightly superior to the baseline serum FSH levels (AUC, 0.753; 95 % CI, 0.641–0.865; p = 0.004) in the patients with DOR
By enter logistic regression analysis, the effects of AHM, FSH and age which were the parameters that influence the number of oocyte higher than 4 were evaluated. The coefficient of the explanatory model showed a good level (80.6 %) and the model was significant. In this model the effects of AHM, FSH and age on oocyte cut-off value of 4 was found to be statistically significant (p <0.01). Odds ratio of AMH effect on oocyte number higher than 4 was found to be 4.527 (95 % CI: 1.797 to 11.403). Odds ratio of FSH effect on oocyte number higher than 4 was found to be 6.615 (95 % CI: 2.605 to 16.802) and odds ratio of age effect on oocyte number higher than 4 was found to be 2.899 (95%CI: 1.243 to 6.761) (Table 2).
Table 2.
Logistic regression analysis of basal markers of ovarian reserve for the prediction of four or more oocytes retrieval in women with high baseline serum FSH >9 IU/mL
| Parameters | %95 CI | |||
|---|---|---|---|---|
| p | Odds ratio | Lower | Upper | |
| AHM ≥1 ng/mL | 0,001** | 4,527 | 1,797 | 11,403 |
| FSH ≤11.5 IU/mL | 0,001** | 6,615 | 2,605 | 16,802 |
| Age <35 years | 0,014* | 2,899 | 1,243 | 6,761 |
CI: confidence interval
With an AMH cutoff level of 1.5 ng/mL, 2 groups with high baseline FSH levels (group 1, AMH <1.5 ng/mL; group 2, AMH ≥1.5 ng/mL) were analysed. The AFCs, duration of stimulation, total dose of gonadotropins used in treatment, and number of retrieved oocytes were significantly different between the groups with AMH levels <1.5 ng/mL and those with AMH levels ≥1.5 ng/mL (p < 0.01) who have elevated baseline FSH levels. Similarly, the numbers of metaphase II oocytes, fertilised oocytes, and cryopreserved embryos were statistically higher in the group with AMH levels ≥1.5 ng/mL than in the group with AMH levels <1.5 ng/mL (p < 0.01; Table 3).
Table 3.
The cycle and group characteristics of patients with an AMH cutoff level of 1.5 ng/mL with high baseline FSH levels
| AHM cut off for ongoing pregnancy | p | ||
|---|---|---|---|
| 1.Group (AMH <1,5 ng/mL) (n = 107) | 2.Group (AMH ≥1,5 ng/mL) (n = 32) | ||
| Mean ± SD (Median) | Mean ± SD (Median) | ||
| Age (years) | 36,49 ± 4,79 (37,00) | 32,50 ± 5,29 (31,50) | 0,001** |
| FSH (IU/mL) | 13,72 ± 5,64 (11,76) | 10,37 ± 2,07 (9,85) | 0,001** |
| BMI (kg/m²) | 26,94 ± 4,33 (27,01) | 25,79 ± 3,37 (26,50) | 0,171 |
| + The total amount of gonadotropins used per cycle (IU) | 2375,56 ± 1557,34 (2100,00) | 2109,80 ± 1012,47 (1858,00) | 0,589 |
| Duration of stimulation (days) | 10,20 ± 1,98 (10,00) | 8,78 ± 1,34 (9,00) | 0,001** |
| E2 at the time of HCG injection (pg/mL) | 717 ± 236 (700) | 977 ± 201 (1000) | 0,001** |
| +Antral follicle counts | 5,28 ± 2,76 (5,00) | 14,31 ± 8,78 (12,00) | 0,001** |
| +Preovulatory follicle counts | 4,88 ± 2,88 (3,00) | 7,66 ± 3,52 (7,50) | 0,001** |
| + The numbers of retrieved oocytes | 2,37 ± 2,58 (2,00) | 9,31 ± 6,88 (9,00) | 0,001** |
| + The numbers of metaphase II oocytes | 1,56 ± 1,88 (1,00) | 7,44 ± 5,00 (6,00) | 0,001** |
| + The numbers of fertilized oocytes | 1,15 ± 1,47 (1,00) | 5,78 ± 3,74 (5,00) | 0,001** |
Student t Test, +Mann Whitney U test, **p < 0,01
Discussion
In this report, we investigated the role of AMH in predicting cycle outcome among women with DOR, as diagnosed by measuring high baseline early follicular FSH levels. We also compared FSH and AMH levels in determining cycle outcome in women with high baseline early follicular FSH levels. To our knowledge, there are only a few reports evaluating AMH level as a predictor of cycle outcome among women with elevated baseline FSH levels [19, 21], and this is the first study to compare the efficacy of random AMH levels and early follicular FSH levels in predicting the outcome of ART cycles among women with high baseline FSH levels.
Our data demonstrated that AMH level is strongly correlated with the accurate prediction of retrieval of 4 or more oocytes and ongoing pregnancy among the women with high early follicular FSH levels.
There are 2 studies investigating the predictive value of serum AMH levels among women with high baseline early follicular FSH levels [19, 21]. Buyuk et al. [19] reported that women with random serum AMH levels ≥0.6 ng/mL demonstrated a greater number of retrieved oocytes, greater number of day 3 embryos, and lower cancellation rates when the women had FSH levels >10 IU/mL. They reported that although the clinical pregnancy rate is also greater among the women with serum AMH levels ≥0.6 ng/mL, this was not statistically significant [19]. The second study of this nature was conducted by Gleicher et al. [21]. They concluded that an AMH cutoff level ≥1.06 ng/mL predicted better delivery chances among women with DOR (where DOR was defined by authors as an abnormally high FSH level or an abnormally low AMH level according to age) [21].
In the present study, we found that with a random serum AMH cutoff level ≥1.5 ng/mL, the ongoing pregnancies were predicted with 83.3 % sensitivity and 82.5 % specificity among the women with baseline early follicular serum FSH levels >9 IU/mL. Similarly, an AMH cutoff level ≥1 ng/mL had a sensitivity of 58.7 % and specificity of 95.1 % in the prediction of retrieval of 4 or more oocytes among the women with early follicular serum FSH levels >9 IU/mL.
In this study, we analysed whether AMH or FSH level better predicts ovarian response and outcome of ART cycles among women with high serum baseline FSH levels. In the present study, although AMH and FSH levels were both found to be significant predictors of retrieval of 4 or more oocytes and ongoing pregnancy, AMH level was seen to be slightly superior to FSH level in the prediction of achieving pregnancy.
Barad et al. compared retrospectively the predictive values of AMH and baseline FSH levels in 76 women for whom AMH and FSH levels were available. AMH level was shown to be significantly more accurate than baseline FSH level in predicting pregnancy. It was also reported that AMH level was a more effective predictor of retrieval of fewer than 4 oocytes in comparison with FSH level in those aged 38 years or older. In younger patients (those younger than 38 years), the predictive values of AMH and FSH levels were not significantly different [22].
In conclusion, accurate assessment of ovarian reserve is one of the main problems encountered in the treatment of women with fertility problems. According to our findings, AMH level is one of the most important parameters available in predicting ovarian reserve and IVF outcome in women with DOR.
Footnotes
Capsule Accurate assessment of ovarian response is one of the main problems in assisted reproductive technology. Random serum Anti- Müllerian hormone seems to a useful tool in prediction of ovarian response in patients with high baseline FSH level.
References
- 1.Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S. Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertil Steril. 2005;84:555–69. doi: 10.1016/j.fertnstert.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 2.Scott RT, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63:1–11. [PubMed] [Google Scholar]
- 3.Bancsi LF, Broekmans FJ, Eijkemans MJ, Jong FH, Habbema JD, Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–36. doi: 10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 4.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 5.Fıçıcıoğlu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–6. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–76. doi: 10.1210/jc.81.2.571. [DOI] [PubMed] [Google Scholar]
- 7.Sahmay S, Cetin M, Ocal P, Kaleli S, Senol H, Birol F, Irez T. Serum anti-müllerian hormone level as a predictor of poor ovarian response in in vitro fertilization patient. Reprod Med Biol. 2011;10:9–14. doi: 10.1007/s12522-010-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooij IA, Broekmans FJ, Velde ER, Fauser BC, Bancsi LF, Jong FH, et al. Serum antimullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 9.Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Antimullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio CA, et al. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 11.Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. The role of antimullerian hormone in predicting outcome after IVF: comparison with antral follicle count. Fertil Steril. 2009;91:705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64:603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 13.Hehenkamp WJ, Looman CW, Themmen AP, Jong FH, Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JE, Jones GS, Acosta AA, Wright G. HMG/hCGfollicular maturation for oocytes aspiration: phase II, 1981. Fertil Steril. 1983;39:174–9. doi: 10.1016/s0015-0282(16)46815-9. [DOI] [PubMed] [Google Scholar]
- 15.Raga F, Bonilla-Musoles F, Casan EM, Bonilla F. Recombinant follicle stimulating hormone stimulation in poor responders with normal basal concentrations of follicle stimulating hormone and oestradiol: improved reproductive outcome. Hum Reprod. 1999;14:1431–4. doi: 10.1093/humrep/14.6.1431. [DOI] [PubMed] [Google Scholar]
- 16.Land J, Yarmolinskaya M, Dumoulin J, Evers J. High-dose human menopausal gonadotropin stimulation in poor responders does not improve in vitro fertilization outcome. Fertil Steril. 1996;65:961–5. doi: 10.1016/s0015-0282(16)58269-7. [DOI] [PubMed] [Google Scholar]
- 17.Karande V, Gleicher N. A rational approach to the management of low responders in IVF. Hum Reprod. 1999;14:1744–9. doi: 10.1093/humrep/14.7.1744. [DOI] [PubMed] [Google Scholar]
- 18.Ferraretti AP, Marca A, Fauser BJCM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–24. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 19.Buyuk E, Seifer DB, Younger J, Grazi RV, Lieman HM. Random anti-Müllerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95:2369–72. doi: 10.1016/j.fertnstert.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 20.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 21.Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94:2824–7. doi: 10.1016/j.fertnstert.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 22.Barad DH, Weghofer A, Gleicher N. Comparing anti-Mullerian hormone (AMH) and follicle- stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91:1553–5. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]