Abstract
Purpose
Retinal pigment epithelium cells derived from human embryonic stem cells (ESCs) could be useful for restoring retinal function in age-related macular degeneration. However the use of non-human feeder cells to support the growth of ESCs for clinical applications raises the concern of possible contamination because of direct contact between animal and human cells.
Methods
In this study, we produced human ESCs using human fibroblast feeder layers isolated from foreskin and abdominal tissues. Using this system, human ESCs differentiated into retinal pigment epithelium cells in differentiation medium.
Results
Seven human ESC lines were established from 18 blastocysts. These human ESCs showed normal morphology, expressed all expected cell surface markers, had the ability to form embryoid bodies upon culture in vitro and teratomas after injection into SCID mice, and differentiated further into derivatives of all three germ layers. Under conditions of committed differentiation, these human ESCs could differentiate into retinal pigment epithelium cells after 2 months in culture.
Conclusions
The results of this study demonstrated that human foreskin/abdominal fibroblasts have the potential to support the derivation and long-term culture of human ESCs, which can then be used to generate retinal pigment epithelium cells with characteristic morphology and molecular markers. This technique avoids the concerns of contamination from animal feeder layers during human ESC derivation, culture and differentiation, and will thus facilitate the development of retinal pigment epithelium cell transplantation therapy.
Keywords: Human embryonic stem cell, Human foreskin fibroblast feeder layer, Human abdominal fibroblast feeder layer, Retinal pigment epithelium differentiation
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in people aged 50 years or older in the developed world. More than 8 million Americans suffer from AMD, and the overall prevalence of advanced AMD is projected to increase by more than 50 % by the year 2020 [5, 13]. Its treatment still depends largely on physical therapies, including photodynamic therapy, laser photocoagulation and radiation therapy, though some drugs, such as anti-vascular endothelial growth factor agents, have been used to treat this disease. However the use of physical methods and chemical drugs is limited, and an effective treatment is still lacking.
The retinal pigment epithelium (RPE) plays a critical role in the development and maintenance of adjacent photoreceptors in the vertebrate retina. Degeneration and/or dysfunction of RPE is involved in the two basic forms of AMD, atrophic and exudative [25]. The RPE is the pigmented cell layer in the human retina located just outside the neurosensory retina. It nourishes the retinal visual cells and is firmly attached to the underlying choroid and overlying retinal visual cells. The functions of the RPE cells are to form a blood-retina barrier, absorb stray light, supply nutrients to the neural retina, regenerate visual pigment and take up and recycle outer segments of photoreceptors [33]. The impairment and progressive loss of the RPE in AMD patients thus leads to choroidal neovascularization and/or photoreceptor depletion, resulting in irreversible blindness [8]. Cell replacement using functional RPE cells provides hope of a cure for AMD caused by degeneration or dysfunction of the RPE.
Successful cell replacement therapy relies on being able to obtain enough cells for transplantation. Human embryonic stem cells (human ESCs) are good models for the in vitro study of disease mechanisms and are the most promising candidates for cell therapy in regenerative medicine, because of their high proliferative capacity and ability to differentiate into lineages of all three embryonic germ layers [35]. RPE cells have been derived from mouse, monkey and human ESCs [26]. Klimanskaya et al. initially studied RPE cell differentiation from human ESCs and compared their gene expression profiles with those of primary RPE tissues and cells [15]. Lu et al. obtained functional RPE cells that were able to rescue visual function after transplantation into dystrophic rats [20]. Gong et al. found that the extracellular matrix and neighboring cells were helpful for the induction of human ESCs into retinal or retinal pigment epithelial progenitors [9], while ldelson et al. suggested that nicotinamide, a member of the transforming growth factor-β superfamily, promoted the differentiation of human ESCs into neural and subsequent RPE fates [12]. These differentiated RPE cells displayed the potential to rescue the retina. However the derivation, growth and propagation of these human ESCs depended on the use of mouse fetal fibroblast feeder cells, which limited progress into the clinical application of these cells.
Feeder cells could support the undifferentiated growth of human ESCs, and mouse fibroblast cells from 12.5 or 13.5 day fetuses have been one of the most popular sources of feeder cells for the derivation and culture of human ESCs [35]. Animal-derived feeder cells are associated with the risk of transmitting animal pathogens to human ESCs, and are therefore not desirable in cell lines intended for transplantation in humans [23]. Attempts have been made to use human fibroblasts from various tissues for the derivation and long-term culture of human ESCs, including fibroblasts from fallopian tubes [29], the uterine endometrium [17], and foreskin tissue [1]. Richards et al. evaluated the effects of various human feeders on the prolonged, undifferentiated growth of human ESCs, and suggested that two adult skin fibroblast cell lines established from abdominal skin biopsies supported their prolonged undifferentiated growth for over 30 weekly passages in culture. Human feeder cells maintained human ESC features, including pluripotency, morphology, and expression of cell-surface markers, for multiple passages [30]. However the committed differentiation of human ESCs grown on human feeder cells has yet to be widely studied, especially in terms of differentiation into RPE cells.
In this study, we studied the derivation and long-term culture of human ESCs using human foreskin and abdominal skin feeder cells, and investigated the potential of these human ESCs for committed differentiation into RPE cells.
Materials and methods
This study was approved by the Institutional Review Board at Tianjin Central Hospital for Obstetrics and Gynecology. All chemicals were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA), unless otherwise stated.
Preparation of human fibroblasts
The isolation of human fibroblasts was performed as in our previous study [21]. Foreskin tissues were collected from one each of 4-, 7- and 12-year-old boys after circumcision, and were donated by the parents. Abdominal skin tissues were collected from 26- and 27-week aborted fetuses and were also donated by the parents. The tissues were washed using Hank’s balanced salt solution (Invitrogen) and minced into pieces of about 1 mm2 using scissors. Tissues were digested with 0.25 % trypsin and 10 U/ml DNase I in DMEM/F-12 (Invitrogen). The disassociated single cells were cultured in culture medium consisting of 85 % Dulbecco’s modified Eagle’s medium (DMEM; high-glucose) (Invitrogen) supplemented with 15 % fetal bovine serum (Invitrogen), 2 mM L-glutamine (Invitrogen), and 1 % penicillin-streptomycin (Invitrogen) in a humidified atmosphere of 5 % CO2 in air at 37.0°C. The human fibroblasts could be propagated in 0.05 % trypsin-EDTA every 3–5 days, and could be expanded for more than 30 passages. Foreskin fibroblasts at passages 10–20 were usually selected for preparation of feeder cells.
Preparation of feeder layers
To prepare a feeder layer, human fibroblasts were inactivated by 10 mg/ml mitomycin C for 2 hr, washed at least five times with phosphate-buffered saline (PBS) and reseeded at a density of 3–5 × 105 cells per well in a 4-well plate coated with 0.1 % gelatin. The fibroblasts were plated 1 day before use and cultured in fibroblast culture medium under a humidified atmosphere of 5 % CO2 in air at 37.0°C, and were used within 3 days of preparation. Two hours before use, the culture medium was changed to human ESC culture medium.
Isolation and culture of human ESCs
Embryos unsuitable for embryo transfer and cryopreservation were collected on day 3 of in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) procedures following IRB approval and the receipt of informed consent by the couples undergoing the IVF treatment. Four or 8-cell embryos were cultured using Gm culture medium (Vitrolife) supplemented with 10 % human serum albumin (Vitrolife) and insulin-like growth factor (IGF)-1, IGF-II and granulocyte-macrophage colony-stimulating factor (GM-CSF). Blastocysts were observed after 2 days and were selected into fresh Gm culture medium for prolonged culture. The fully expanded blastocysts were collected for isolation of the inner cell mass (ICM).
After removal of the zona pellucida using acidic Tyrode solution, trophoblast cells were removed by immunosurgery using anti-human whole serum (1:3) and guinea pig complement (1:10) in 50-μl droplets under oil. The ICM was washed twice, and immediately transferred onto the mitomycin-C mitotically inactivated human fibroblast feeder layer in human ESC culture medium, consisting of knockout DMEM (KO-DMEM; Invitrogen) supplemented with 20 % knockout serum replacement (KO-SR; Invitrogen), 2 m L-glutamine (Invitrogen), 1× minimal essential medium non-essential amino acids (Invitrogen), 1 % penicillin-streptomycin (Invitrogen), 0.1 mM β-mercaptoethanol (Millipore) and 10 ng/ml basic fibroblast growth factor (bFGF) 1 % penicillin-streptomycin (Invitrogen).
After 5 days, the human ICM formed a small colony, and the culture medium was changed every 2 days. After 10–15 days, the ICM clump was disassociated mechanically into 3–4 small clumps using a micropipette, and these were replated onto a fresh human fibroblast feeder layer. After 4–5 days, the resulting colonies were propagated mechanically into pieces containing about 200 ES-like cells on a fresh human fibroblast feeder layer, and cultured under a humidified atmosphere of 5 % CO2 in air at 37.0°C. After five passages, the human ES-like colonies were propagated using 1 mg/ml type IV collagenase every 4–7 days. To date, the ESC line cjes001 has been maintained under these culture conditions for 12 months (passage 74).
Differentiation, enrichment, and culture of pigmented cells
Human ESCs at passages 20, 40 and 60 were used in differentiation experiments. The ESCs were cultured in 6-well culture plates for 5–7 days until the borders of the ESC colonies contacted each other, after which the human ESCs culture medium was removed and changed to differentiation medium (human ES culture medium without bFGF). The typical morphology of human ESC colonies slowly disappeared and the colonies gradually became multilayered. After 3–5 days, these pigmented clusters were mechanically isolated using a glass pipette under a dissecting microscope (SMZ1000, Nikon), and replated into 4-well culture dishes coated with Matrigel (BD Biosciences, diluted 1:30), when pigmented foci were observed. The pigmented clusters were cultured in differentiation medium. Expanded monolayers of pigmented foci were cultured for a further 2 months, after which the cells displayed a hexagonal shape and contained melanin. These differentiated cells were cultured under a humidified atmosphere of 5 % CO2 in air at 37.0°C.
Alkaline phosphatase (AP) and immunofluorescence staining
For alkaline phosphatase staining, human ESCs on human fibroblast feeder layers were fixed with 10 % formalin and then incubated with the AP substrate BCIP/NBT. The resulting precipitate was observed using an inverted microscope (TE100, Nikon).
For immunofluorescence staining, ESCs were initially passaged on human fibroblast feeder layers in 4-well plates for 2–4 days, fixed with 4 % paraformaldehyde and incubated with primary antibodies (1:100) overnight at 4°C. The primary antibodies included human ESC antibodies against stage-specific embryonic antigen (SSEA)-1, SSEA-3, and SSEA-4 (R&D), TRA-1-60 and TRA-1-81 (BD Biosciences), and a RPE cell marker (ZO-1). TX-conjugated goat anti-mouse and TRITC-conjugated goat anti-rat immunoglobulin antibodies were used as secondary antibodies (1:200). Human ESC nuclei were stained using Hoechst 33342. The cells were examined with a confocal laser scanning microscope (LSM710, Zeiss).
Karyotyping analysis
Human ESCs were passaged onto a culture dish plated with Matrigel (BD Biosciences) for 3–4 days prior to karyotyping analysis. Human ESCs were incubated for 4 h in ESC medium containing colchicine (Sigma), and then rinsed twice with PBS. ESCs were collected for centrifugation (200 g, 10 min) after detachment from the culture dish by trypsinization. The nuclear membrane was disrupted by hypotonic treatment with 8 mM potassium chloride for 30 min, after which the cells were fixed with methanol:glacial acetic acid, dropped carefully onto glass slides, and dried overnight.
Chromosome spreads were stained with 0.4 % (w/v) Giemsa in buffered methanol solution (pH 6.8) and photographed. The chromosomes were visualized using standard G-band staining. At least 100 metaphase cells were examined from each sample.
Teratoma formation
For teratoma formation, 5 × 106 human ESCs were prepared and dissociated with trypsin. The cells were then injected subcutaneously into the hind leg of 6–8 week-old non-obese diabetic/severe-combined immunodeficient (NOD-SCID) mice. After the teratomas had grown to over 3 cm in diameter (usually 10–12 weeks), the mice were euthanized and the lesions were surgically removed. The tumors were fixed overnight in 4 % paraformaldehyde at 4°C. Paraffin sections were prepared and processed for histological examination.
Embryoid body (EB) formation
For EB formation, human ESCs were plated onto gelatin-coated tissue culture dishes (at ratios of 1:5) and grown for 5–7 days until 80–90 % subconfluent. The cells were trypsinized and cultured in EB medium (human ES culture medium without bFGF) on low adherent plates. The EBs were cultured for 15–20 days and RNA was then extracted for identification of the three germ layers.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from undifferentiated human ESC or EB samples according to the manufacturer’s recommended protocol. cDNA was synthesized from about 1 mg of total RNA using SuperScript Reverse Transcriptase (Invitrogen) and was subjected to PCR amplification using primers for the human ESC plurioptent genes OCT4 and Nanog, and for differentiation markers for the three germ layers (AFP, HBZ, and NEUOD-1). The PCR primers used were partly referred to in a previous study. The PCR products were size fractionated using 2 % agarose gel electrophoresis and visualized by staining with ethidium bromide. Human fibroblasts were used as negative controls.
Results
Derivation of human ESCs using human fibroblast feeder layers
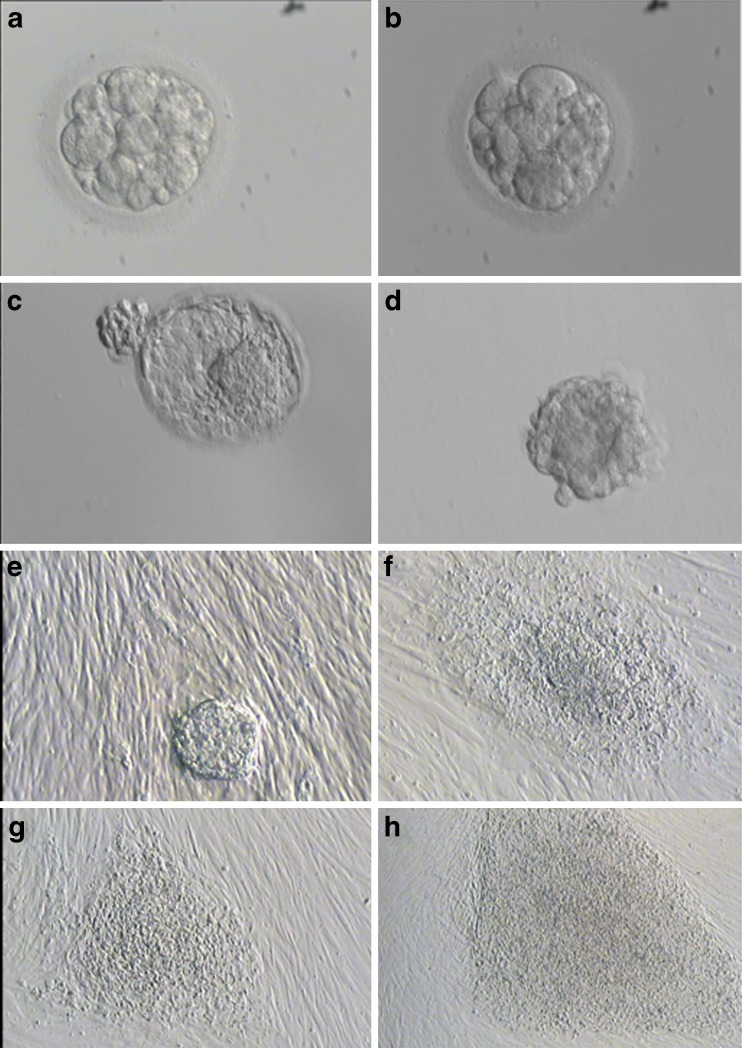
A total of 134 embryos discarded during IVF or ICSI procedures were collected and cultured in Gm medium (Fig. 1a and b). Twenty-six embryos formed a blastocoel in the early blastocyst stage, and 18 blastocysts were fully expanded on days 6–9 for immunosurgery (Fig. 1c and d). After 5 days, 15 ICMs were attached to the human fibroblast feeder layer (Fig. 1e). Nine colonies were observed after 10–15 days and some clumps of every colony were cut and reseeded onto fresh human fibroblast feeder layers (Fig. 1f). Finally, seven ESC lines (H-TJ6-8 using foreskin feeder layer and H-TJ9-12 using human abdominal feeder layer) were established and demonstrated stable growth rates and morphologies after 60 passages (Table 1) (Fig. 1g and h).
Fig. 1.
Morphology of human fertilized embryos, blastocysts, ICM and human ESCs. a, b Discarded low-quality fertilized embryos that did not meet the embryo transfer criteria in IVF or ICSI cycles. c Blastocyst on day 6.5 before ICM isolation. d Isolated ICM. e Attached ICM on human foreskin fibroblast feeder layer on day 1. f Primary colony of human ESCs on day 14. g Colony of human ESCs at passage 2. h Colony of human ESCs at passage 20
Table 1.
Information of established human ESCs lines
| Blastocysts | Day | Grading | Feeder layer | Attachment | Outgrowth | ES cell lines |
|---|---|---|---|---|---|---|
| 1 | 6 | 4BC | Foreskin | Y | Y | Y |
| 2 | 8 | 4CC | Foreskin | |||
| 3 | 8 | 5BB | Foreskin | Y | ||
| 4 | 7 | 3BA | Foreskin | Y | Y | Y |
| 5 | 7 | 4CC | Foreskin | |||
| 6 | 7 | 5CB | Foreskin | Y | ||
| 7 | 9 | 3CB | Foreskin | Y | Y | |
| 8 | 6 | 3AB | Foreskin | Y | Y | Y |
| 9 | 6 | 5BC | Abdomen | Y | Y | |
| 10 | 8 | 2BA | Abdomen | Y | ||
| 11 | 7 | 4CB | Abdomen | Y | Y | Y |
| 12 | 7 | 5BB | Abdomen | Y | Y | Y |
| 13 | 9 | 3CC | Abdomen | |||
| 14 | 9 | 3CC | Abdomen | Y | ||
| 15 | 8 | 4BA | Abdomen | Y | ||
| 16 | 6 | 3BA | Abdomen | Y | Y | Y |
| 17 | 8 | 5CB | Abdomen | Y | ||
| 18 | 6 | 5AB | Abdomen | Y | Y | Y |
| Total 18 | 15 | 9 | 7 |
Characteristics of human ESCs on human fibroblast feeder layers
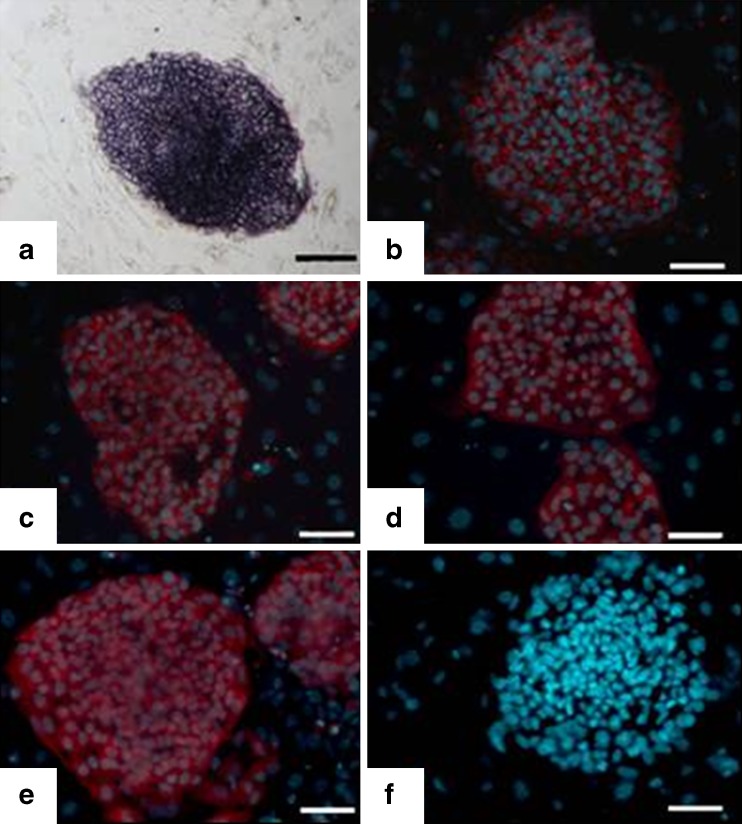
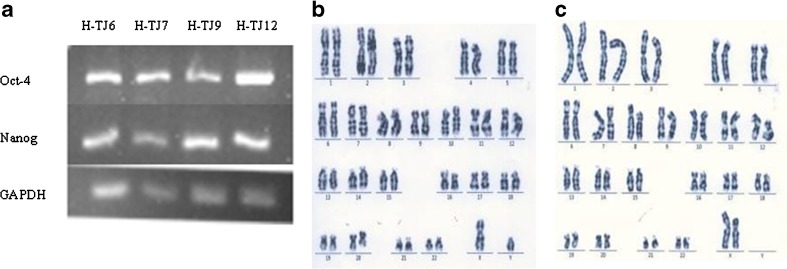
H-TJ6-12 showed the normal morphological characteristics of human ESCs, including an increased nucleus/cytoplasm ratio, formation of tightly-packed colonies, and distinct borders, relative to the feeder cells (Fig. 1h). H-TJ6-12 also displayed high levels of AP activity (Fig. 2a). All seven human ESC lines expressed SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 (representative expressions for H-TJ6 are shown in Fig. 2b–e), but did not express the negative cell surface marker SSEA-1 (Fig. 2f). RT-PCR studies provided further evidence of the stem cell pluripotent characteristics of H-TJ6 cell lines by identifying the expression of many genes, including OCT4 and Nanog (Fig. 3a). These seven cell lines showed normal karyotypes (2 were 46, XX and 5 were 46, XY) at passages 20, 40 and 60. The karyotypes of H-TJ6 and H-TJ10 at passage 20 are shown in Fig. 3b and c. At least 100 cells from each line were examined, and each one possessed normal human 46XX or 46XY karyotypes.
Fig. 2.
Expression of characteristic cell markers in human ESCs (H-TJ6, passage 20). Line P-TJ had high alkaline phosphatase activity (a) and expressed SSEA-3 (b), SSEA-4 (c), TRA-1-60 (d), and TRA-1-81 (e) but not SSEA-1 (f). Bar: 100 μm
Fig. 3.
RT-PCR analysis of the expression of pluripotent genes in human ESCs (a) and karyotyping analysis (b, c). The expression of pluripotent genes was examined in four human ESC lines, H-TJ6, H-TJ7, H-TJ9 and H-TJ12 (a). Karyotyping analysis was performed using in human ESC lines, H-TJ6 and H-TJ10. H-TJ6 had a normal 46, XY karyotype (b) and H-TJ10 had a normal 46, XX karyotype (C)
In-vitro and in-vivo differentiation potentials of human ESCs
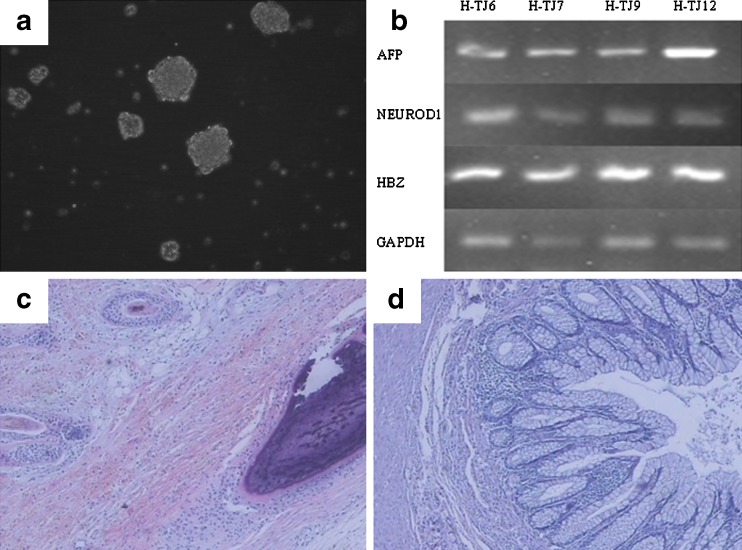
To identify their in-vitro differentiation potential, human ESCs were induced to form EBs by suspension culture in differentiation culture medium (Fig. 4a). After spontaneous differentiation for 7 days, RNA was extracted from the EBs for RT-PCR. Markers for all three embryonic germ layers were identified successfully, including AFP (endoderm), NEUOD-1 (ectoderm) and HBZ (mesoderm) (Fig. 4b). The abilities of H-TJ6-12 to form EBs were identified at passage 20, and marker expression and morphological changes occurred in all seven human ESC lines during differentiation.
Fig. 4.
Identification of differentiation potential of human ESCs in vitro and in vivo (H-TJ6). a EBs in suspension culture at day 4. Bar: 100 μm. b RT-PCR analysis of the expression of specific markers for the three embryonic germ layers in EBs, including AFP (endoderm), NEUOD-1 (ectoderm) and HBZ (mesoderm). c, d Histopathological analysis of teratomas resulting from in vivo differentiation of human ESCs, including hair follicles (ectoderm) (c), cartilage tissue (mesoderm) (c) and intestinal epithelium (endoderm) (d)
To identify the in-vivo differentiation potential, 5 × 106 human ESCs were collected and injected into SCID mice. Mice with tumors were sacrificed and tumors were removed at 8–12 weeks, once they reached a diameter of about 3 cm. All seven human ESC lines derived in this research formed teratomas at passages 25. Each teratoma contained tissues representative of the three embryonic germ layers, including hair follicles (ectoderm), cartilage tissue (mesoderm) and intestinal epithelium (endoderm). The tissues formed in these teratomas are shown in Fig. 4c (hair follicle and cartilage tissue) and D (intestinal epithelium).
Committed RPE differentiation of human ESCs
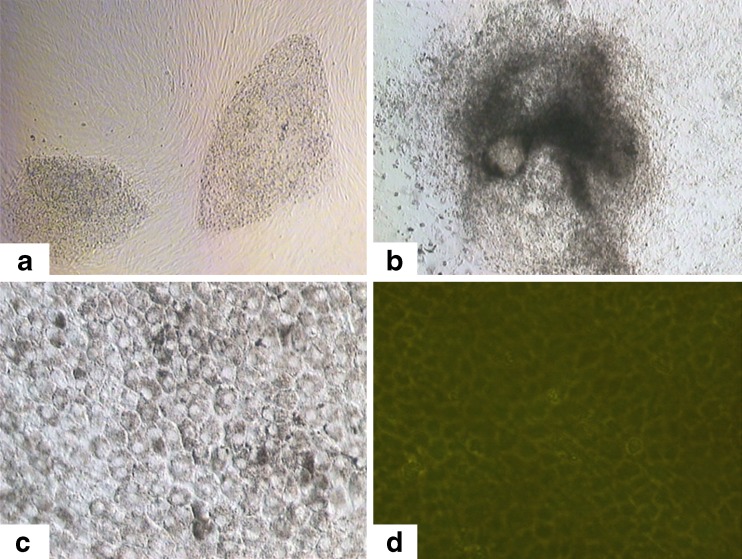
Human ESCs at passages 20, 40 and 60 were used in differentiation experiments to identify their potential to form RPE cells (Fig. 5a). When human ESCs were allowed to spontaneously differentiate in culture with differentiation medium, the colonies slowly lost their typical undifferentiated morphology and formed multi-layered structures (Fig. 5b). Pigmented cells arose spontaneously within 3–4 weeks, and the pigmented clusters increased in size and number after 6–8 weeks, by which time polygonal pigmented cells coexisted in cultures with cells of other phenotypes. The pigmented clusters matured with proliferation of polygonal pigmented cells, and the dense pigmentation gradually spread to the periphery. Some cells lost pigmentation during this process, as they divided and migrated. The pigmented clusters formed an expanded monolayer of pigment foci for more 2 months, and the resulting cells displayed a hexagonal shape and contained melanin. The epithelial morphology and expression of pigment suggested the differentiated state of these human ESC-derived RPE cells (Fig. 5c and d).
Fig. 5.
RPE differentiation and identification in culture of human ESC-derived pigmented cells. a H-TJ6 at passage 40. b EB outgrowth at 1 week. c Primary culture of pigmented epithelial cells. d RPE cells expressed ZO-1 protein, which is a tight junction marker of epithelium cells
Discussion
In this study, seven primary human ESC lines were derived from fertilized embryos using human fibroblast feeder cells; three were derived using human foreskin fibroblast feeder layers and four using human abdominal fibroblast feeder layers. The embryos were donated by couples undergoing IVF or ICSI, because their qualities did not meet the required criteria for embryo transfer or cryopreservation, according to Gardner et al. [7]. Derivation of human ESCs using lower quality blastocysts has been reported, but two differences between the previous and current studies should be noted. The derivation efficiencies were 20 % (2/10) [24], 11 % (2/19) [3] and 8.5 % (8/94) [18], respectively, however seven human ESC lines were derived from 18 blastocysts in the current study, giving an efficiency of 38.9 %, which is almost twice that in any previous study. This may be attributed to the addition of growth factors, including GM-CSF and IGF-1, to the embryo culture medium; these growth factors have been shown to have beneficial effects on human blastocysts [19, 31]. Another possible factor is that human ESCs in previous studies were isolated and derived using mitomycin-treated mouse embryonic fibroblast (MEF) feeder layers. However, Martin et al. demonstrated that certain mouse glycoproteins can be identified on the surface of human ESCs cultured on MEFs, and that these glycoproteins, if maintained on the surface of the cells, could result in a severe immune reaction against any transplanted cells [23]. The current study used human feeder layers from foreskin and abdominal tissues collected from discarded tissue after circumcision and from aborted fetuses, respectively. These tissues were easier to obtain than donated fallopian tube epithelium or uterine endometrium, though these tissues also have the potential to support the derivation and propagation of human ESCs.
The human ESCs lines derived here showed similar properties to those described by several other groups. They showed a similar morphology to already established human ESCs and typical human ESC characteristics, including AP staining, expression of cell surface markers (including SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81 but not the marker, SSEA-1) and pluripotent genes (including OCT-4, Nanog and SOX2). These human ESCs could be passaged sequentially for more than 70 passages, and retained a normal karyotype and undifferentiated human ESC characteristics. In addition to self-renewal characteristics, the cells also possessed another defining feature of human ESCs in terms of the ability to differentiate into derivatives of all three germ layers both in vitro and in vivo. This capacity was assessed by evaluating the formation of teratomas in vivo and by identifying the formation of EBs in vitro.
AMD is the leading cause of irreversible blindness in people aged 50 years or older in the developed world, and no effective treatments are currently available. Degeneration and/or dysfunction of the RPE are closely involved in AMD, suggesting that cell replacement therapy might provide new hope for AMD treatment [25]. The quality and quantity of transplanted cells are keys to the successful outcome of cell transplantation therapy. Pluripotent stem cells, including human ESCs and induced plurioptent stem cells (iPSCs) have been successfully induced to differentiate into cells for treating several diseases, such as dopamine neurons for Parkinson’s disorder [11, 27], pancreatic β-cells for diabetes [16, 32], hematopoietic colony-forming cells for hemopathies [4, 14] and hepatocytes for liver disease [28, 34]. RPE cells have recently been differentiated using human ESCs and iPSCs, and the functions of these differentiated cells have been identified using animal AMD-disease models, which demonstrated that transplantation of differentiated cells could rescue visual function in a rat model of retinal degeneration caused by RPE dysfunction [2, 10, 22]. However established human ESCs and functional RPE cells differentiated from these human ESCs are little used in clinical practice because of their derivation using mouse feeder layers, which increase the potential for heterologous contamination. The current study partly resolved this issue by establishing feeder layers using human fibroblasts. The human ESCs derived using these human feeder layers demonstrated normal morphology and the expression profiles of key human ESC markers, differentiated RPE cells could be induced effectively and identified using the specific ZO-1 marker, a tight junction protein.
Two key points need to be considered regarding the use of differentiated RPE cells in clinical settings. First, it is necessary to avoid immune rejection. The optimal solution would be to use transplanted cells that originate from the patient. This could be achieved by human somatic cell nuclear transfer or by using iPSCs. iPSCs have been derived using forced expression of exogenous pluripotent genes and have demonstrated the potential to differentiate into RPE cells [2]. The use of human ESCs from cloned blastocysts by somatic cell nuclear transfer has not been successful, although the human cloned blastocysts could be obtained [36]. The second issue concerns safety. Lu et al. evaluated the long-term safety and function of RPEs differentiated from human ESCs using a mouse model over more than 220 days [21]. No teratomas or tumors developed, suggesting that these RPE cells could serve as a potentially safe and inexhaustible source for the effective treatment of a range of retinal degenerative diseases. Fukuda et al., however, suggested that there was a risk of tumor formation as a result of contamination with undifferentiated cells [6], and Hirami et al. also found that at least 0.6 % of mouse iPSCs remained undifferentiated in culture, even at 15 days after retinal cell differentiation [10]. The differentiation efficiency of RPE cells in previous reports ranged from 70–80 %, and improving differentiation efficiency by removing non-RPE cells and/or improving the purity of RPE cells is thus key to the clinical application of differentiated RPE cells.
In summary, we established two types of human feeder layers using foreskin fibroblasts and abdominal fibroblasts, respectively, and demonstrated that human ESCs could be derived using these two feeder layers. Moreover, RPE cells could differentiate from these human ESCs. Further studies will focus on the purity and functional identification of RPE cells. The present findings may facilitate the development of human ESC-based transplantation therapies for retinal diseases.
Acknowledgement
This work was supported in part by the National Natural Science Foundation of China of Young Scholars (No. 81100404) to L.Z.Y, by the National Natural Science Foundation of China (No. 30973255) to L.X.R, and by the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20110001120008) to Y.Y.
Footnotes
Capsule
Human embryonic stem cells were successfully derived and propagated using human foreskin and abdomen fibroblast cells as feeder cells, and these embryonic stem cells could differentiate to the retinal pigment epithelium cells in vitro.
Contributor Information
Yun-Shan Zhang, Phone: +86-022-58287436, FAX: +86-022-58287436, Email: tjzys@hotmail.com.
Zhen-Yu Lu, Phone: +86-022-58287436, FAX: +86-022-58287436, Email: luzhenyu.tj@gmail.com.
References
- 1.Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–6. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Qian K, Hu J, Liu D, Lu W, Yang Y, et al. The derivation of two additional human embryonic stem cell lines from day 3 embryos with low morphological scores. Hum Reprod. 2005;20:2201–6. doi: 10.1093/humrep/dei010. [DOI] [PubMed] [Google Scholar]
- 4.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–71. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 8.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Sagiv O, Cai H, Tsang SH, Priore LV. Effects of extracellular matrix and neighboring cells on induction of human embryonic stem cells into retinal or retinal pigment epithelial progenitors. Exp Eye Res. 2008;86:957–65. doi: 10.1016/j.exer.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A; 107:4335–40. [DOI] [PMC free article] [PubMed]
- 12.Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–21. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–45. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 16.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 17.Lee JB, Song JM, Lee JE, Park JH, Kim SJ, Kang SM, et al. Available human feeder cells for the maintenance of human embryonic stem cells. Reproduction. 2004;128:727–35. doi: 10.1530/rep.1.00415. [DOI] [PubMed] [Google Scholar]
- 18.Lerou PH, Yabuuchi A, Huo H, Takeuchi A, Shea J, Cimini T, et al. Human embryonic stem cell derivation from poor-quality embryos. Nat Biotechnol. 2008;26:212–4. doi: 10.1038/nbt1378. [DOI] [PubMed] [Google Scholar]
- 19.Lighten AD, Moore GE, Winston RM, Hardy K. Routine addition of human insulin-like growth factor-I ligand could benefit clinical in-vitro fertilization culture. Hum Reprod. 1998;13:3144–50. doi: 10.1093/humrep/13.11.3144. [DOI] [PubMed] [Google Scholar]
- 20.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–35. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Zhu W, Yu Y, Jin D, Guan Y, Yao R, et al. Derivation and long-term culture of human parthenogenetic embryonic stem cells using human foreskin feeders. J Assist Reprod Genet; 27:285–91. [DOI] [PMC free article] [PubMed]
- 22.Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–99. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 23.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 24.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, et al. Human embryonic stem cell lines derived from discarded embryos. Stem Cells. 2003;21:521–6. doi: 10.1634/stemcells.21-5-521. [DOI] [PubMed] [Google Scholar]
- 25.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–26. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–24. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 27.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–8. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 29.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–6. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 30.Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–56. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- 31.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–76. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- 32.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–4. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology; 51:329–35. [DOI] [PMC free article] [PubMed]
- 35.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Mai Q, Chen X, Wang L, Gao L, Zhou C, et al. Assessment of the developmental competence of human somatic cell nuclear transfer embryos by oocyte morphology classification. Hum Reprod. 2009;24:649–57. doi: 10.1093/humrep/den407. [DOI] [PubMed] [Google Scholar]