Abstract
The selective reaction of one functional group in the presence of others is not a trivial task. A noteworthy amount of research has been dedicated to the chemoselective reaction of the hydroxyl moiety. This group is prevalent in many biologically important molecules including natural products and proteins. However, targeting the hydroxyl group is difficult for many reasons including its relatively low nucleophilicity in comparison to other ubiquitous functional groups such as amines and thiols. Additionally, many of the developed chemoselective reactions cannot be used in the presence of water. Despite these complications, chemoselective transformation of the hydroxyl moiety has been utilized in the synthesis of complex natural product derivatives, the reaction of tyrosine residues in proteins, the isolation of natural products and is the mechanism of action of myriad drugs. Here, methods for selective targeting of this group, as well as applications of several devised methods, are described.
Introduction
The development of chemoselective reactions has seen great interest in the last several decades owing to the important roles that these transformations play throughout both chemistry and biology.1–3 Selective reactions have been used extensively in the field of bioconjugation for many applications including antibody modification, protein visualization studies, DNA labeling and protein-protein conjugation to name a few.4 The ability to selectively transform small molecules is also critical. From a synthetic standpoint, use of chemoselective reactions is ideal for both atom and step economy.5,6 Despite the utility of selective transformations, it is often difficult to formulate conditions that affect only one functional group class given the similarity in reactivity of several moieties. As a result, the vast majority of chemoselective transformations target the most reactive groups. In biological applications, reactions are directed towards thiols and amines owing to their nucleophilic character and ketones/aldehydes due to their electrophilicity.7,8 To expand the chemoselective toolkit, methods to address functional groups that are not at the extremes of the reactivity spectrum are needed. Here, we discuss progress towards the generation of chemoselective strategies directed at the hydroxyl moiety.
The hydroxyl group is present in many biologically significant molecules including ~65% of known natural products,9 ~40% of drugs,9 several classes of lipids and three of the 20 common amino acids. In human proteins, serine (Ser) and threonine (Thr) comprise approximately 7.5% and 6% of the amino acid content, respectively, while tyrosine (Tyr) represents ~3%.10 The hydroxyl moiety is also present in glycans that have long been addressed with periodate oxidation11 and boronate-mediated reactions12 for purification and bioconjugation applications.4,13 It is clear that tools to selectively transform this moiety will find utility; however, transformation of the hydroxyl group is challenging given that it is less nucleophilic than other common moieties including the amine, thiol or carboxylic acid. In addition, methods for targeting of alcohols in biological samples are severely hindered by the overwhelming presence of another hydroxyl group-containing molecule, water. Despite these impediments, successful strategies have been developed and will be highlighted here. We will provide a brief overview of selective hydroxyl-directed transformations with an emphasis on those that have or may find utility in the field of chemical biology. Several recent examples of biological studies that were enabled by the chemoselective targeting of a hydroxyl group will then be discussed.
Chemoselective Transformation of Hydroxyl Groups in Small Molecule Substrates
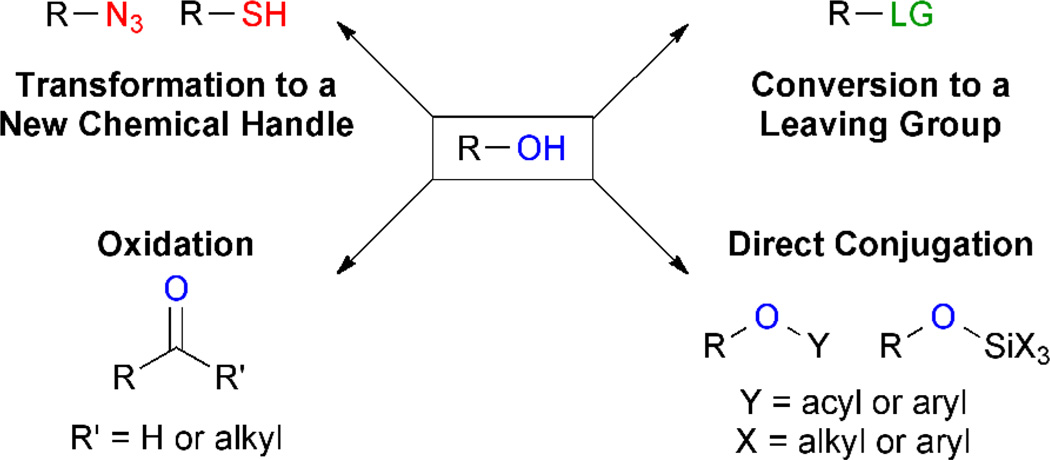
Chemoselectivity is of critical importance in synthetic chemistry;14 however, a comprehensive summary of strategies to target the hydroxyl moiety is out of the scope of this review. Instead, we will focus on methods of particular interest to the chemical biology community, that is, those that could facilitate the swift generation of inhibitor analogs to enable identification of their protein target(s).15 This can be accomplished by total synthesis but is more favorably achieved by selective conjugation of a tag (e.g. biotin, photocrosslinker, solid support) to the molecule of interest. Here, we will discuss reactions that could enable connection of two molecular fragments through an alcohol. We have categorized these reactions into subsets: direct conjugation, oxidation, activation by generation of a leaving group (e.g., Cl, tosyl) and transformation to an alternative chemical handle (i.e., azide or thiol; Figure 1). Many of the presented examples have only been applied to relatively simple substrates to date or only afford partial selectivity, highlighting the difficulty in identification of conditions for specific conversion of a hydroxyl moiety amidst an array of more reactive functional groups. However, progress towards this goal has been steady and many of the described strategies may find utility in more complex molecules including natural products and proteins in the future.
Figure 1.
Chemoselective transformation of the hydroxyl group. Four categories of conversions are described: direct conjugation reactions, oxidation, activation by generation of a leaving group and transformation to an alternative chemical handle.
Direct conjugation reactions
The reactions discussed in this section are those that promote the linkage of two molecular fragments by selective arylation or acylation of the hydroxyl group (Figure 1, bottom right).16 A significant amount of effort has been put into development of strategies to facilitate the selective reaction of an alcohol in the presence of an amine given the abundance of compounds that contain both of these functional groups and the relatively low nucleophilicity of the hydroxyl group. Generally, primary amines must be protected prior to reaction of the alcohol and later unmasked. Several synthetic strategies have been devised to circumvent this roundabout approach with particular focus on amino alcohols, which are important starting materials for a number of total syntheses.
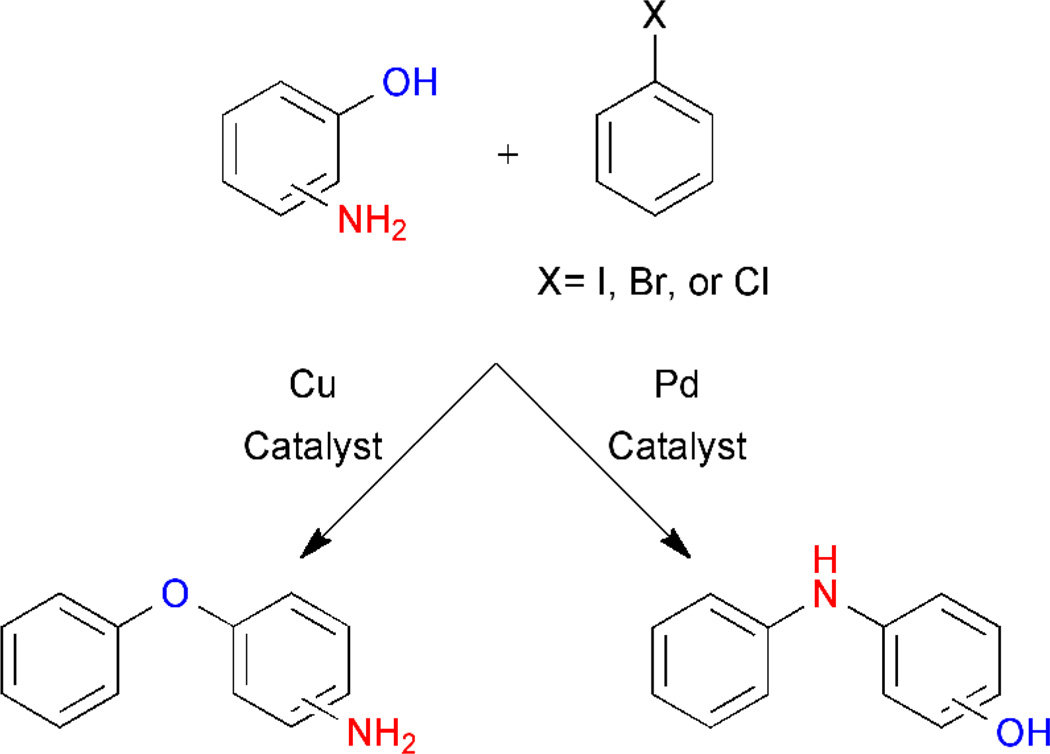
Ullmann-type couplings have been widely used to form C-C, C-N, and C-O bonds and can be tuned for either hydroxyl or amine selectivity.17 Buchwald and coworkers developed a variant for the O-arylation of β-amino alcohols by tailoring solvent, temperature and the base utilized. Initially, alcohol selectivity was achieved only at high temperature (125 °C).18 However, screening of several ligands to chelate the requisite Cu(I) species resulted in the identification of a method for the chemoselective O-arylation of a variety of amino alcohols at lower temperatures (90 °C; >15:1 selectivity).19 Additionally, they reported the ability to differentiate between N- and O-arylation of 3- and 4-aminophenols by utilizing either a palladium or copper catalyst, respectively (Figure 2).20
Figure 2.
Selective O- or N-arylation can be achieved by use of the appropriate catalyst. Copper-mediated catalysis favors formation of the O-arylation products while utilization of palladium yields the N-arylation products.
Selective O-acylation has also been accomplished using metal-mediated processes. A zinc-cluster catalyst was developed to facilitate chemoselective formation of O-acetylated products from aliphatic starting materials, even in the presence of primary and secondary amines.21,22 An alternative to employing metal catalysts to achieve selectivity is to take advantage of the pKa differences between alcohols and amines. For example, to synthesize peracetylated carbohydrate intermediates from D-glucamine without acetylating the amino group, Hamilton and coworkers utilized acetyl chloride under acidic conditions, which prevented acylation of the primary amine by formation of the ammonium chloride salt.23 The field of carbohydrate chemistry has encouraged the development of strategies for the regioselective acylation of either primary or secondary hydroxyl groups. For example, modified 4-(N,N'-dimethylamino)pyridine (DMAP)- and peptide-based catalysts have been explored to selectively functionalize carbohydrates on their secondary hydroxyl groups.24–26 None of these methods were applied in the presence of a primary amine, and therefore may not be chemoselective for the hydroxyl functional group. Finally, several studies have sought to devise methods to differentiate between aliphatic alcohols and the more acidic phenol group. Strategies for both selective arylation27 and acylation have been reported.28 However, exploration of the ability of these methods to target only hydroxyl groups when other nucleophiles are present has not been pursued.
Enzymes have also been explored for their utility as chemoselective catalysts. Lipases, which endogenously acylate hydroxyl groups, have been employed to process both small molecule- and peptide-based substrates. These proteins can be used to achieve both regio- and chemoselective O-acylation on a wide variety of substrates.29,30 Of particular interest is the ability of many lipases to function in organic solvents, which allows the enzymes to retain their specificity while catalyzing reactions that would not be possible in water.31 Selective O-acylation of a model peptide containing both serine and lysine residues has been achieved using a lipase isolated from Pseudomonas sp.32 The acylation of serine was shown to be faster than acylation of lysine, and after 1.5 hours the peptide, mono-acylated at the O-position, was obtained in a 98% yield. Attaining selectivity over thiol-containing substrates has proven to be challenging but has seen some success.33 Investigation of the ability of several lipases to generate only the O-acylated product of 3-mercapto-1-propanol led to the identification of catalysts that produced the desired product in 66–92% yields.
Alcohol oxidation
Oxidation of primary or secondary alcohols to produce the electrophilic aldehyde or ketone, respectively, is an important reaction for both bioconjugation applications and synthesis of biologically active molecules (Figure 1, bottom left). Production of an electrophilic moiety often provides a group with orthogonal reactivity to the remainder of the molecule, facilitating selectivity in subsequent transformations. Unfortunately, development of oxidation conditions that are functional group tolerant is exceptionally difficult given the large number of moieties that are susceptible to oxidation or that inhibit effective conversion (e.g., alkenes, thiols, heterocycles). As a result, strategies to oxidize alcohols without the need for protecting groups are scarce.
Although chemoselective oxidation conditions are lacking, a significant amount of effort has been dedicated to generating methods that selectively oxidize primary alcohols in the presence of secondary alcohols. It has been reported that application of a mixture of trichloroisocyanuric acid and catalytic amounts of TEMPO will convert primary alcohols to their corresponding aldehydes and secondary alcohols to ketones.34 However, these authors noted that they were able to preferentially oxidize the primary hydroxyl because the relative rate of the oxidation of a secondary hydroxyl was much slower. Selective TEMPO-mediated oxidation of primary alcohols has also been reported by Stahl and Hoover who utilized this reagent in conjunction with Cu(I).35 Importantly, this aerobic oxidation method produces aldehydes in the presence of secondary alcohols, heterocycles, alkynes, alkenes, aryl halides and even unprotected anilines.
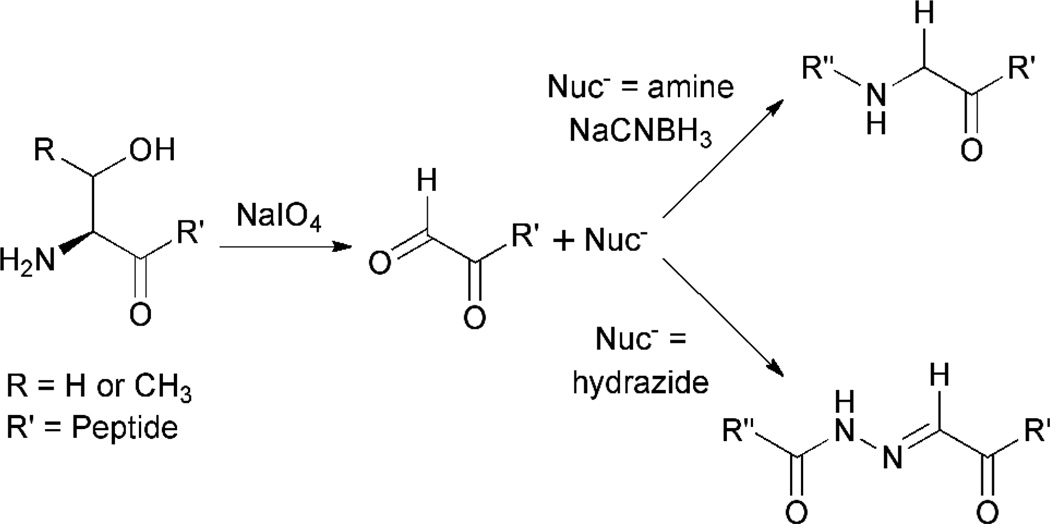
Selective conditions for the oxidation of hydroxyl-containing amino acids have not been reported. However, the most common modification of Ser/Thr residues does involve oxidation. These amino acids are specifically targeted when they reside at the N-terminus of a protein or peptide. In this position, Ser/Thr residues provide a β-amino alcohol motif that can be chemoselectively transformed with sodium periodate to yield a glyoxyloyl moiety (Figure 3).36 This electrophile can then be conjugated to a variety of molecules including primary amines, hydrazides and hydroxylamines and can also be converted into a glycyl group by transamination.4,37,38
Figure 3.
Oxidation of an N-terminal Ser or Thr residue of a protein or peptide. An electrophilic site is specifically generated at the N-terminus of the substrate enabling selective bioconjugation at this position. The requirement for the β-amino alcohol motif precludes the use of this transformation is a wide variety of substrates.
Activation by generation of a leaving group
Conversion of the hydroxyl moiety to a good leaving group is another strategy to attain the chemoselective conversion of a hydroxyl group (Figure 1, upper right). One of the most common ways to achieve this is by replacement with a halogen. Standard conditions for this transformation require the use of harsh reagents, such as phosphorous halides, which are generally not selective and can lead to significant side-reactions when applied to complex molecules. Thus, the development of more gentle and selective reagents is needed. 2,4,6-Trichloro[1,3,5]triazine (TT) has been explored for this purpose and has lead to rapid and quantitative conversion of primary, secondary, tertiary, and benzylic alcohols to the corresponding alkyl chloride.39 This method was also found to be applicable to allyl alcohols and β-amino alcohols, without loss of the N-protecting group as can be seen with conventional methods. Conversion to the bromide was accomplished by addition of NaBr; however, no product was observed with the addition of NaI.
A second method for the direct conversion of a hydroxyl moiety to a halogen uses a visible-light-activated photocatalyst, Ru(bpy)3Cl2, and CBr4/NaBr for bromination or CHI3/NaI for iodination.40 Primary alcohols are transformed into their corresponding bromide or iodide in a 77–98% yield. This strategy shows great tolerance for a wide variety of functional groups including ethers, silyl ethers, alkenes, alkynes, carbamates, esters, and phenols. Secondary alcohols were also converted, but at a slower rate as compared to primary alcohols.
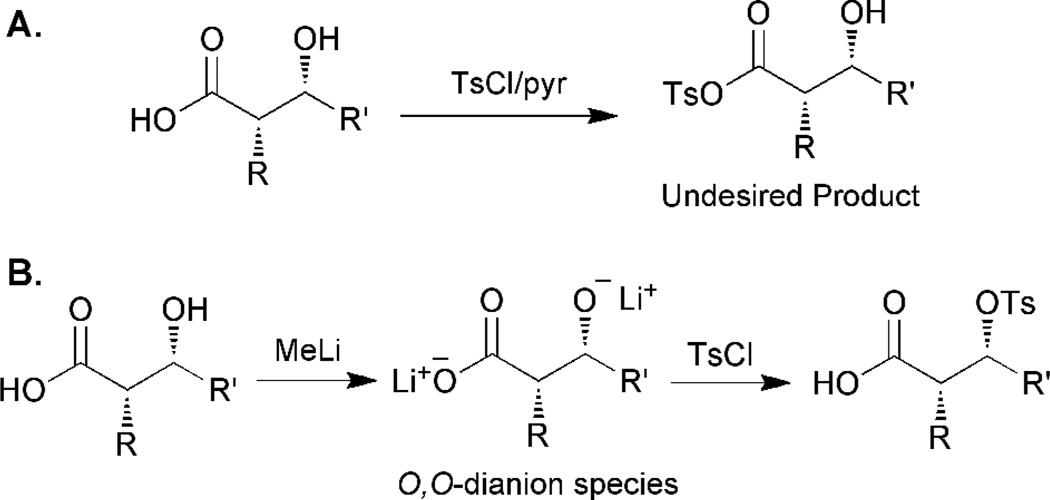
Activation of hydroxyl groups with tosyl chloride is another important goal. Despite the widespread use of this method, it is not chemoselective since molecules that contain a hydroxyl and a carboxylic acid moiety can be tosylated at both positions.41 To prevent carboxylic acid tosylation, a dianion-based strategy has been reported.42 Treatment of a substrate that contains both a carboxylic acid and a hydroxyl functional group with methyllithium yields an O,O-dianion species (Figure 4). Since the carboxylate is now occupied with the strong lithium counter ion, it does not react with the tosyl chloride, while the hydroxyl moiety is capable of undergoing reaction. The carboxylic acid moiety is then regenerated during the work-up, yielding a free carboxylic acid and the corresponding tosylate.
Figure 4.
Tosylation of the hydroxyl moiety in the presence of a carboxylic acid functional group. Application of a strong base deprotonates both groups, but the corresponding ion pair is weaker for the hydroxyl moiety and can be exchanged with a tosyl group.
Transformation to an alternative chemical handle
Hydroxyl groups can also be converted to functionalities that are commonly used in bioconjugation strategies. Here, we will describe methods that facilitate its transformation to either an azide or a thiol group (Figure 1, upper left). Installation of an azide has become commonplace in many chemical biology applications due to the bioorthogonality of this group and its ability to participate in the Huïsgen azide-alkyne 1,3-dipolar cycloaddition, which is commonly referred to as “click chemistry.” This bioorthogonal reaction has been widely used for the modification of natural products, peptides, proteins, carbohydrates, polymers, and other bioconjugation applications.43
Many of the procedures for conversion of a hydroxyl group to the azide are not selective and require harsh conditions and/or the use of explosive reagents. Tosylation of a hydroxyl group followed by displacement with sodium azide is not completely selective.44 Mitsounobu conditions are often utilized for azidination, but also result in modification of thiols. To make a broadly applicable method, reaction conditions that are chemoselective for hydroxyl conversion and that use reagents that are safe and convenient are needed. Nowrouzi and coworkers reported use of a mixture of Ph3P:2,3-dichloro-5,6- dicyanobenzoquinone (DDQ):n-Bu4NN3 for this application.45 They demonstrated that a wide range of alcohols was converted to azides in no more than 15 minutes. A related strategy utilizes TT as the oxidizing agent instead of DDQ.46 TT is commercially available, less expensive, and is reported to promote fewer side reactions. This method generates azides from a wide-range of alcohol substrates, including primary, second, and tertiary alcohols. Importantly, this strategy affords highly selective conversion of alcohols in the presence of thiols.
Conditions to facilitate the conversion of a hydroxyl group to the more nucleophilic thiol have also been reported.47 Davis and coworkers utilized Lawesson’s reagent (LR), an aryl thiophosphine sulfide, for the conversion of allylic alcohols and the C-1 hydroxyl of carbohydrates to the corresponding thiols.47,48 LR has been widely used as a thionation reagent and is known to react with ketones, aldehydes, esters, and various other functional groups. However, under mild conditions, such as moderate temperatures (e.g. 80°C), low equivalents of LR, and short reaction times, chemoselective modification of hydroxyl groups was observed. The authors report conversion of allylic alcohol-functionalized isoprenes into thiols to enable facile conjugation to proteins via disulfide bond formation. This technique facilitates ready access to discreetly modified protein species. LR can also be used to modify reducing sugars. Glycosyl thiols can be produced from unprotected sugars, which can then be directly used to glycosylate proteins all in a one-pot procedure.48
Natural Product Analog for Target Identification
An important application of many chemoselective transformations is to enable the facile synthesis of complex small molecules. The use of chemoselective methods can prevent the need for protecting groups during total synthesis efforts.2 It can also enable modification of complex molecules without the requirement of total chemical synthesis. This is of particular importance for studies that necessitate functionalization of natural products, which often have complex, densely functionalized scaffolds and are available in limited quantities.
The discovery of most natural products is guided by the identification of compounds that possess a bioactivity of interest. Despite this fact, the protein partners that a given molecule interacts with to elicit this biological effect are often unknown due to the technical difficulty of elucidation. A standard way to identify the target(s) of a natural product is to generate an analog, such as a biotinylated variant, that can be used in a pull-down assay.49 In the absence of a strategy to selectively append this tag to a discrete functionality, the natural product analog must be produced by total synthesis. However, use of a chemoselective reaction could enable direct conjugation of the natural product to the tagging reagent without requiring a time-consuming and difficult synthesis.
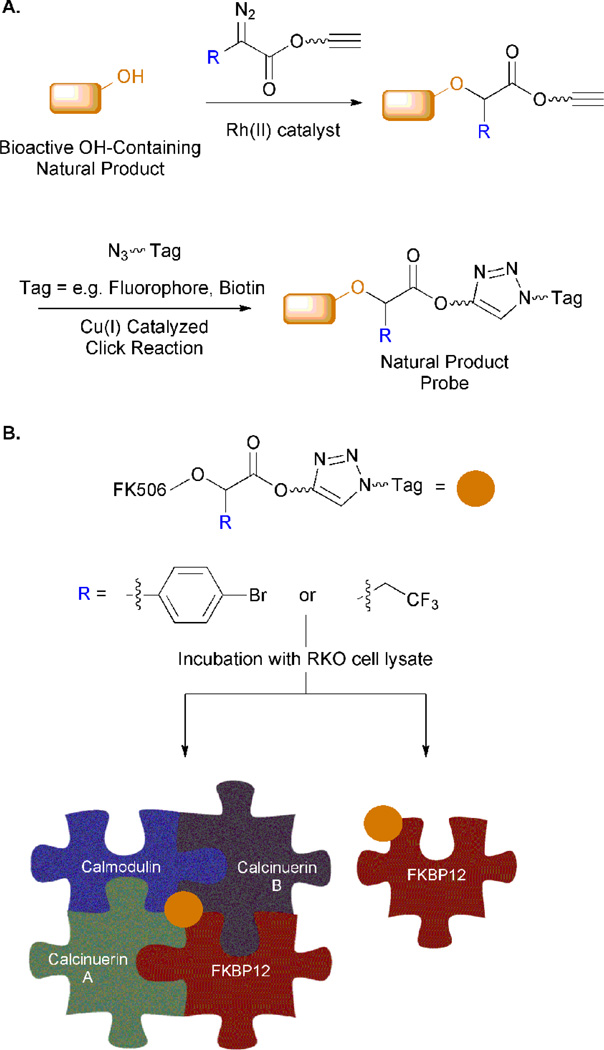
Romo and coworkers have reported a hydroxyl group-selective strategy to facilitate direct conjugation of a tag to natural products containing this functional group. This work utilizes a rhodium catalyst and a diazoester-functionalized coupling partner (Figure 5A).50 A metallocarbene is formed via the metal-catalyzed decomposition of the diazo esters. This carbene readily inserts into the O-H bond. Selective O-H insertion is performed on a variety of natural products to “arm” them with a terminal alkyne, which enables subsequent conjugation to a biotin or a fluorophore. This tag ultimately facilitates detection of the natural product-protein complex.
Figure 5.
Hydroxyl group-targeted method to generate natural product analogs for protein target identification. A. A rhodium catalyzed reaction between an alcohol and a diazoester-functionalized coupling partner results in incorporation of an alkyne moiety. B. FK506 was used as a model natural product to demonstrate the utility of the devised strategy. Following functionalization of FK506 with an alkyne handle, this probe was incubated with RKO cells to facilitate the identification of the binding partner(s) of the natural product probe. Probe design dramatically altered the results of these experiments.
The utility of this method was shown by chemoselectively and regioselectively modifying a hydroxyl moiety on FK506, an immunosuppressive natural product that targets the peptidyl–prolyl cis/trans isomerase, FKBP (FK506 binding protein; Figure 5B). Using the alkyne-modified FK506, the authors performed a pull-down in the lysate of RKO cells, a human colon carcinoma cell line. Interestingly, the exact structure of the diazoester-modifier dramatically affected the results of this experiment. The first generation of this technology utilized a diazoester containing a p-bromophenyl group (Figure 5B, left arrow). This probe was shown to pull-down a ternary complex that was composed of FKBP12 (FK506’s natural substrate), calcinuerin A and B, and calmodulin.50 A second generation probe was devised that displays a smaller substituent on the diazoester, an α-trifluoroethyl moiety (Figure 5B, right arrow).51 Instead of pulling-down the entire ternary complex, this probe variant yielded isolation of only FKBP12. These authors postulate that is due to more specific interactions between the natural product probe and protein target that are made possible by the smaller substituent.
Generation of strategies that hasten our ability to identify the protein targets of complex natural products is an important goal. Chemoselective transformations will be critical in this effort, and particularly those that target functional groups such as the hydroxyl group, which are prevalent in natural products. The aforementioned example has made large strides towards the generation of a selective strategy for functionalization of many alcohol-containing compounds. It was shown to afford selective modification of a variety of molecules with the exception of amine-containing compounds, which were also reactive under the described reaction conditions. Additionally, Romo and coworkers have also devised strategies to selectively target arenes52 and alkenes53 for similar applications.
Functional Group-Mediated Enrichment of Alcohols
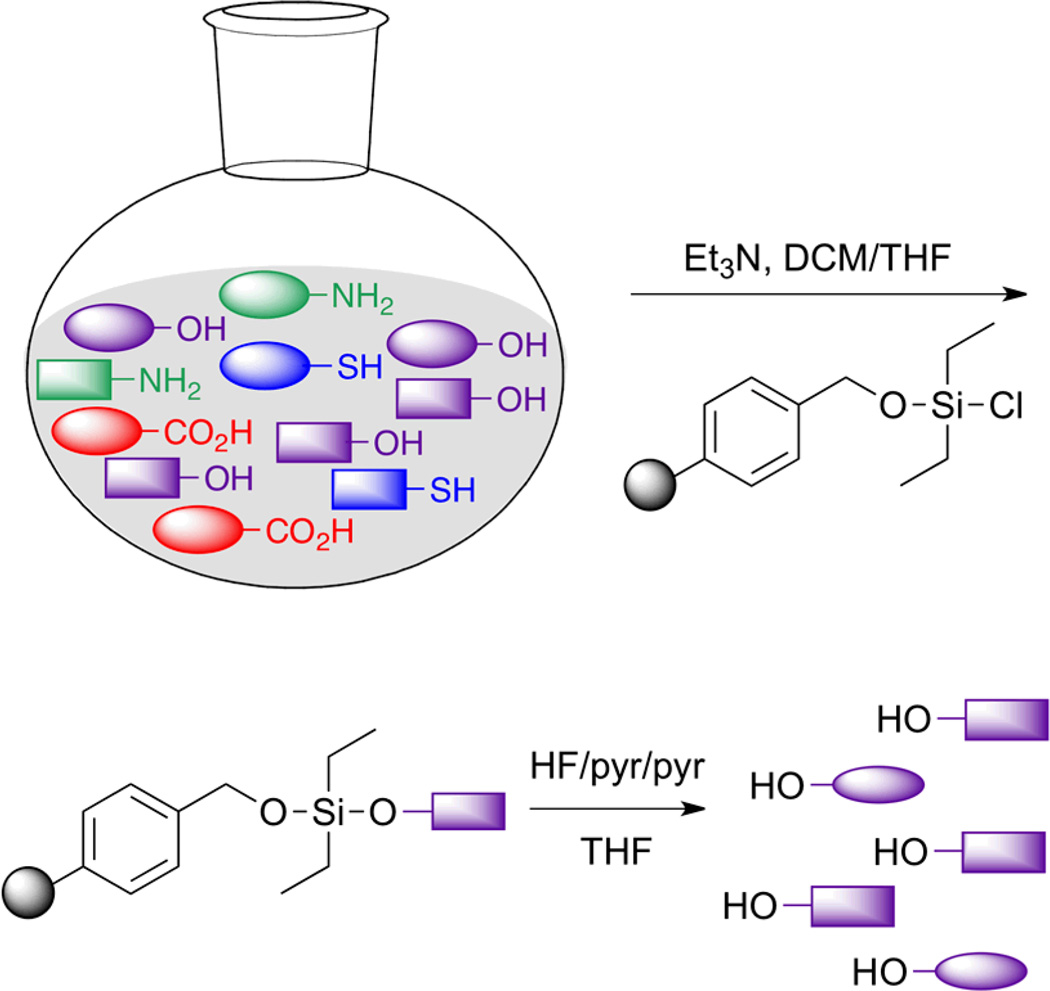
The reaction between a hydroxyl group and an activated trialkyl(aryl)silane has been widely used in total synthesis and solid phase synthesis. Organosilanes are heavily utilized because of the ease with which they can be both added to and removed from a molecule.54 Additionally, silicon’s oxophilic nature yields a stable silyl ether bond almost exclusively in many systems. Taking advantage of this chemistry, we recently developed an application that is dependent upon compound immobilization through silyl ether bond formation, purification of small molecules. In particular, our efforts have focused on the enrichment of hydroxyl group-containing natural products from their source materials.
Natural products are important molecular scaffolds in many areas of medicine. Nearly 50% of the drugs currently on the market are either natural products or natural product-inspired, suggesting that continued efforts towards discovery of new compounds are warranted.55,56 However, the purification of a single molecule from a complex matrix remains a formidable challenge. We sought to develop an isolation strategy that is orthogonal to existing methods as a means to expedite this process. Accordingly, a chemoselective purification method that targets only those molecules in the crude mixture that contain a specific functional group was designed.57 The devised separation technology utilizes polymer supported enrichment tags that are derivatized with a reactive group to capture small molecules (Figure 6). In the case of the hydroxyl group, a solid-supported benzylic chlorodiethylsilane moiety, which selectively forms a silyl ether bond even in the presence of other nucleophilic groups such as amines, thiols and carboxylic acids, was utilized. A traceless cleavage was performed to free the enriched natural products from the solid support after all unreacted compounds had been washed away.
Figure 6.
Chemoselective enrichment of hydroxyl-containing molecules through the formation of a silyl ether bond. Hydroxyl group-containing compounds are then retained on the solid-support resin while molecules deplete of this functional group are rinsed away. Upon traceless cleavage of the enriched molecules they can be immediately subjected to functional and structural characterization.
A chemoselective isolation approach is advantageous because it provides a means for discovery of natural products that are unlikely to be identified by traditional strategies due to low isolation yields, poor compound resolution, and/or the presence of interfering compounds. This strategy was used to isolate a wide variety of model hydroxyl-containing molecules with impressive recovery yields and complete chemoselectivity. More importantly, when applied to the enrichment of an endogenously produced natural product, anisomycin, from the broth of Streptomyces griselous, we obtained equivalent recovery yields and saw no capture of undesired functional groups. We also noted that utilization of a chemoselective isolation technique led to generation of a less complicated mixture (i.e. compound enrichment), which we expect will increase the rate of the discovery of low abundance molecules that can get lost when using traditional purification methods. Work is underway to apply this strategy, and our recently published method for carboxylic acid enrichment,58 to natural product discovery.
Chemoselective Protein Bioconjugation
Efficient and selective means to perform bioconjugation reactions are essential. Often, the most nucleophilic groups in a protein are targeted, the amine and thiol. Scores of methods for reaction with lysine residues have been reported and are heavily utilized.4 To achieve site selectivity, the dramatically less abundant amino acid cysteine is the target of choice. Chemo- and site-selective protein modifications have also been facilitated by installation of a bioorthogonal group such as an azide, alkyne or aldehyde.59 Still, there is need to devise strategies to address other natural amino acids including those that contain hydroxyl groups. Several enzyme-mediated strategies have been reported to target proteins at their hydroxyl moieties.60–63 We will focus our discussion on chemical transformations.
Chemoselective targeting of alcohols is particularly difficult in the context of bioconjugate chemistry. For proteins, N-terminal Ser/Thr oxidation has been successfully employed as shown in Figure 3. However, this strategy is only applicable to β-amino alcohols making other methods necessary. Two reagents have been used routinely to activate the hydroxyl moiety, 1,1'-carbonyldiimidazole (CDI) and N,N'-disuccinimidyl carbonate (DSC),4 but neither of these reagents is selective for the hydroxyl group, preferentially reacting with amines or carboxylates. Additionally, anhydrous conditions are preferred with these reagents making them poorly applicable to many bioconjugation applications. This example highlights the two major difficulties in targeting of alcohols in a protein, the modest reactivity of these groups and a potentially even more problematic issue, the requirement that the devised reactions are water compatible. Reasonably speaking, these challenges will make development of strategies to selectively address serine and threonine residues exceptionally difficult. However, a number of methods have been devised to address the phenolic amino acid, tyrosine.
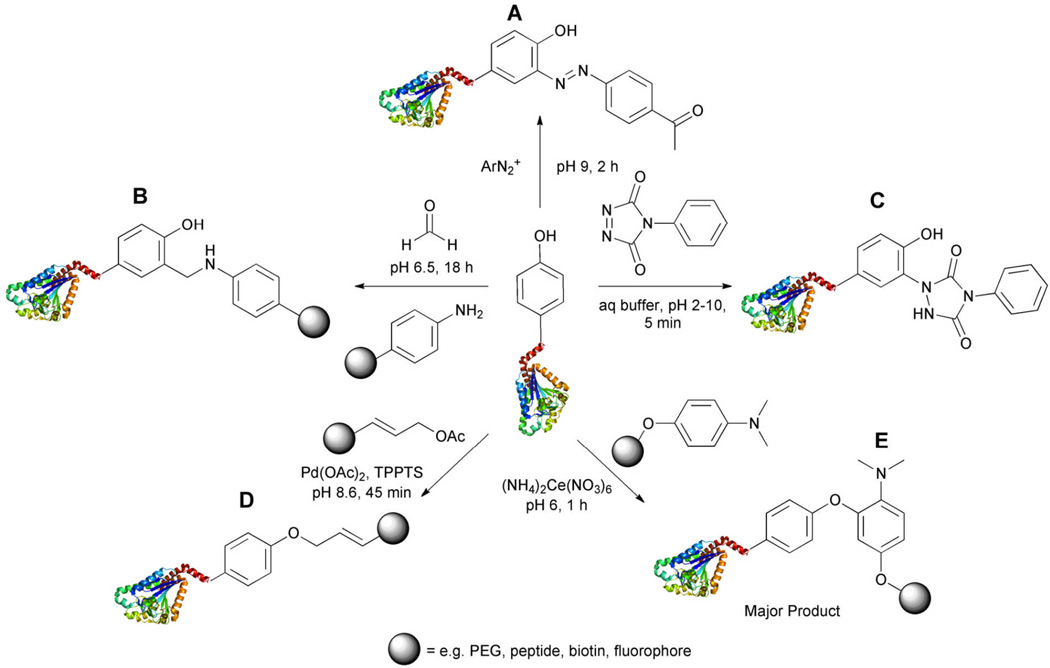
Tyrosine represents an attractive target for bioconjugation as it is only modestly prevalent and is often buried within the protein structure, which may enable selective labeling of the limited number of solvent exposed residues. The first report of a chemoselective strategy for tyrosine targeting was presented by the Francis research group in 2004.64 These and subsequent studies established that electron deficient diazonium salts could be added to tyrosine residues by electrophilic aromatic substitution (Figure 7A).65 The appended moiety could, for example, contain a ketone that can be reacted in a second step resulting in conjugation to a larger component through oxime formation.
Figure 7.
Tyrosine residues have been targeted utilizing several different strategies, reaction with the electron-rich aromatic ring at the position ortho to the hydroxyl or direct targeting of the phenol hydroxyl group. A. Coupling to an electron deficient diazonium salt. B. A three component Mannich-type reaction yields an o-substituted tyrosine moiety. C. An ene-type reaction is performed between tyrosine and a cyclic diazocarboxamide yielding a highly stable product. D. Metal-mediated coupling facilitates reaction at the phenolic site. Palladium promotes addition of allylic substrates. E. Cerium promotes a one-electron oxidative coupling to tyrosine.
A three-component Mannich-type coupling reaction has also been utilized for tyrosine targeting (Figure 7B).66 An imine is formed in situ from reaction of an aldehyde and an electron-rich aniline. The hydroxyl group of tyrosine is then thought to direct formation of a cyclic transition state between the amino acid and the imine, ultimately yielding an o-substituted residue. Importantly, this reaction can be performed at lower pH values (pH=6.5) than the diazonium-based strategy (pH=9). Chymotrypsinogen A, lysozome, and RNase A were selectively modified with this strategy. This method has also been exploited to append synthetic peptides to proteins with accessible tyrosine residues, a strategy that will find utility for the study of cellular uptake mechanisms and trafficking, facilitation of protein purification, and incorporation of unnatural amino acids.67 A two-component variant of this reaction was published by the Tanaka group in 2008.68 In this example, a cyclic imine coupling partner can be used over a wide pH range.
Barbas and coworkers have reported the development of an ene-type reaction as a selective strategy for tyrosine modification (Figure 7C).69 A cyclic diazodicarboxamide reacts chemoselectively with the phenol yielding a 1,2,4-triazoldine-3,5-dione, which was shown to be more hydrolytically and thermally stable than those products formed utilizing the Mannich-type reactions. This transformation was applied to the conjugation of an integrin binding cyclic RGD peptide to the therapeutic antibody herceptin. Their studies demonstrated that modification of herceptin through tyrosine did not decrease its ability to recognize its natural substrate. In addition, the generated antibody conjugate also bound the RGD partner, integrin αvβ3, suggesting that this selective transformation could be a general route to synthesis of antibodies with multiple specificities.
Two transition metal catalyzed methods for tyrosine modification have also been reported.70,71 Based upon the precedent that allylation of phenol groups could be performed using electrophilic π-allyl complexes, Francis and coworkers generated a water compatible strategy for tyrosine conjugation using π-allylpalladium (Figure 7D).70 When a model protein, chymotrypsinogen A, was exposed to a rhodamine-labeled allylic acetate, Pd(OAc)2, and triphenylphosphine tris(sulfonate) (TPPTS), the protein was 50–65% labeled. Further analysis showed that only one of chymotrypsinogen A’s three tyrosines was modified demonstrating that this method selectively labels only solvent accessible tyrosine residues. Using this strategy, hydrophobic groups were appended to a protein leading to “solubility switching.” A previously hydrophilic protein was made hydrophobic, which facilitated its incorporation into lipid membranes.
Recently, a cerium-based strategy was also reported by the Francis group (Figure 7E).71 This tactic takes advantage of the susceptibility of electron rich aromatic rings, such as the tyrosine, to oxidative coupling. The authors report use of cerium (IV) ammonium nitrate as a one-electron oxidant to facilitate coupling with electron rich anilines. In comparison to previously disclosed methods, this strategy is performed near neutral pH, at relatively low substrate concentrations and proceeds with faster conversion. Importantly, this coupling reaction was combined with cysteine modification to yield doubly modified viral capsid proteins. Thiol conversion was performed with Oregon green maleimide to functionalize the protein with an imaging tag. Next, the protein was modified at a tyrosine residue with the appropriately derivatized cyclic RGD peptide to facilitate cell targeting through binding of integrin αvβ3. The bifunctionalized protein was successfully generated indicating the orthogonality of these two bioconjugation reactions.
Hydroxyl Groups in Enzyme Active Sites
Although design of chemical strategies to affect transformation of Ser/Thr residues is likely to be challenging, aliphatic alcohol-containing amino acids can be targeted when they are present in the active site of an enzyme, often due to both perturbation of their pKa value and proximity effects. This phenomenon is well illustrated by the number of drugs that evoke their biological response by specifically and covalently interacting with hydroxyl group-containing amino acids. A widely recognized class of inhibitors that function through this type of mechanism is the β-lactams, which acylate an active site serine in their targets, the penicillin binding proteins (PBPs).72 Synthesis of the bacterial cell wall structure is largely mediated by PBPs making these enzymes critical for cell survival. Aspirin or acetylsalicylic acid also modifies its protein targets in a similar, irreversible fashion.73 Cyclooxygenase 1 and 2 (COX-1 and COX-2) are both inhibited by aspirin-mediated acetylation of an active site serine residue, Ser530 and Ser516, respectively.74 COX-1 and COX-2 are essential enzymes in the synthesis of prostanoids and their inhibition can lead to a reduction of inflammation and pain.
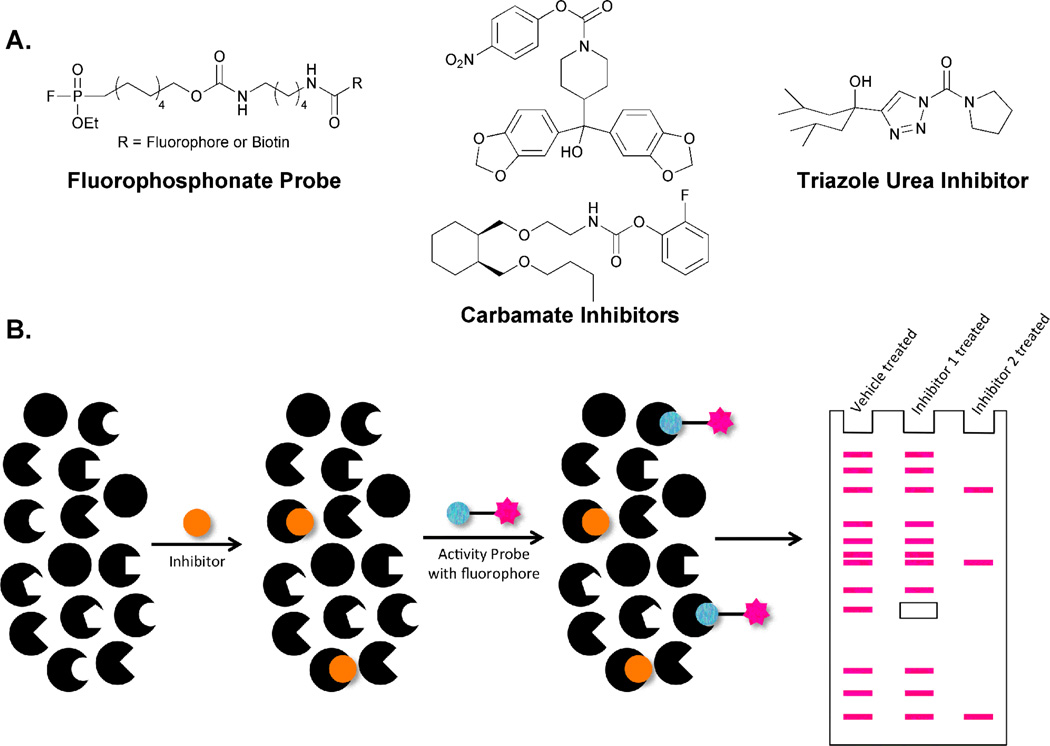
Use of active site-promoted hydroxyl labeling has also been applied to the generation of activity-based protein profiling (ABPP), a method specifically tailored to indicate the catalytic state of a given protein class. ABPP utilizes chemoselective probes, which target one or only a small number of protein classes through covalent modification of an active site residue.75 Cravatt and coworkers first reported a probe to target an enzyme class dependent upon an active site hydroxyl group, the serine hydrolases, in the late 1990s.76,77 This family of enzymes is one of the largest and most diverse in both the eukaryotic and prokaryotic proteomes.78 As their name suggests, all members of this class contain a nucleophilic serine residue that is required for catalytic activity. Fluorophosphonate (FP)-containing probes have been shown to selectively react with this active serine residue, enabling profiling of tens or even hundreds of serine hydrolases simultaneously (Figure 8A). In fact, the high reactivity of the FP group leads to labeling of nearly all mammalian serine hydrolases.79 While this reactivity is beneficial in an ABPP probe, it limits the use of this electrophilic functionality in compounds intended for the analysis of specific serine hydrolases. In the last decade, studies have been ongoing to search for specific inhibitors of several members of the serine hydrolase family.80,81 Carbamates, which still covalently modify the active site serine, have emerged as important scaffolds in these efforts and several compounds have been discovered that target a single protein facilitating detailed study of these family members (Figure 8A).80–82 The attenuated reactivity of the carbamate is likely responsible for the ability of these compounds, but not FPs, to achieve selectivity. Still, potent and selective inhibitors for many serine hydrolases are lacking and additional classes of serine-targeted probes are needed.
Figure 8.
Activity-based protein profiling can be used to target enzymes that utilize hydroxyl group-containing active site nucleophiles. A. Serine hydrolases can be targeted by several compound classes. Fluorophosphonate-based probes are promiscuous within the protein family. Compounds with attenuated reactivity such as carbamates and ureas enable selective examination of one or a small number of family members. B. The global nature of FP labeling can be utilized in the development of an assay to identify highly specific serine hydrolase inhibitors. Vehicle treated samples will display labeling of all FP-susceptible proteins. Addition of potential inhibitors will prevent susceptible enzymes from reacting with the FP probe, leading to a loss of a band or bands during gel-based analysis. Selective inhibitors will cause the loss of only one protein band (i.e., inhibitor 1) while promiscuous inhibitors will promote the loss of many (i.e., inhibitor 2).
To address this challenge, Cravatt and coworkers utilized a strategy called competitive ABPP (Figure 8B). This screening method takes advantage of the global nature of FP labeling by using this molecule as a readout of whether or not the active site of each serine hydrolase has been blocked by an inhibitor. Proteome samples are first treated with a potential inhibitory agent and then labeled with FP. Vehicle treated samples will display labeling of all FP-susceptible proteins. Inhibitor-bound proteins will no longer be able to react with the FP probe, leading to a loss of a band or bands during gel-based analysis. Selective inhibitors will cause the loss of only one protein band (i.e., Figure 8B, inhibitor 1) while promiscuous inhibitors will promote the loss of many (i.e., Figure 8B, inhibitor 2). Using this strategy, compounds containing a 1,2,3-triazole urea were identified as selective inhibitors of several diverse serine hydrolase family members in mouse brain proteome (Figure 8A).83 Again, these compounds function through covalent modification of the active site serine but display altered reactivity profiles when compared to carbamates or FPs. One of the targeted enzymes was an acyl-peptide hydrolase (APEH). In vivo evaluation of the identified triazole-containing inhibitor in mice showed that APEH inhibition promotes T cell proliferation and accumulation of N-acetylated proteins validating this screening strategy for the identification of compounds with potent activity. This report also solidifies the promise of 1,2,3-triazole ureas as a privileged scaffold for future development of additional serine hydrolase inhibitors and highlights the influence that subtle alterations in the reactivity of catalytic components, such as a serine, can have on probe development.
Conclusions and Future Perspectives
The toolkit for chemoselective targeting of many functional groups has grown substantially in the last several decades. Unfortunately, progress in the development of hydroxyl group-selective methods has been comparatively slow. The attenuated reactivity of this functional group is largely to blame for this lag in progress. Despite this challenge, several inventive strategies have been devised as discussed here. Although chemoselective targeting of the hydroxyl group may never be as routine as many other functional groups, continued efforts to generate novel selective transformations are sure to be important in many future applications. Selective methods will enable synthesis of compounds with better efficiency, targeting of precise sites on a biomolecule and generation of new chemical probes. Success in this area will likely come from a combination of creative application of existing synthetic strategies, development of novel selective reactions and additional methods that take advantage of hydroxyl groups with atypical properties, like those found in enzyme active sites.
Acknowledgements
Thanks to L. Brown, K. Brown and K. Garber for critical reading of the manuscript. This work was supported by NIH R00GM82983, NIH DP2OD008592, a Pew Biomedical Scholar Award (E.E.C.) and an IU Carmack Fellowship (D.J.T.).
Biographies

Darci J. Trader graduated in 2007 from Southern Illinois University-Edwardsville with her B.S. in chemistry and then in 2008 with a master’s degree in organic chemistry under the direction of Prof. James McClure. She started her doctoral work at Indiana University-Bloomington in the fall of 2008 with Prof. Erin Carlson in the area of bioorganic chemistry. Darci’s thesis research involves the design, synthesis and utilization of solid-phase chemoselective enrichment tags that can be employed to isolate natural products based upon their functional group composition.

Erin E. Carlson received her B.A. at St. Olaf College in 2000. She went on to the University of Wisconsin - Madison and earned a Ph.D. in organic chemistry in 2005 under the direction of Professor Laura L. Kiessling. Subsequently, Dr. Carlson pursued postdoctoral studies at The Scripps Research Institute with Professor Benjamin F. Cravatt. In 2008, Dr. Carlson joined the chemistry faculty at Indiana University. Her research group works at the interface of chemistry and biology, focusing on the development and application of new technologies to explore the mechanisms of bacterial pathogenesis and identify potential therapeutic agents.
Footnotes
Published as part of a themed issue dedicated to Emerging Investigators.
Literature Cited
- 1.McGrath NA, Raines RT. Acc. Chem. Res. 2011;44:752. doi: 10.1021/ar200081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenvi RA, O'Malley DP, Baran PS. Acc. Chem. Res. 2009;42:530. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiefenbrunn TK, Dawson PE. Biopolymers (Pept Sci) 2010;94:95. doi: 10.1002/bip.21337. [DOI] [PubMed] [Google Scholar]
- 4.Hermanson GT. Bioconjugate Techniques. 2nd ed. Academic Press; 2008. [Google Scholar]
- 5.Young IA, Baran PS. Nature Chem. 2009;1:193. doi: 10.1038/nchem.216. [DOI] [PubMed] [Google Scholar]
- 6.Trost BM. Science. 1983;219:245. doi: 10.1126/science.219.4582.245. [DOI] [PubMed] [Google Scholar]
- 7.Sletten EM, Bertozzi CR. Angew. Chem. Int. Ed. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackenberger CPR, Schwarzer D. Angew. Chem. Int. Ed. 2008;47:10030. doi: 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- 9.Henkel T, Brunne RM, Muller H, Reichel F. Angew. Chem. Int. Ed. 1999;38:643. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Doolittle RF. In: Prediction of protein structure and the principles of protein conformation. Fasman GD, editor. New York: Plenum Press; 1989. p. 599. [DOI] [PubMed] [Google Scholar]
- 11.Bobbitt JM. Adv. Carbohydr. Chem. 1956;48:1. doi: 10.1016/s0096-5332(08)60115-0. [DOI] [PubMed] [Google Scholar]
- 12.Weith HL, Wiebers JL, Gilham PT. Biochemistry. 1970;9:4396. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]
- 13.Qian R, Ding L, Bao L, He S, Ju H. Chem. Commun. 2012;48:3848. doi: 10.1039/c2cc18167c. [DOI] [PubMed] [Google Scholar]
- 14.Afagh NA, Yudin AK. Angew. Chem. Int. Ed. 2010;49:262. doi: 10.1002/anie.200901317. [DOI] [PubMed] [Google Scholar]
- 15.Raida M. Curr. Opin. Chem. Biol. 2011;15:570. doi: 10.1016/j.cbpa.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Nahmany M, Melman A. Org. Biomol. Chem. 2004;2:1563. doi: 10.1039/b403161j. [DOI] [PubMed] [Google Scholar]
- 17.Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2009;48:6954. doi: 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]
- 18.Job GE, Buchwald SL. Org. Lett. 2002;4:3703. doi: 10.1021/ol026655h. [DOI] [PubMed] [Google Scholar]
- 19.Shafir A, Lichtor PA, Buchwald SL. J. Am. Chem. Soc. 2007;129:3490. doi: 10.1021/ja068926f. [DOI] [PubMed] [Google Scholar]
- 20.Maiti D, Buchwald SL. J. Am. Chem. Soc. 2009;131:17423. doi: 10.1021/ja9081815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohshima T, Iwasaki T, Maegawa Y, Yoshiyama A, Mashima K. J. Am. Chem. Soc. 2008;130:2944. doi: 10.1021/ja711349r. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki T, Maegawa Y, Hayashi Y, Ohshima T, Mashima K. Synlett. 2009;10:1659. [Google Scholar]
- 23.Paik I, Tapriyal D, Enick RM, Hamilton AD. Angew. Chem. Int. Ed. 2007;46:3284. doi: 10.1002/anie.200604844. [DOI] [PubMed] [Google Scholar]
- 24.Kurahashi T, Mizutani T, Yoshida J. Tetrahedron. 2002;58:8669. [Google Scholar]
- 25.Kattnig E, Albert M. Org. Lett. 2004;6:945. doi: 10.1021/ol0364935. [DOI] [PubMed] [Google Scholar]
- 26.Griswold KS, Miller SJ. Tetrahedron. 2003;59:8869. [Google Scholar]
- 27.Maiti D. Chem. Commun. 2011;47:8340. doi: 10.1039/c1cc12694f. [DOI] [PubMed] [Google Scholar]
- 28.Sabitha G, Reddy BS, Reddy GK, Yadav JS. New J. Chem. 2000;24:63. [Google Scholar]
- 29.Bjorkling F, Godtfredsen SE, Kirk O. J. Chem. Soc. Chem. Commun. 1989:934. [Google Scholar]
- 30.Rodriguez-Perez T, Fernandez S, Sanghvi YS, Detorio M, Schinazi RF, Gotor V, Ferrero M. Bioconjugate Chem. 2010;21:2239. doi: 10.1021/bc1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therisod M, Kilibanov AM. J. Am. Chem. Soc. 1987;109:3977. [Google Scholar]
- 32.Gardossi L, Bianchi D, Kilbanov AM. J. Am. Chem. Soc. 1991;113:6329. [Google Scholar]
- 33.Iglesias LE, Baldessari A, Gros EG. Bioorg. Med. Chem. Lett. 1996;6:853. [Google Scholar]
- 34.De Luca L, Giacomelli G, Porcheddu A. Org. Lett. 2001;3:3041. doi: 10.1021/ol016501m. [DOI] [PubMed] [Google Scholar]
- 35.Hoover JM, Stahl SS. J. Am. Chem. Soc. 2011 doi: 10.1021/ja206230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon HBF. J. Protein Chem. 1984;3:99. [Google Scholar]
- 37.Lempens EHM, Helms BA, Merkx M, Meijer EW. Chembiochem. 2009;10:658. doi: 10.1002/cbic.200900028. [DOI] [PubMed] [Google Scholar]
- 38.Chelius D, Shaler TA. Bioconjugate Chem. 2003;14:205. doi: 10.1021/bc025605u. [DOI] [PubMed] [Google Scholar]
- 39.De Luca L, Giacomelli G, Porcheddu A. Org. Lett. 2002;4:553. doi: 10.1021/ol017168p. [DOI] [PubMed] [Google Scholar]
- 40.Dai C, Narayanam JMR, Stephenson CJR. Nature Chem. 2011;3:140. doi: 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]
- 41.Mulzer J, Bruntrup G, Chucholowski A. Angew. Chem. Int. Ed. 1979;18:622. [Google Scholar]
- 42.Wu Y, Sun YP. Chem. Commun. 2005:1906. doi: 10.1039/b416383d. [DOI] [PubMed] [Google Scholar]
- 43.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Kumar ST, Madsen AS, Wengel J, Hrdlicka PJ. J. Org. Chem. 2006;71:4188. doi: 10.1021/jo060331f. [DOI] [PubMed] [Google Scholar]
- 45.Iranpoor N, Firouzabadi H, Akhlaghinia B, Nowrouzi N. Tetrahedron Lett. 2004;45:3291. doi: 10.1021/jo035238v. [DOI] [PubMed] [Google Scholar]
- 46.Akhlaghinia B, Samiei S. J. Braz. Chem. Soc. 2007;18:1311. [Google Scholar]
- 47.Gamblin DP, Kasteren S, Bernardes GJ, Chalker JM, Oldham NJ, Fairbanks AJ, Davis BG. Mol. Biosyst. 2008;4:558. doi: 10.1039/b802199f. [DOI] [PubMed] [Google Scholar]
- 48.Bernardes GL, Gamblin DP, Davis BG. Angew. Chem. Int. Ed. 2006;45:4007. doi: 10.1002/anie.200600685. [DOI] [PubMed] [Google Scholar]
- 49.Burdine L, Kodadek T. Chem. Biol. 2004;11:593. doi: 10.1016/j.chembiol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Peddibhotla S, Dang Y, Liu JO, Romo D. J. Am. Chem. Soc. 2007;129:12222. doi: 10.1021/ja0733686. [DOI] [PubMed] [Google Scholar]
- 51.Chamni S, He QL, Dang Y, Bhat S, Liu JO, Romo D. ACS Chem. Biol. 2011;6:1175. doi: 10.1021/cb2002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou CY, Li J, Peddibhotla S, Romo D. Org. Lett. 2010;12:2104. doi: 10.1021/ol100587j. [DOI] [PubMed] [Google Scholar]
- 53.Robles O, Serna-Saldívar SO, Gutiérrez-Uribe JA, Romo D. Org. Lett. 2012 doi: 10.1021/ol300105q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crouch RD. Tetrahedron. 2004;60:5833. [Google Scholar]
- 55.Clardy J, Walsh C. Nature. 2004;432:829. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 56.Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odendaal AY, Trader DJ, Carlson EE. Chem. Sci. 2011;2:760. doi: 10.1039/C0SC00620C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trader DJ, Carlson EE. Org. Lett. 2011;13:5652. doi: 10.1021/ol202376m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carrico IS. Chem. Soc. Rev. 2008;37:1423. doi: 10.1039/b703364h. [DOI] [PubMed] [Google Scholar]
- 60.Bellucci G, Chiappe C, Pucci L, Gervasi PG. Chem. Res. Toxicol. 1996;9:871. doi: 10.1021/tx9600053. [DOI] [PubMed] [Google Scholar]
- 61.Minamihata K, Goto M, Kamiya N. Bioconjugate Chem. 2011 doi: 10.1021/bc1003982. [DOI] [PubMed] [Google Scholar]
- 62.Ramakrishnan B, Boeggeman E, Qasba PK. Bioconjugate Chem. 2007;18:1912. doi: 10.1021/bc7002346. [DOI] [PubMed] [Google Scholar]
- 63.Sunbul M, Yin J. Org. Biomol. Chem. 2009;7:3361. doi: 10.1039/b908687k. [DOI] [PubMed] [Google Scholar]
- 64.Hooker JM, Kovacs EW, Francis MB. J. Am. Chem. Soc. 2004;126:3718. doi: 10.1021/ja031790q. [DOI] [PubMed] [Google Scholar]
- 65.Schlick TL, Ding Z, Kovacs EW, Francis MB. J. Am. Chem. Soc. 2005;127:3817. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- 66.Joshi NS, Whitaker LR, Francis MB. J. Am. Chem. Soc. 2004;126:15942. doi: 10.1021/ja0439017. [DOI] [PubMed] [Google Scholar]
- 67.Romannini DW, Francis MB. Bioconjugate Chem. 2008;19:153. doi: 10.1021/bc700231v. [DOI] [PubMed] [Google Scholar]
- 68.Minakawa M, Guo HM, Tanaka F. J. Org. Chem. 2008;73:8669. doi: 10.1021/jo8017389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ban H, Gavrilyuk J, Barbas CF. J. Am. Chem. Soc. 2010;132:1523. doi: 10.1021/ja909062q. [DOI] [PubMed] [Google Scholar]
- 70.Tilley SD, Francis MB. J. Am. Chem. Soc. 2006;128:1080. doi: 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]
- 71.Seim KL, Obermeyer AC, Francis MB. J. Am. Chem. Soc. 2011;133:16970. doi: 10.1021/ja206324q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konaklieva MI. Curr. Med. Chem. - Anti-Infective Agents. 2002;1:215. [Google Scholar]
- 73.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegman RA, Pak JY, Gildehaus D, Iyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Nature. 1996;284:644. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 74.Kalgutkar AS, Kozak KR, Crews BC, Hochgesang P, Marnett LJ. J. Med. Chem. 1998;41:4800. doi: 10.1021/jm980303s. [DOI] [PubMed] [Google Scholar]
- 75.Cravatt BF, Wright AT, Kozarich JW. Annu. Rev. Biochem. 2008;77:383. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Patricelli MP, Cravatt BF. Proc. Natl. Acad. Sci. 1999;96:14694. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simon GM, Cravatt BF. J. Biol. Chem. 2010;285:11051. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rawlings ND, Barrett AJ, Bateman A. Nucleic Acids Res. 2010;38:D227. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bachovchin DA, Ji T, Li W, Simon GM, Blankman JL, Adibekian A, Hoover H, Niessen S, Cravatt BF. Proc. Natl. Acad. Sci. 2012;107:20941. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Science. 2002;298:1793. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- 81.Kienesberger PC, Oberer M, Lass A, Zechner R. J. Lipid Res. 2009;50:S63. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guengerich FP, Wu ZL, Bartleson CJ. Biochem. Biophys. Res. Commun. 2005;338:465. doi: 10.1016/j.bbrc.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 83.Adibekian A, Martin BR, Wang C, Hsu KL, Bachovchin DA, Niessen S, Hoover H, Cravatt BF. Nat. Chem. Biol. 2011;7:469. doi: 10.1038/nchembio.579. [DOI] [PMC free article] [PubMed] [Google Scholar]