Abstract
We review recent evidence that acetylation and deacetylation of cellular proteins, including transcription factors and nuclear cofactors, may be involved in the regulation of muscle mass. The level of protein acetylation is balanced by histone acetyltransferases (HATs) and histone deacetylases (HDACs) and studies suggest that this balance is perturbed in muscle wasting. Hyperacetylation of transcription factors and nuclear cofactors regulating gene transcription in muscle wasting may influence muscle mass. In addition, hyperacetylation may render proteins susceptible to degradation by different mechanisms, including intrinsic ubiquitin ligase activity exerted by HATs and by dissociation of proteins from cellular chaperones. In recent studies, inhibition of p300/HAT expression and activity and stimulation of SIRT1-dependent HDAC activity reduced glucocorticoid-induced catabolic response in skeletal muscle, providing further evidence that hyperacetylation plays a role in muscle wasting. It should be noted, however, that although several studies advocate a role of hyperacetylation in muscle wasting, apparently contradictory results have also been reported. For example, muscle atrophy caused by denervation or immobilization may be associated with reduced, rather than increased, protein acetylation. In addition, whereas hyperacetylation results in increased degradation of certain proteins, other proteins may be stabilized by increased acetylation. Thus, the role of acetylation and deacetylation in the regulation of muscle mass may be both condition- and protein-specific. The influence of HATs and HDACs on the regulation of muscle mass as well as methods to modulate protein acetylation are important areas for continued research aimed at preventing and treating muscle wasting.
Keywords: Histone acetyl transferases (HATs), histone deacetylases (HDACs), muscle atrophy, p300, SIRT1, transcription factors, nuclear cofactors
1. Introduction
Under normal conditions, protein homeostasis in skeletal muscle is maintained by equal rates of protein synthesis and degradation. When this balance is perturbed with protein degradation rates exceeding those of protein synthesis, loss of muscle mass occurs. Loss of muscle mass, in part reflecting stimulated ubiquitin-proteasome-dependent and autophagy-lysosomal protein breakdown, is commonly seen in patients with cancer, sepsis, and severe injury [1–5]. Patients with diabetes and renal failure may also suffer from muscle wasting [6,7]. Critical illness requiring care in the intensive care unit (ICU) and prolonged mechanical ventilatory support is associated with muscle wasting. In these patients, muscle mass is lost as a combined result of the underlying condition, bed rest, muscle inactivity, and mechanical ventilation [8,9]. Patients in intensive care can lose about 2% of their muscle mass per day and some patients lose up to 50% of the muscle mass during their stay in the ICU. Muscle atrophy in elderly patients (sarcopenia) affects the quality of life in an increasing number of individuals as the elderly population keeps growing [10]. In addition, muscle strength is commonly lost as a result of the metabolic syndrome in older people [11].
Muscle wasting is associated with multiple severe clinical consequences, most of which are related to loss of muscle function and strength caused by the breakdown of myofibrillar contractile proteins. Muscle weakness in critically ill patients makes ambulation difficult, prolonging bed rest and increasing the risk for thromboembolic and pulmonary complications. Involvement of respiratory muscles in the catabolic response further increases the risk for pulmonary complications, including atelectases and pneumonia, and also increases the need for ventilatory support [12]. Mechanical ventilation in itself results in a rapid onset of atrophy and weakness of respiratory muscles [13] thus creating a vicious circle. Weakness of respiratory muscles and respiratory failure are common in patients with advanced cancer and muscle wasting has been reported to contribute to the death in up to 50% of patients with cancer [4].
The consequences of muscle wasting are long-lasting. Studies suggest that objective evidence of muscle wasting and weakness persists for several years after discharge from care in the ICU [8,9], including reduced walking speed and endurance and increased need for support in daily social activities.
Because of the significant consequences of muscle wasting, methods to prevent and treat this debilitating condition will have important clinical implications. Despite intensive research efforts in the field of muscle wasting during the last couple of decades, no effective treatment of muscle wasting exists. Continued efforts to find remedies for the loss of muscle mass in catabolic patients will to a great extent rely on increased knowledge of molecular mechanisms regulating muscle mass. In the present review, we discuss recent evidence suggesting that hyperacetylation of cellular proteins, including regulatory proteins such as transcription factors and nuclear cofactors, is an important mechanism of protein breakdown and muscle wasting. In addition, we review new methods to prevent hyperacetylation that may become an essential component of the prevention and treatment of muscle wasting.
2. Protein acetylation is regulated by a balance between histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities
Most cellular proteins are subjected to posttranslational modifications that may influence both their function and turnover. Although phosphorylation and ubiquitination are the most extensively studied posttranslational modifications with regard to muscle wasting, other modifications are probably also involved [14,15]. Recent studies suggest that protein acetylation may play a role in the regulation of muscle mass [16–19].
The reversible acetylation of cellular proteins may be as important as phosphorylation in cellular regulation [20]. Whereas different amino acids (e.g., threonine, serine, and tyrosine) can be phosphorylated, acetylation occurs on lysine residues. Interestingly, lysine is the target not only for acetylation, but for ubiquitination and sumoylation as well, setting the stage for a potential “traffic jam” at lysine residues for posttranslational modifications in muscle wasting [21]. The level of protein acetylation is regulated by enzymes that increase (HATs) or decrease (HDACs) acetylation. When the balance between these mechanisms is altered, the net result can be protein hyperacetylation or deacetylation (Fig 1).
Fig 1.
Protein acetylation is an important posttranslational modification regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities.
Among different HATs, the nuclear cofactor p300 has attracted great interest because of its interaction with a large number of transcription factors and other regulatory proteins [22,23]. p300 is a large protein with a molecular weight of approximately 300 kDa. It is homologous with another enzyme having HAT activity, CREB-binding protein (CBP), and the two molecules are commonly referred to as p300/CBP although they also have certain distinct biological activities [22]. Other enzymes with HAT activity include members of the Gcn5-related N-acetyltransferase (GNAT) family (Gcn5, PCAF) and the MYST group (MOZ, YBF2/SAS3, and TIP 60).
It was initially believed that the only (or at least the most important) function of p300 and other HATs was to catalyze the acetylation of histone lysine residues, thereby changing the configuration of chromatin and increasing the access of transcription factors to DNA binding sites. Studies have shown, however, that proteins other than histones are also acetylated by p300 and a more generic name (lysine acetyltransferases, KATs) has therefore been proposed to describe this group of enzymes [24]. In the present review, the term HAT is used since it is still most commonly used in the literature. The p300 and CBP HATs have more than 70 defined histone and nonhistone substrates [22].
Although protein acetylation is regulated by a number of different HATs, the present review is focused mainly on p300 since this nuclear cofactor has been implicated in the regulation of muscle cell differentiation [25] and muscle wasting [16–19]. p300 regulates the acetylation of both histones and nonhistone proteins, including transcription factors and nuclear cofactors involved in the regulation of muscle mass, such as NF-kB/p65 [26], FOXO transcription factors [27], C/EBPβ [28], and PGC-1α [29]. p300 HAT activity involves initial binding of the enzyme to acetyl-CoA. Recent determination of the high-resolution x-ray structure of the p300 molecule suggests that a narrow tunnel in p300 accommodates acetyl-CoA [30]. The acetyl-CoA/p300 complex transiently binds to the positively charged target lysine in the protein to be acetylated and dissociates from the acetylated lysine immediately after acetyl transfer. This “hit-and-run” mechanism has been the basis for the development of selective small-molecule inhibitors [31].
Protein hyperacetylation can be caused not only by increased HAT activity but also by reduced HDAC activity or a combination of these changes (see Fig 1). Enzymes regulating deacetylation (HDACs) constitute a family consisting of at least 18 members divided into three classes [32]. Interestingly, HATs and HDACs intereact not only by regulating the acetylation and deacetylation of the same target protein but also by a complex cross talk. For example, to maximize enzymatic activity, p300 not only acetylates itself by autoacetylation [22] but also acetylates the histone deacetylase SIRT2, a process that inactivates SIRT2 which in turn further increases the acetylation (and activity) of p300 [33,34].
3. Hyperacetylation stimulates protein degradation
A number of recent reports suggest that both HATs and HDACs can regulate the degradation of cellular proteins and that the acetylation status of the protein is a factor that determines degradation rates [35,36]. Some studies suggest that acetylation of a lysine residue may reduce the degradation of certain proteins by “locking” a lysine residue, thereby blocking its ubiquitination and the proteasome-dependent degradation of the protein [35,36]. In contrast, other studies provided evidence that lysine acetylation is a signal that stimulates the degradation of many proteins, including hypoxia-inducible factor 1α (HIF-1α) [37], the SV40 T antigen [38], and the retinoblastoma tumor suppressor protein RB [39].
Mechanisms involved in acetylation-induced protein degradation are complex. In addition to direct regulation of the degradation of proteins by their acetylation, there are several indirect mechanisms by which acetylation and deacetylation may influence protein degradation. One such mechanism is the activation of certain transcription factors and other proteins regulating the transcription of genes involved in muscle wasting. Indeed, regulation of NF-kB/p65, C/EBPβ, and FOXO transcription factors by acetylation is well documented [26–28] and may be a mechanism by which they regulate muscle mass in various muscle wasting conditions [40]. Interestingly, a recent study suggests that acetylated FOXO1 stimulates autophagy [41], an important observation considering the role of autophagy/lysosomal proteolysis in muscle wasting [5].
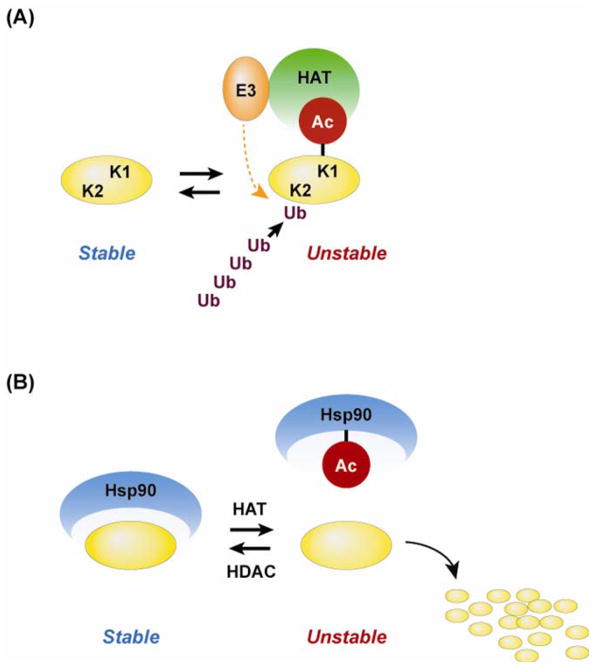
An additional potential mechanism by which HATs influence protein degradation is their relationships with the ubiquitin-proteasome proteolytic pathway. Studies suggest that several HATs (including p300, CBP, and PCAF) exert intrinsic ubiquitin ligase and polyubiquitination activities in addition to their acetyltransferase activity [35,36]. For example, a previous report [42] identified p53 as a target of p300/CBP-regulated polyubiquitination activity and other studies have shown that the N-terminal of the p300/CBP-associated factor, PCAF, has a domain with ubiquitin ligase activity [43]. A potential mechanism by which acetylation may interact with ubiquitination of proteins and stimulate proteasome-dependent proteolysis is illustrated in Fig 2A.
Fig 2.
Acetylation may influence protein degradation by multiple mechanisms. (A) HATs may acetylate lysine residues (exemplified by lysine residue K1 in the figure) and may increase the ubiquitination of other lysine residues (exemplified by K2) through intrinsic ubiquitin ligase (E3) activity. (B) Acetylation of cellular chaperones, for example Hsp90, may result in dissociation of the chaperone from proteins, resulting in destabilization and degradation of the proteins. The figure is based on reports by Khochbin and co-workers [35,36].
Besides HATs having dual activities (i.e., acetyltransferase and ubiquitination activities), there is evidence that individual HATs and HDACs can form complexes with different enzymes regulating protein ubiquitination [35,36]. Several examples of interactions between ubiquitin ligase complexes and HATs [44] and HDACs [45] have been reported and although it is not known how these complexes stimulate protein degradation, it is possible that HAT- and HDAC-regulated acetylation (and activation) of the associated ubiquitin ligases play a role.
An additional example of an indirect mechanism by which acetylation results in increased protein degradation is acetylation of the chaperone protein HSP90. Similar to other chaperones, HSP90 stabilizes multiple cellular proteins. Recent studies suggest that acetylation of HSP90 reduces its protective effects leading to accelerated degradation of several HSP90-interacting proteins [46,47] as illustrated in a schematic form in Fig 2B.
It is obvious, then, that hyperacetylation may stimulate protein degradation by multiple and complex mechanisms. Because apparently contradictory results have also been reported suggesting that hyperacetylation stabilizes certain proteins [35,36,48], it is possible that the regulation of protein degradation by acetylation varies with different proteins and in different cell types. The mechanisms by which HATs and HDACs regulate protein breakdown in muscle wasting conditions are not known but are important subjects for future studies. A recent study from our laboratory suggests that p300-dependent acetylation of multiple muscle wasting-related transcription factors may be involved in the loss of muscle mass during catabolic conditions [40].
4. Muscle wasting is regulated by p300-dependent hyperacetylation
Our laboratory was the first to report evidence suggesting that muscle wasting may at least in part be regulated by hyperacetylation. When cultured L6 myotubes were treated with dexamethasone (an experimental model that replicates sepsis- and glucocorticoid-induced muscle protein degradation, activation of the ubiquitin-proteasome pathway and muscle atrophy), we found a time- and dose-dependent increase in p300 protein levels, mainly reflecting stimulated synthesis of p300 [16].
In subsequent experiments, we found evidence that the increased p300 expression in dexamethasone-treated myotubes was accompanied by increased HAT activity [17]. In the same study, treatment of the myotubes with dexamethasone resulted in reduced expression of HDAC3 (a class I HDAC) and HDAC6 (a class II HDAC) and decreased HDAC activity, providing additional mechanisms of hyperacetylation.
In additional experiments in the same study [17], molecular evidence was found that p300 and its HAT activity are involved in dexamethasone-induced muscle proteolysis. Thus, when p300 expression was knocked down in cultured myotubes with p300 siRNA, the dexamethasone-induced increase in protein degradation was abolished. Furthermore, when the cultured muscle cells were transfected with a mutated p300 plasmid lacking HAT activity, the dexamethasone-induced increase in protein degradation was abolished. Taken together, our observations in dexamethasone-treated myotubes suggest that glucocorticoid-induced muscle wasting is at least in part regulated by p300 and that the role of p300 reflects its HAT activity.
Our observations suggesting a role of p300 in glucocorticoid-induced muscle wasting were confirmed in a recent study by Tobimatsu et al [18]. In that study, blocking p300 by transfecting cultured muscle cells with a plasmid expressing wild-type CBP/p300-interacting transactivator with ED-rich tail 2 (Cited2), which binds to the cysteine-histidine-rich region 1 of p300, prevented dexamethasone-induced atrophy of the muscle cells and the induction of atrogin-1 and MuRF1. The myotube-sparing effects were less pronounced with a mutated form of Cited2 that lacked the ability to bind p300, further supporting the role of p300/HAT in glucocorticoid-induced muscle wasting.
In more recent experiments, we found that septic peritonitis in rats resulted in increased p300 expression and HAT activity in skeletal muscle [19], similar to our observations in dexamethasone-treated myotubes [16,17]. In the same study [19], sepsis in rats resulted in reduced expression of HDAC3, HDAC6, and SIRT1 (a class III HDAC). In more recent experiments, the reduction of muscle SIRT1 expression in septic rats was particularly pronounced and more long-lasting than the reduction of other HDACs (unpublished observations). Importantly, the reduced expression of SIRT1 and other HDACs was accompanied by reduced HDAC activity in skeletal muscle. Interestingly, the downregulation of HDAC expression and activity occurred earlier than the increase in p300/HAT expression and activity, possibly reflecting activation of p300 caused by hyperacetylation secondary to reduced HDAC activity. Taken together, our results suggest that muscle wasting in a clinically relevant model of sepsis (septic peritonitis in rats) is associated with increased p300/HAT expression and activity and reduced expression and activity of HDACs.
5. Induction of hyperacetylation with HDAC inhibitor stimulates muscle proteolysis
Inhibition of HDAC activity shifts the balance between HATs and HDACs and results in protein hyperacetylation. A number of drugs inhibiting HDAC activity have been described [49] and have been of particular interest for cancer treatment [50]. In addition, drugs that inhibit HDAC activity are important tools to test the role of hyperacetylation in metabolic events.
Trichostatin A (TSA) is a well known inhibitor of class I and II HDACs [49]. Although the inhibition of HDAC activity in itself results in protein hyperacetylation, studies suggest that additional mechanisms contribute to TSA-induced hyperacetylation. For example, in a recent study, treatment of cultured T47D cells (a breast adenocarcinoma cell line) and HeLa cells (a human cervical carcinoma cell line) with TSA resulted in increased acetylation of p300, stimulated p300/HAT activity, and increased stability (reduced degradation) of p300 [51]. Thus, treatment with TSA may increase protein acetylation by multiple mechanisms, including inhibition of HDAC activity, acetylation and activation of p300, and increased amounts of p300 secondary to increased stability of p300.
Based on our recent observations that dexamethasone treatment of cultured myotubes [17] and induction of sepsis in rats [19] resulted in increased p300/HAT and reduced HDAC3 and 6 expression and activity in skeletal muscle, we hypothesized that induction of hyperacetylation by treatment with TSA would stimulate muscle proteolysis. Indeed, when cultured myotubes were treated with TSA, protein degradation was increased [17]. The effects of TSA and dexamethasone on protein degradation were almost identical and no further (additive) effects were noted when the myotubes were treated with both dexamethasone and TSA, suggesting that the drugs stimulate protein degradation by the same mechanism (i.e., hyperacetylation).
In other experiments, treatment of rats in vivo with TSA also resulted in stimulated muscle protein breakdown [19]. Interestingly, the increase in muscle proteolysis was associated with increased expression of atrogin-1, suggesting that hyperacetylation may upregulate ubiquitin-proteasome-dependent muscle proteolysis. Of note, MuRF1 expression was not increased in TSA-treated rats [19]. A differential regulation of atrogin-1 and MuRF1 in muscle wasting was reported previously, possibly reflecting differential involvement of transcription factors regulating the expression of the ubiquitin ligases [52,53] or a complex crosstalk between the ubiquitin ligases and their regulators [54,55]. The mechanisms involved in the selective upregulation of atrogin-1 by hyperacetylation remain to be determined.
It should be pointed out that although our recent observations suggest that treatment with TSA may induce muscle wasting secondary to increased protein breakdown [17,19], apparently contradictory results have been reported. For example, Narver et al [56] found that TSA had beneficial effects in a mouse model of spinal muscular atrophy. Even though the reasons for these apparently contradictory results are not known at present, they may reflect different roles of hyperacetylation in different muscle wasting conditions. It should also be noted that in the study by Narver et al [56], TSA treatment was combined with aggressive nutritional support which complicates the interpretation of the results as far as the role of hyperacetylation in itself is concerned.
6. HDAC6 expression is reduced in skeletal muscle during sepsis and may regulate muscle mass through multiple mechanisms
Among different HDACs, the expression of HDAC6 (together with SIRT1) was reduced in skeletal muscle from septic rats [19]. Previous studies reported HDAC6-related mechanisms that may be involved in muscle wasting in addition to its deacetylating activity. One such mechanism reflects the presence of a ubiquitin binding domain in the N-terminal of the HDAC6 molecule [45]. High affinity binding of HDAC6 to ubiquitin may prevent the ubiquitination of other proteins (by competition) and delay their processing by the proteasome [57]. Therefore, it is possible that reduced levels of HDAC6, as observed in dexamethasone-treated myotubes [17] and in muscle from septic rats [19], may increase the ubiquitination and degradation of certain proteins.
An additional interesting mechanism by which HDAC6 may regulate muscle proteolysis is its interaction with p97/valosin-containing protein (p97/VCP). The HDAC6 partner p97/VCP is a chaperone involved in the regulation of a number of different cellular functions, many of them dependent on its ability to disassemble various complexes, including those containing ubiquitinated proteins. Studies suggest that p97/VCP may influence protein degradation by multiple mechanisms [reviewed in 58,59]. First, p97/VCP may act as a chaperone transferring ubiquitinated proteins to the proteasome. Second, p97/VCP may be a component of the 26S proteasome. Third, p97/VCP may participate in the unfolding of ubiquitinated proteins before their injection into the proteasome. Interestingly, p97/VCP may be necessary for the degradation of IkBα resulting in NF-kB activation [60]. Although most previous studies on the role of p97/VCP in ubiquitin-proteasome-dependent proteolysis were performed in non-muscle cells, we proposed already 10 years ago that p97/VCP may be involved in muscle wasting [58].
Recent studies suggest that the equilibrium between HDAC6 and p97/VCP cellular concentrations may be critical in the determination of how ubiquitinated proteins are handled [57]. An excess of p97/VCP over HDAC6 facilitates the release of ubiquitin-bound HDAC6 and the delivery of ubiquitinated proteins to the proteasome. In contrast, a relative excess of HDAC6 over p97/VCP results in the accumulation of ubiquitinated and unfolded proteins, resulting in the formation of aggresomes and stimulation of autophagy.
Of note, the present discussion was focused on HDAC6 because we found recently that sepsis in rats resulted in reduced levels of HDAC6 mRNA and protein in skeletal muscle accompanied by reduced HDAC activity [19]. Our observations do not rule out that reduced expression and activity of other HDACs are also involved in muscle wasting during sepsis and other catabolic conditions. Indeed, in our recent study, the protein expression of SIRT1 was also reduced in skeletal muscle during sepsis [19] and results from other experiments in our laboratory suggest that reduced expression and activity of SIRT1 is an important mechanism of glucocorticoid-induced muscle wasting [61].
7. Prevention of hyperacetylation may preserve muscle mass
Protein hyperacetylation may be reversed by inhibition of HAT activity and/or stimulation of HDAC activity (see Fig 1). A number of different drugs with p300 inhibitory properties have been described in the literature. Several of those inhibitors are natural products or derivatives of those products, including curcumin (isolated from turmeric), garcinol (from garlic), the garcinol derivative LTK-14, and anacardic acid (from cashew nuts) [62–67]. Like many pharmacologic inhibitors, most of these drugs are not specific in their actions. For example, curcumin inhibits NF-kB activity and is an oxygen radical scavenger in addition to being a p300 inhibitor [68]. Of note, we used curcumin in a recent study to block sepsis-induced muscle wasting [67] and in that report, we interpreted the effects of curcumin as being caused mainly by inhibition of NF-kB. The possibility that the muscle sparing effects of curcumin in that study reflected inhibition of p300 can not be ruled out, and if so, it is not known if curcumin exerted its effects by blocking p300-dependent acetylation of NF-kB/p65, explaining why NF-kB activity was reduced [26,67], or by blocking acetylation of other protein(s).
Given the lack of specificity of many drugs with p300 inhibitory properties, recent research has focused on small molecules with a substantially higher degree of specificity. Lys-CoA is a specific p300 inhibitor with high potency but its use is limited by not being cell permeable [69]. Cole and co-workers [70] reported recently on a selective small molecule p300 inhibitor, C646, which is a competitive p300/HAT inhibitor with a Ki of 400 nM and is selective versus other acetyltransferases. The drug was identified by using virtual ligand screening and built on knowledge of the high-resolution X-ray structure of p300. C646 docks into a narrow tunnel in the p300 molecule that otherwise accommodates acetyl-CoA and the interaction between C646 and p300 therefore excludes acetyl-CoA from p300 explaining why C646 is a competitive inhibitor of p300. The effects of C646 on sepsis- and glucocorticoid-induced hyperacetylation, muscle wasting, and muscle weakness remain to be determined.
In recent studies, we and others found genetic evidence that inhibition of p300/HAT expression and activity can prevent glucocorticoid-induced protein degradation in cultured myotubes [17,18]. In additional experiments, we found recently that downregulation of p300 expression reduced dexamethasone-induced acetylation of several muscle wasting-related transcription factors, upregulation of MuRF1 expression, and atrophy in cultured myotubes [40]. Those observations lend further support to the concept that inhibition of p300 may prevent muscle wasting, at least muscle wasting regulated by glucocorticoids.
An alternative method to reduce protein hyperacetylation is to stimulate HDAC activity. Small molecule HDAC activators were described recently [71]. In addition, several natural products, including resveratrol, have been reported to activate HDACs. Resveratrol is a polyphenolic compound mainly found in the skin of grapes and is present in red wine [72,73]. It has been shown to significantly increase SIRT1 activity through an allosteric interaction, resulting in increased affinity for both NAD+ and the acetylated substrate [74]. Although resveratrol is frequently used as a SIRT1 activator, it has other important properties as well, including antioxidant properties [75], inhibition of NF-kB [76], and activation of the AMP-activated protein kinase (AMPK) [77].
In recent experiments, we found evidence suggesting that resveratrol can prevent glucocorticoid-induced muscle wasting through a SIRT1-dependent mechanism [61]. In those experiments, resveratrol prevented dexamethasone-induced acetylation of FOXO1, expression of atrogin-1 and MuRF1, and protein degradation in cultured myotubes. In addition, dexamethasone-induced atrophy of myotubes was blocked by resveratrol. In the same study, resveratrol activated SIRT1 and downregulation of SIRT1 expression with SIRT1 siRNA blocked the beneficial effects of resveratrol. Taken together, those results support the concept that activation of the histone deacetylase SIRT1 with resveratrol can prevent glucocorticoid-induced hyperacetylation and muscle wasting. Additional studies support the beneficial effects of resveratrol in various conditions characterized by a catabolic response in skeletal muscle [78–82]. The role of resveratrol in the treatment of metabolic diseases associated with muscle wasting, such as obesity and type 2 diabetes mellitus, is being increasingly recognized [83].
8. Several muscle wasting-associated transcription factors are regulated by acetylation
There is evidence that genes regulating ubiquitin-proteasome-dependent and autophagy-lysosomal protein breakdown during muscle wasting are under the control of multiple transcription factors, including FOXO transcription factors [53,84–86], members of the C/EBP family of transcription factors [87–89], and NF-kB [52,90–93]. The activity of these transcription factors is regulated by different posttranslational modifications, including acetylation.
Several studies suggest that FOXO transcription factors are regulated by acetylation mediated by both p300-dependent acetylation [27] and SIRT1-dependent deacetylation [94,95]. Although some reports suggest that acetylation of FOXO transcription factors results in decreased activity [96–98], several other reports provided evidence that FOXO activity is increased by acetylation [27,99,100]. It is possible that previous, apparently conflicting results with regards to the functional consequences of FOXO acetylation at least in part reflect different cell types being studied and differential effects of individual HATs and HDACs regulating the acetylation of different lysine residues. In addition, there is evidence to suggest that acetylation may enhance or repress FOXO transcription factors in a target gene-specific manner [94,97]. The complex regulation of FOXO activity by acetylation and deacetylation as well as by protein-protein interaction with other transcription factors and nuclear cofactors was reviewed recently [101].
A role of FOXO acetylation and deacetylation in the regulation of metabolism has been reported in different cell types, such as cardiac myocytes [102]. In recent experiments in our laboratory, treatment of cultured myotubes with dexamethasone resulted in a robust increase in cellular levels of acetylated FOXO1 and FOXO3a [40]. Those were important observations because FOXO1 and FOXO3a were found in previous reports to regulate the transcription of the atrogin-1 and MuRF1 genes as well as genes involved in autophagy-lysosomal muscle proteolysis [53,84–86].
In our recent study, we found that nuclear levels of NF-kB/p65 acetylated at Lys 310 were increased in dexamethasone-treated myotubes [40]. This was a significant finding because NF-kB/p65 is activated by acetylation of Lys 310 [26] and several previous reports provided strong evidence that NF-kB/p65 is involved in muscle wasting during different catabolic conditions [52,90,91,93]. Of note, the dexamethasone-induced acetylation of p65 was inhibited by p300 siRNA [40], providing further support for the concept that p300 expression and activity are involved in muscle wasting.
A role of C/EBP transcription factors in muscle wasting, in particular C/EBPβ, was first reported in studies from our laboratory [87,88,103]. Those observations were recently corroborated by Zhang et al [89] who reported that C/EBPβ regulates muscle mass in cancer-induced muscle wasting.
In our previous study in which we found evidence that p300 expression was upregulated in dexamethasone-treated myotubes, co-immunoprecipitation also indicated that p300 formed a complex with C/EBPβ [16]. Although we did not determine levels of acetylated C/EBPβ in that study, more recent experiments in our laboratory suggest that C/EBPβ is acetylated in dexamethasone-treated myotubes in a dose- and time-dependent manner and that the acetylation is at least in part regulated by p300 [40]. Of note, additional acetyltransferases have been reported to acetylate C/EBPβ, including CBP, PCAF, and GCN5 [28].
Previous studies suggest that C/EBPβ acetylation is regulated not only by increased HAT activity but by reduced HDAC activity as well. For example, studies in cultured adipocytes suggest that treatment with glucocorticoids may increase C/EBPβ acetylation through a combination of increased PCAF/GCN5 and reduced HDAC activity [104,105].
There is evidence that several lysine residues in the C/EBPβ molecule can be acetylated and that acetylation of different lysines may have different functional consequences. For example, acetylation of C/EBPβ Lys 39 may be particularly important for transcriptional activation whereas acetylation of Lys 215 and 216 regulates C/EBPβ DNA binding activity [28]. It will be important in future experiments to determine whether C/EBPβ is acetylated in muscle wasting-related conditions other than dexamethasone-treated myotubes, and to define which lysines that are acetylated as well as the functional consequences of their acetylation.
Taken together, reports from our and other laboratories suggest that FOXO transcription factors, NF-kB/p65, and C/EBPβ may be involved in the regulation of muscle wasting-associated genes and that the activity of these transcription factors may be regulated by hyperacetylation.
9. Multiple studies, but not all, support a role of hyperacetylation in protein degradation and muscle wasting
Although in the present review, several studies from our own laboratory were discussed, reports from other groups support the concept that protein hyperacetylation promotes protein degradation and may be involved in muscle wasting [35,36]. The recent report by Tobimatsu et al [18] confirmed the role of p300 and its HAT activity in glucocorticoid-induced atrophy of cultured muscle cells. In another study, Jeong et al [106] reported that activation of CBP and inhibition of HDAC1 resulted in acetylation and autophagy-lysosomal degradation of Huntingtin protein (Htt). Other studies suggest that hyperacetylation results in increased degradation of E2F1 [107], hypoxia-inducible factor-1 (HSF-1) [37], the SV40 T antigen [38], and the retinoblastoma tumor suppressor protein RB [39]. Several studies, in addition to a recent report from our laboratory [61], suggest that the SIRT1 activator resveratrol (the activity of which reduces the levels of acetylated proteins) exerts beneficial effects in various conditions associated with muscle atrophy [78–82].
Although multiple studies support the concept that hyperacetylation may result in increased protein degradation both in muscle cells and in other cell types, apparently contradictory results have also been reported. For example, recent studies suggest that HDAC1- and SIRT1-dependent deacetylation promotes ubiquitination and degradation of the transcription factor Smad7 [48]. Additional proteins that have been reported to be stabilized by increased acetylation include p53, Runx3, and MYC [reviewed in 36]. Therefore, the role of acetylation and deacetylation in the regulation of protein turnover may be protein-specific.
Important for the present review suggesting that hyperacetylation may be an important factor in muscle wasting, some reports seem to contradict that notion. For example, inhibition of class I and II HDACs in cultured C2C12 and human primary myocytes (setting the stage for increased levels of acetylated proteins) resulted in the formation of myotubes with increased cell size and abundance of muscle proteins [108]. In other studies, treatment with TSA resulted in functional and morphological recovery of muscles in mice with muscular dystrophy [109] and had beneficial effects in mice with spinal muscular atrophy [56]. Results in additional studies suggest that increased, rather than decreased, expression and activity of HDAC4 and 5, class IIa HDACs, are involved in denervation-induced muscle atrophy [110–112] and that exercise-induced muscle hypertrophy is associated with reduced HDAC4 and 5 activity [113]. Thus, the role of hyperacetylation in the regulation of muscle mass may be different in denervation- and exercise-induced changes than in muscle wasting caused by catabolic conditions, such as sepsis and high levels of glucocorticoids.
In a recent study by Senf et al [114], transfection of rat soleus muscle with a dominant-negative p300, which lacks HAT activity and inhibits endogenous p300 HAT activity, increased FOXO reporter activity and induced the expression of atrogin-1. In contrast, transfection of wild-type p300 or CBP inhibited the increase in FOXO activity and atrogin-1 expression caused by 3 days of cast immobilization of both hind limbs in rats and by treatment of cultured C2C12 with dexamethasone or “starvation” induced by replacement of culture medium with salt solution. In addition, results in the same study [114] suggested that different members of the FOXO family of transcription factors may be differentially regulated by p300 with increased p300 expression repressing FOXO3a nuclear localization and activity but increasing FOXO1 nuclear translocation.
Although the report by Senf et al [114] may seem to contradict a role of p300-mediated hyperacetylation in muscle wasting, the results in that study need to be interpreted with caution for several reasons. First, the regulation of p300 expression and activity during muscle atrophy caused by immobilization may be different than the regulation of p300 during muscle wasting seen in various catabolic conditions, such as cancer, sepsis, and severe injury. Interestingly, muscle wasting caused by these conditions is typically more pronounced in white, fast-twitch than in red, slow-twitch skeletal muscle [115]. In contrast, red, slow-twitch muscle is more sensitive to the effects of immobilization, suggesting that different mechanisms may be involved in muscle wasting caused by catabolic conditions and immobilization. Of note, the experiments reported by Senf et al [114] were performed in the red, slow-twitch soleus muscle and it remains to be determined whether similar effects of p300 overexpression occur in white, fast-twitch muscle. Second, HAT activity was not determined in the different models of muscle atrophy used in the study by Senf et al [114], i.e., 3-day hind limb immobilization and dexamethasone-treated or nutrient-deprived C2C12 muscle cells. Also, the influence of the wild-type or dominant-negative p300 plasmids on HAT activity was not determined. Finally, in light of our recent observation that sepsis-induced muscle wasting was associated with activation of FOXO1 but not FOXO3a or FOXO4 [85], it was interesting to note that p300 overexpression resulted in increased nuclear localization (and most likely activation) of FOXO1 in the study by Senf et al [114].
A recent study by Bertaggia et al [98] supported the concept that FOXO3 activity in skeletal muscle is negatively regulated by acetylation. In that study, muscle atrophy was induced by denervation and the influence of catabolic conditions was not investigated. In addition, the effects of acetylation on FOXO1 activity were not examined.
Previous results supporting or contradicting a role of hyperacetylation in loss of muscle mass are summarized in Tables 1 and 2. Regardless of the reasons for the apparently contradictory results with regards to the role of hyperacetylation in muscle wasting, it is obvious that additional studies are needed to clarify the exact role of acetylation in different conditions characterized by loss of muscle mass.
Table 1.
Observations supporting a role of hyperacetylation in muscle wasting
| Increased p300/HAT expression and activity in dexamethasone-treated myotubes and septic rats [16,17,19]. |
| Decreased expression and activity of HDAC3 and 6 and SIRT1 in dexamethasone-treated myotubes and septic rats [17,19]. |
| Prevention of glucocorticoid-induced muscle atrophy by inhibition of p300/HAT expression and activity [17,18]. |
| SIRT1-dependent prevention of glucocorticoid-induced muscle atrophy by resveratrol [61]. |
| p300-dependent nuclear translocation of FOXO1 in skeletal muscle [114]. |
| Hyperacetylation-induced degradation of cellular proteins [35–39,106,107]. |
| Acetylation-mediated activation of muscle wasting-related transcription factors, including NF-kB/p65, C/EBPβ, and FOXO1 [26–28]. |
| Acetylation of multiple muscle wasting-related transcription factors in dexamethasone- treated myotubes [40]. |
| Stimulation of autophagy by acetylated FOXO1 [41]. |
| Ubiquitin ligase and polyubiquitination activities by HATs [35,36,42,43]. |
| Increased muscle protein degradation and atrogin-1 expression following treatment with the HDAC inhibitor TSA [17,19]. |
Table 2.
Observations contradicting a role of hyperacetylation in muscle wasting
| Decreased degradation of certain hyperacetylated proteins [35,36,48]. |
| Increased cell size and protein content in cultured muscle cells by HDAC inhibition [108]. |
| Recovery of muscles in mice with muscular dystrophy by treatment with TSA [109]. |
| Beneficial effects of TSA in mice with spinal muscular atrophy [56]. |
| p300- and acetylation-dependent repression of FOXO3 activity in skeletal muscle [98,114]. |
| Increased expression and activity of HDAC4 and 5 in denervation-induced muscle atrophy [110–112]. |
| Reduced HDAC4 and 5 activity in exercise-induced muscle hypertrophy [113]. |
10. Summary and conclusions
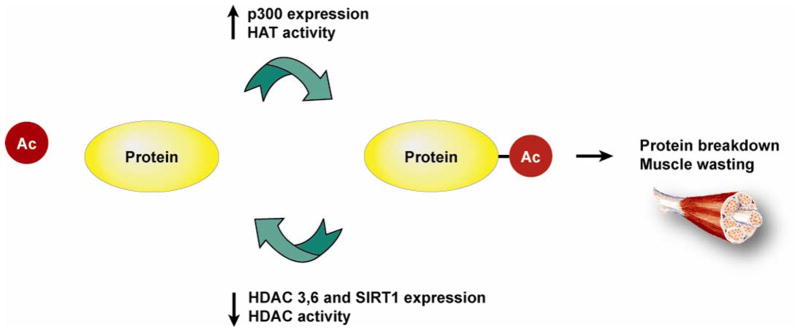
Multiple recent studies, both from our laboratory and other laboratories, support a role of protein hyperacetylation in stimulated muscle proteolysis and loss of muscle mass during various catabolic conditions. Based on literature reviewed in this report, we propose a model in which increased HAT activity (at least in part regulated by increased expression of p300) and reduced HDAC activity (at least in part reflecting reduced expression and activity of HDAC3 and 6 and SIRT1) cooperate in acetylation of cellular proteins, including muscle wasting-related transcription factors, resulting in increased muscle protein breakdown and muscle atrophy (Fig 3). Importantly from a clinical standpoint, protein hyperacetylation may be reduced by p300/HAT inhibitors and HDAC activators, for example the SIRT1 activator resveratrol [61], and these therapeutic modalities may prove fruitful in the prevention and treatment of muscle wasting. The present review, therefore, has obvious translational implications.
Fig 3.
Muscle wasting, mainly reflecting stimulated ubiquitin-proteasome-dependent and autophagy-lysosomal myofibrillar protein breakdown, may at least in part reflect hyperacetylation of cellular proteins regulated by increased p300/HAT expression and activity and decreased expression and activity of HDAC3 and 6 and SIRT1. Of note, whereas this model may reflect mechanisms involved in muscle wasting during various catabolic conditions, such as sepsis and high levels of glucocorticoids, the role of hyperacetylation may be different or even opposite in other conditions characterized by loss of muscle mass, for example denervation and immobilization.
It is important to emphasize again that some controversy exists with regards to the role of hyperacetylation in muscle wasting. A weakness of the current review and the argument for a role of hyperacetylation in muscle wasting is the lack of clinical evidence for prevention of muscle wasting by inhibition of acetylation. Despite this weakness, the present review will hopefully stimulate continued research on the role of protein acetylation for the loss of muscle mass in catabolic patients.
Acknowledgments
ZA was supported in part by the Department of Clinical Medicine, Sapienza, University of Rome, Rome, Italy. EC was supported in part by Gobierno Vasco, Spain (BFI2010-240).
Funding
Work described in this article and performed in our laboratory was supported in part by NIH R01 DK37908.
Abbreviations
- AMPK
AMP-activated protein kinase
- CBP
CREB-binding protein
- C/EBP
CCAAT/enhancer binding protein
- FOXO
Forkhead box O
- GCN5
General control non-depressible 5
- GNAT
Gen5-related N-acetyltransferase
- HAT
Histone acetyltransferase
- HDAC
Histone deacetylase
- HIF-1
Hypoxia-inducible factor-1
- HSP90
Heat shock protein 90
- MOZ
Monocytic leukemia zinc-finger protein
- MuRF1
Muscle RING-finger protein 1
- NF-kB
Nuclear factor kappa B
- PCAF
p300/CBP-associated factor
- PGC-1
Proliferator-activated receptor γ coactivator-1
- RB
Retinoblastoma protein
- Runx3
Runt-related transcription factor 3
- SIRT
Sirtuin
- SV40
Simian virus 40
- TIP 60
Tat-interactive protein 60 kDa
- TSA
Trichostatin A
- VCP
Valosin-containing protein
Footnotes
None of the authors has a conflict of interest to report
Contributions
Nima Alamdari, Zaira Aversa, Estibaliz Castillero, and Per-Olof Hasselgren contributed to the design and conduct of the study, collection, analysis, and interpretation of data, and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasselgren PO, Menconi MJ, Fareed MU, et al. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–68. doi: 10.1016/j.biocel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–19. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 5.Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol. 2010;298:C1291–7. doi: 10.1152/ajpcell.00531.2009. [DOI] [PubMed] [Google Scholar]
- 6.Roberts-Wilson TK, Reddy RN, Bailey JL, et al. Calcineurin signaling and PGC-1α expression are suppressed during muscle atrophy due to diabetes. Biochim Biophys Acta. 2010;1803:960–7. doi: 10.1016/j.bbamcr.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muscaritoli M, Molfino A, Bollea MR, et al. Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care. 2009;12:378–83. doi: 10.1097/MCO.0b013e32832c7ae1. [DOI] [PubMed] [Google Scholar]
- 8.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37:S354–67. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweikert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–9. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 10.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–99. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Yang EJ, Lim S, Lim Y, et al. Association between muscle strength and metabolic syndrome in older Korean men and women: the Korean longitudinal study on health and aging. Metabolism. 2012;61:317–24. doi: 10.1016/j.metabol.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–15. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 13.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 14.Hasselgren PO. Ubiquitination, phosphorylation, and acetylation – triple threat in muscle wasting. J Cell Physiol. 2007;213:679–89. doi: 10.1002/jcp.21190. [DOI] [PubMed] [Google Scholar]
- 15.Lunke S, El-Osta A. The emerging role of epigenetic modifications and chromatin remodeling in spinal muscular atrophy. J Neurochem. 2009;109:1557–69. doi: 10.1111/j.1471-4159.2009.06084.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Menconi MJ, Wei W, et al. Dexamethasone upregulates the expression of the nuclear cofactor p300 and its interaction with C/EBPβ in cultured myotubes. J Cell Biochem. 2005;94:1058–67. doi: 10.1002/jcb.20371. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Wei W, Menconi M, et al. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent. Am J Physiol Regul Integr Comp Physiol. 2007;292:R337–44. doi: 10.1152/ajpregu.00230.2006. [DOI] [PubMed] [Google Scholar]
- 18.Tobimatsu K, Noguchi T, Hosooka T, et al. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun. 2009;378:399–403. doi: 10.1016/j.bbrc.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 19.Alamdari N, Smith IJ, Aversa Z, et al. Sepsis and glucocorticoids upregulate p300 and downregulate HDAC6 expression and activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;299:R509–20. doi: 10.1152/ajpregu.00858.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? Embo J. 2000;19:1176–9. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiman RN, Tjian R. Regulating the regulators: lysine modifications make their mark. Cell. 2003;112:11–7. doi: 10.1016/s0092-8674(02)01278-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Tang Y, Cole PA, et al. Structure and chemistry of the p300/CBP and Rtt 109 histone acetyltransferases: implications for acetyltransferase evolution and function. Curr Opin Struct Biol. 2008;18:741–7. doi: 10.1016/j.sbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–61. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allis CD, Berger SL, Cote J, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–6. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 25.McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev. 2001;11:497–504. doi: 10.1016/s0959-437x(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 26.Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kB transcription factor complex by acetylation. J Mol Med. 2003;81:549–57. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- 27.Perrot V, Rechler MM. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol Endocrinol. 2005;19:2283–98. doi: 10.1210/me.2004-0292. [DOI] [PubMed] [Google Scholar]
- 28.Cesena TI, Cardinaux JR, Kwok R, et al. CCAAT/enhancer-binding protein (C/EBP)β is acetylated at multiple lysines. Acetylation of C/EBPβ at lysine 39 modulates its ability to activate transcription. J Biol Chem. 2007;282:956–67. doi: 10.1074/jbc.M511451200. [DOI] [PubMed] [Google Scholar]
- 29.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. Embo J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Wang L, Zhao K, et al. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008;451:846–50. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- 31.Ott M, Verdin E. HAT trick: p300, small molecule, inhibitors. Chem Biol. 2010;17:417–8. doi: 10.1016/j.chembiol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 32.DeRuijter AJM, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black JC, Mosley A, Kitada T, et al. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell. 2008;32:449–55. doi: 10.1016/j.molcel.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Y, Jin YH, Kim YJ, et al. Acetylation of Sirt2 by p300 attenuates its deacetylase activity. Biochem Biophys Res Commun. 2008;375:576–80. doi: 10.1016/j.bbrc.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 35.Caron C, Boyault C, Khochbin S. Regulatory cross-talk between lysine acetylation and ubiquitination: role in the control of protein stability. BioEssays. 2005;27:408–15. doi: 10.1002/bies.20210. [DOI] [PubMed] [Google Scholar]
- 36.Sadoul K, Boyault C, Pabion M, et al. Regulation of protein turnover by acetyltransferases and deacetylases. Biochim. 2008;90:306–12. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1α by ARD1-mediated acetylation. Cell. 2002;111:709–20. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 38.Shimazu T, Komatsu Y, Nakayama KI, et al. Regulation of SV40 large T-antigen stability by reversible acetylation. Oncogene. 2006;25:7391–400. doi: 10.1038/sj.onc.1209731. [DOI] [PubMed] [Google Scholar]
- 39.Leduc C, Claverie P, Eymin B, et al. p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene. 2006;25:4147–54. doi: 10.1038/sj.onc.1209446. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain W, Gonnella P, Alamdari N, et al. Multiple muscle wasting-related transcription factors are acetylated in dexamethasone-treated muscle cells. Biochem Cell Biol. doi: 10.1139/o11-082. epub 2012 Jan 31. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Yang J, Liao W, et al. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–75. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 42.Grossman SR, Deato ME, Brignone C, et al. Polyubiquitination of p53 by a ubiquitin ligase activity of p300. Science. 2003;300:342–4. doi: 10.1126/science.1080386. [DOI] [PubMed] [Google Scholar]
- 43.Linares LK, Kiernan R, Triboulet R, et al. Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat Cell Biol. 2007;9:331–8. doi: 10.1038/ncb1545. [DOI] [PubMed] [Google Scholar]
- 44.Turnell AS, Stewart GS, Grand RJ, et al. The APC/C and CBP/p300 cooperate to regulate transcription and cell cycle progression. Nature. 2005;438:690–5. doi: 10.1038/nature04151. [DOI] [PubMed] [Google Scholar]
- 45.Seigneurin-Berney D, Verdel A, Curtet S, et al. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21:8035–44. doi: 10.1128/MCB.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 47.Scroggins BT, Robzyk K, Wang D, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–9. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonsson M, Heldin CH, Ericsson J, et al. The balance between acetylation and deacetylation controls Smad7 stability. J Biol Chem. 2005;280:21797–803. doi: 10.1074/jbc.M503134200. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida M, Matsuyama A, Komatsu Y, et al. From discovery to the coming generation of histone deacetylase inhibitors. Curr Med Chem. 2003;10:2351–8. doi: 10.2174/0929867033456602. [DOI] [PubMed] [Google Scholar]
- 50.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 51.Kim SH, Kang HJ, Na H, et al. Trichostatin A enhances acetylation as well as protein stability of ERα through induction of p300 protein. Breast Cancer Res. 2010;12:R22. doi: 10.1186/bcr2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai D, Frantz JD, Tawa NE, et al. IKKβ/NF-kB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waddell DS, Baehr LM, van den Brandt J, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–97. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usui S, Maejima Y, Pain J, et al. Endogenous muscle atrophy F-box mediates pressure overload-induced cardiac hypertrophy through regulation of nuclear factor-kappa B. Circ Res. 2011;109:161–71. doi: 10.1161/CIRCRESAHA.110.238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narver HL, Kong L, Burnett BG, et al. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann Neurol. 2008;64:465–70. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyault C, Zhang Y, Fritah S, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–81. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun. 2002;290:1–10. doi: 10.1006/bbrc.2001.5849. [DOI] [PubMed] [Google Scholar]
- 59.Boyault C, Sadoul K, Pabion M, et al. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–76. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 60.Dai RM, Chen E, Longo DL, et al. Involvement of valosin-containing protein, an ATPase co-purified with IkBα and 26S proteasome, in ubiquitin-proteasome-mediated degradation of IkBα. J Biol Chem. 1998;273:3562–73. doi: 10.1074/jbc.273.6.3562. [DOI] [PubMed] [Google Scholar]
- 61.Alamdari N, Aversa Z, Castillero E, et al. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun. 2012;417:528–33. doi: 10.1016/j.bbrc.2011.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balasubramanyam K, Varier RA, Altaf M, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, suppresses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–71. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 63.Morimoto T, Sunagawa Y, Kawamura T, et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–78. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balasubramanyam K, Altaf M, Varier RA, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–26. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 65.Mantelingu K, Reddy BAA, Swaminathan V, et al. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol. 2007;14:645–57. doi: 10.1016/j.chembiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Ghizzoni M, Boltjes A, de Graaf C, et al. Improved inhibition of the histone acetyltransferase PCAF by an anacardic acid derivative. Bioorg Med Chem. 2010;18:5826–34. doi: 10.1016/j.bmc.2010.06.089. [DOI] [PubMed] [Google Scholar]
- 67.Poylin V, Fareed MU, O’Neal P, et al. The NF-kB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat Inflam. 2008;2008:317851. doi: 10.1155/2008/317851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alamdari N, O’Neal P, Hasselgren PO. Curcumin and muscle wasting – a new role for an old drug? Nutrition. 2009;25:125–9. doi: 10.1016/j.nut.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau OD, Courtney AD, Vassilev A, et al. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J Biol Chem. 2000;275:1953–9. doi: 10.1074/jbc.M003219200. [DOI] [PubMed] [Google Scholar]
- 70.Bowers EM, Yan G, Mukherjee C, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: Identification of a selective small molecule inhibitor. Chem Biol. 2010;17:471–82. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soleas GJ, Diamandis EP, Goldberg DM. The world of resveratrol. Adv Exp Med Biol. 2001;492:159–82. doi: 10.1007/978-1-4615-1283-7_13. [DOI] [PubMed] [Google Scholar]
- 73.Burns J, Yokota T, Ashikara H, et al. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 74.Borra MT, Smith BC, Denn JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–95. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 75.Jackson JR, Ryan MJ, Hao Y, et al. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gatsrocnemius muscles of young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1572–81. doi: 10.1152/ajpregu.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmes-McNary M, Baldwin AS. Chemo-preventive properties of trans-resveratrol are associated with inhibition of activation of the IkB kinase. Cancer Res. 2000;60:3477–83. [PubMed] [Google Scholar]
- 77.Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011;301:E922–30. doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- 78.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 80.Momken I, Stevens L, Bergouignan A, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–60. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 81.Chen LL, Zhang HH, Zheng J, et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism. 2011;60:1598–609. doi: 10.1016/j.metabol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Kang W, Hong HJ, Guan J, et al. Reveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–33. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Mantzoros C, Kim YB. Resveratrol: is selectivity opening the key to therapeutic effects? Metabolism. 2012;61:289–90. doi: 10.1016/j.metabol.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting Foxo transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 85.Smith I, Alamdari N, O’Neal P, et al. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol. 2010;42:701–11. doi: 10.1016/j.biocel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mammacuri C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–6. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 87.Yang H, Mammen J, Wei W, et al. The expression and activity of C/EBPβ and δ are upregulated by dexamethasone in skeletal muscle. J Cell Physiol. 2005;204:219–26. doi: 10.1002/jcp.20278. [DOI] [PubMed] [Google Scholar]
- 88.Gonnella P, Alamdari N, Tizio S, et al. C/EBPβ regulates dexamethasone-induced muscle cell atrophy and expression of atrogin-1 and MuRF1. J Cell Biochem. 2011;112:1737–48. doi: 10.1002/jcb.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang G, Jin B, Li YP. C/EBPβ mediates tumour-induced ubiquitin ligase atrogin-1/MAFbx upregulation and muscle wasting. Embo J. 2011;30:4323–35. doi: 10.1038/emboj.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Penner CG, Gang G, Wray C, et al. The transcription factors NF-kB and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun. 2001;281:1331–6. doi: 10.1006/bbrc.2001.4497. [DOI] [PubMed] [Google Scholar]
- 91.Li YP, Reid MB. NF-kB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1165–70. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 92.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 93.Van Gammeren D, Damrauer JS, Jackman RW, et al. The IkB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009;23:362–70. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FoxO transcription factors by the Sirt1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 95.An BS, Tavera-Mendoza LE, Dimitrov V, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fukuoka M, Daitoku H, Hatta M, et al. Negative regulation of fork head transcription factor AFX (FOXO4) by CBP-induced acetylation. Int J Mol Med. 2003;12:503–8. [PubMed] [Google Scholar]
- 97.Van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–6. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Bertaggia E, Coletto L, Sandri M. Posttranslational modifications control FoxO3 activity during denervation. Am J Phsyiol Cell Physiol. 2012;302:C587–96. doi: 10.1152/ajpcell.00142.2011. [DOI] [PubMed] [Google Scholar]
- 99.Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 100.Yang Y, Hon H, Haller EM, et al. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. Embo J. 2005;24:1021–32. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813:1954–60. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1–13. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Penner G, Gang G, Sun X, et al. C/EBP DNA binding activity is upregulated by a glucocorticoid-dependent mechanism in septic muscle. Am J Physiol Regul Integr Comp Physiol. 2002;282:R439–44. doi: 10.1152/ajpregu.00512.2001. [DOI] [PubMed] [Google Scholar]
- 104.Wiper-Bergeron N, Wu D, Pope L, et al. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. Embo J. 2003;22:2135–45. doi: 10.1093/emboj/cdg218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiper-Bergeron N, Abdon Salem H, Tomlinson J, et al. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPβ by GCN5. Proc Natl Acad Sci USA. 2007;104:2703–8. doi: 10.1073/pnas.0607378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeong H, Then F, Melia TJ. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galbiati L, Mendoza-Maldonado R, Gutierrez MI, et al. Regulation of E2F-1 after DNA damage by p300-mediated acetylation and ubiquitination. Cell Cycle. 2005;4:930–9. doi: 10.4161/cc.4.7.1784. [DOI] [PubMed] [Google Scholar]
- 108.Iezzi S, Cossu G, Nervi C, et al. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. Proc Natl Acad Sci USA. 2002;99:7757–62. doi: 10.1073/pnas.112218599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Minetti GC, Colussi C, Adami R, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–50. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 110.Cohen TJ, Waddell DS, Barrientos T, et al. The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. J Biol Chem. 2007;282:33752–9. doi: 10.1074/jbc.M706268200. [DOI] [PubMed] [Google Scholar]
- 111.Tang H, Macpherson P, Marvin M, et al. A histone deacetylase 4/myogenin positive feed back loop coordinates denervation gene induction and suppression. Mol Biol Cell. 2009;20:1120–31. doi: 10.1091/mbc.E08-07-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moresi V, Williams AH, Meadows E, et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell. 2010;143:35–45. doi: 10.1016/j.cell.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGee SL, Fairlie E, Garnham AP, et al. Exercise-induced histone modifications in human skeletal muscle. J Physiol. 2009;587.24:5951–8. doi: 10.1113/jphysiol.2009.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Senf SM, Sandesara PB, Reed SA, et al. p300 acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am J Physiol Cell Physiol. 2011;300:C1490–501. doi: 10.1152/ajpcell.00255.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hasselgren PO, James JH, Benson DW, et al. Total and myofibrillar protein breakdown in different types of skeletal muscle: effects of sepsis and regulation by insulin. Metabolism. 1989;38:634–40. doi: 10.1016/0026-0495(89)90100-5. [DOI] [PubMed] [Google Scholar]