Aside from humans, songbirds have by far the most elaborate communicative sounds of any organism. Most complex of all are their songs (e.g., Fig. 1), which are learned and culturally transmitted between generations, often enriched by invention and improvisation (1–3). The specialized subsystem in the songbird brain that sustains this behavior, first described in 1976 (4), eventually became known as the song system (SS) (Fig. 2) (5, 6). One circuit, including the nuclei HVC (high vocal center) and RA (robust nucleus of the archistriatum), is essential for producing song motor patterns throughout life; the other, the anterior forebrain circuit, is more important for song development and plasticity (7–9). Electrophysiological studies have demonstrated that these circuits contain neurons active during singing and neurons responsive to the sounds of song (10–13).
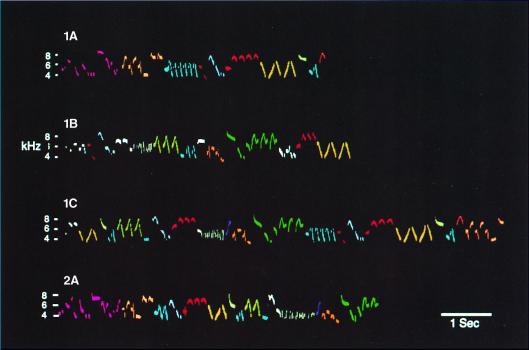
Figure 1.
The winter wren, Troglodytes troglodytes, has one of the most elaborate bird songs. A male has a repertoire of 5–10 song types, each up to 10 sec in duration. Song types share many phrases, patterned in different sequences, as in these three songs of male 1 (1 A–C). Male 2 (2A), a neighbor, shares some phrases, but arranges them differently (54).
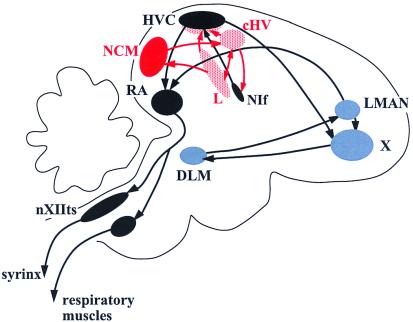
Figure 2.
A schematic cross section of the songbird brain depicting the traditionally defined song system and associated auditory areas. A network of discrete areas is devoted to song learning and production: the “motor pathway” (in black) is required throughout life for normal song production; the anterior forebrain circuit (in blue) is more important for song learning and plasticity. The NCM is one of the interconnected telecephalic auditory areas (in red), including the thalamorecipient area field L, that project directly and indirectly to the song system. For song system abbreviations, see Brenowitz et al. (3).
Methods for functional localization using activation of immediate early genes (IEGs) by song have provided information about additional brain areas that might be involved in learning to sing (14–17). In a recent issue of PNAS, Bolhuis et al. (18) have taken this work a step farther by analyzing induction of the IEGs c-fos and ZENK in the zebra finch brain in response to stimulation with the particular song with which birds were tutored. They show that tutor song, as shown previously for other songs, does not induce these IEGs within the traditional SS, but rather outside it, especially in the caudal part of the neostriatum (NCM) (Fig. 2), one of a complex network of brain regions that lie earlier in the auditory processing pathways than the conventional song nuclei (19–22). This result serves to remind us that the memorization of songs early in the learning process may involve extensive processing before internalized representations of them are ready for the transformation into a produced signal.
Both song memorization and the conversion of a song heard into a song uttered depend on hearing (ref. 23; Fig. 3), as does song maintenance after crystallization (24–27). Although the evidence seems to indicate that the SS is critical for the processes of sensorimotor integration associated with learning to produce a song, there is only limited evidence of direct involvement of the SS with tutor recognition and more general sensory aspects of vocal communication (28–31). For a time, electrophysiological studies suggested that a simple neural implementation of the song memory might reside within the SS. Neurons were found in the high vocal center (HVC), the lateral part of the magnocellular nucleus of the anterior neostriatum (LMAN), and area X that responded to sound, reacting most strongly to the bird's own song (BOS) (11, 12). But then individually invented inflections or experimentally induced abnormalities in the bird's own production, absent from the tutor song, were shown to be critical to BOS stimulus potency (11, 35), with BOS sensitivity emerging in parallel with the bird's own motor production (32–35). Thus, the evidence appears to link BOS auditory responses to the production side of the learning process.
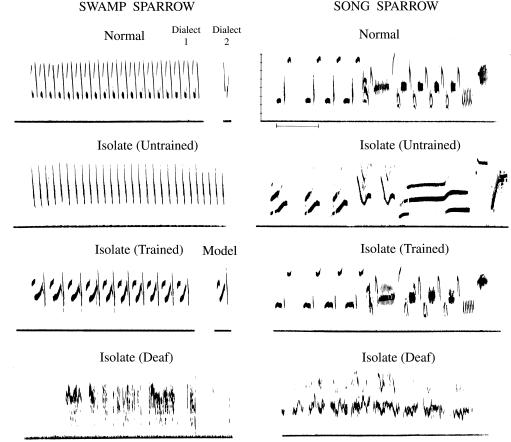
Figure 3.
Audition plays a double role in song development, for birds to hear a tutor and to listen to their own voices. At the top are normal songs of adult male swamp and song sparrows (Melospiza georgiana and M. melodia). Males raised in isolation with no song stimulation have simpler songs. Songs of males raised in isolation but tutored with tape-recorded song during the sensitive period are normal. The inset shows one syllable of the swamp sparrow tutor song. The song sparrow tutor was the normal song shown above. Much more amorphous songs are developed by males deafened before song production (55, 56). Highly degraded songs are produced after early deafening, whether the bird was tutored beforehand or not (23). Thus auditory feedback is necessary for production of both normal song and isolate song. Frequencies are marked in 1-kHz intervals. The time marker is 0.5 sec.
Curiously, auditory sensitivity to the original tutor song sometimes can be found in anterior forebrain neurons, but is seen primarily in the same neurons that respond to BOS, even when the tutor song and the BOS are quite different (35). Thus two aspects of sensory experience critical to song learning, both the tutor song and BOS, may in some sense be represented in single SS neurons. Neurons in the SS also display strong activity during singing (10, 13, 36), apparently tied to the motor act of singing, although a sensory response to BOS could be embedded within this activity. Strikingly, many SS neurons show less sensory response to song when birds are awake than when they are asleep or anesthetized (37, 38) and are thus “gated” by the bird's behavioral state, perhaps designed to be most responsive to song when the bird is singing itself.
Along the same lines, the IEGs studied thus far do not appear to be induced in the SS of awake birds by acoustic stimuli (14–17), even by the tutor song (as Bolhuis et al. report for the first time in ref. 18), but are strongly activated in SS nuclei when the bird sings (39, 40). Of course, specific genes that are responsive to acoustic stimulation in the SS may yet be identified; a song playback-induced increase in the high vocal center (HVC) of a DNA-binding protein linked to many activity-induced genes, phosphorylated CREB (cAMP response element binding protein), hints at this possibility (41). Moreover, a lack of gene expression does not necessarily indicate a lack of neuronal excitation. Nonetheless, both the electrophysiological and gene-induction data suggest that the song-responsive units in the SS are far from being simple auditory neurons, but rather reflect multiple sensory and motor aspects of song learning. This is a reminder of how complicated and intertwined sensory and motor processes are in this learned vocal behavior. In this and other respects, bird song is reminiscent of human speech (42); electrical stimulation of a single language area can affect both production and perception of speech (43), and some cortical neurons respond differently to the same word spoken by the subjects themselves, or by someone else (44).
But brain areas that process sounds of others independent of one's own vocal production also must exist. Auditory responsiveness is required not just for memorizing the tutor's song, but also for such functions as vocal identification of mates, group members, neighbors, and strangers (45, 46). Moreover, these functions are just as important to female birds as to males. In species in which only males sing, the female song system is typically much reduced (47), but there seems to be no evidence that variation in song system development correlates with general auditory recognition abilities. Instead, NCM and the other complex auditory areas identified by IEG induction (14–18), which, unlike the song system, appear equally represented and equally responsive in males and females (14, 48), seem like plausible locations for high-level auditory processing of songs.
Numerous studies have related NCM to auditory responsiveness. ZENK activation and increased neural firing have been shown in NCM in adult zebra finches of both sexes in response to song stimulation (14, 49, 50). Repeated presentation of the same individual's song leads to stimulus-specific habituation, both of IEG activation and neurophysiological responsiveness (49, 50). By both measures, substitution of a different individual's song reinstated NCM responsiveness. Evidently songs of individuals are discriminable in parts of the brain outside the classical SS, at least by this assay. Another feature of IEG induction by song in NCM is that same-species songs are more potent than songs of other species (14). This is reminiscent of inborn behavioral preferences for same-species song that influence the selectivity of song learning in many birds (51). Recognition learning also displays a degree of species-specific selectivity and an ability to learn to discriminate between same-species songs more rapidly than other-species songs (52, 53). Thus, both recognition learning and learning for vocal production could benefit from the species selectivity of NCM.
Bolhuis and colleagues (18) went beyond the issue of species specificity, however, with the important step of testing for IEG induction using vocal stimuli of known behavioral significance to their subjects. Young male zebra finches were individually tutored with tape-recorded song, kept in isolation until their own songs developed, and then restimulated with their tutor song. A correlation was discovered between the accuracy of song imitation and the degree of ZENK and c-fos activation, which was greatest in the NCM of the best imitators. The interpretation of this striking result is somewhat clouded by the lack of control birds stimulated with a nontutor song. It might be that ZENK induction in NCM reflects a predisposition for good learners to attend closely to any song stimulus. In this case the same individual differences would emerge with activation by a nontutor song; attentional differences between birds could, of course, contribute to the ability to learn well. Another crucial question is whether the gene induction seen here actually reflects BOS responses, because the songs of good learners will resemble their tutors most closely; very similar correlations of neural response with similarity to tutor song exist within the song nuclei (34). Controls with other songs as stimuli also would help to evaluate the possible influence on NCM activation of the necessarily unusual rearing of these birds: they were raised without companions much of the time, hearing only their own song and the tutor song. In normally reared birds, NCM typically is activated by any conspecific song stimulus, and it is unusual that some birds in this experiment (critical to the observed correlation) showed virtually no ZENK induction by song in NCM. If there was a decrease or delay in the development of ZENK induction by song, as has been noted in young birds raised in isolation (16), some of the birds raised by Bolhuis et al. might be unresponsive not just to the tutor, but to any song. Nonetheless, the authors' demonstration of individual differences in brain physiology that correlate with differences in a learned behavior is an important step forward.
As a final point, we note that actually learning to produce a vocal imitation differs in a number of ways from the memorization of a song, whether for recognition or production. Memorization can take place rapidly, but developing a learned song is a protracted process. It necessarily involves a series of complex interactions between motor activities, feedback from those activities, and the acquisition of skill in converging on an internalized target that may itself have undergone transformation from the original auditory inputs. This undertaking alone may be a sufficient challenge to have called forth in evolution the complexities of the avian SS, with much of the sensory processing and memorizing of songs as auditory stimuli perhaps taking place elsewhere in the brain.
Footnotes
See companion article on page 2282 in issue 5 of volume 97.
References
- 1.Kroodsma D E. Acoustic Communication in Birds, Vol. 2: Song Learning and Its Consequence. New York: Academic; 1982. pp. 1–23. [Google Scholar]
- 2.Marler P. Trends Neurosci. 1991;14:199–206. doi: 10.1016/0166-2236(91)90106-5. [DOI] [PubMed] [Google Scholar]
- 3.Brenowitz E A, Margoliash D, Nordeen K W. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- 4.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 5.Wild J M. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Bottjer S W, Johnson F. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 9.Sohrabji F, Nordeen K W, Nordeen E J. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 10.McCasland J S. J Neurosci. 1987;7:23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margoliash D. J Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doupe A J. J Neurosci. 1997;17:1147–1167. doi: 10.1523/JNEUROSCI.17-03-01147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessler N A, Doupe A J. J Neurosci. 1999;19:10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mello C V, Vicario D S, Clayton D F. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mello C V, Ribeiro S. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Clayton D F. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 17.Clayton D F. J Neurobiol. 1997;33:549–571. [PubMed] [Google Scholar]
- 18.Bolhuis J J, Zijlstra G G O, den Boer-Visser A M, Van der Zee E A. Proc Natl Acad Sci USA. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheich H, Langner G, Bonke D. J Comp Physiol. 1979;132:257–276. [Google Scholar]
- 20.Vates G E, Broome B M, Mello C V, Nottebohm F. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Wild J M, Karten H J, Frost B J. J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- 22.Fortune E S, Margoliash D. J Comp Neurol. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- 23.Konishi M. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 24.Leonardo A, Konishi M. Nature (London) 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 25.Nordeen K W, Nordeen E J. Behav Neurol Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 26.Wooley S M N, Rubel E W. J Neurosci. 1997;17:6380–6390. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okanoya K, Yamaguchi A. J Neurobiol. 1997;33:343–356. [PubMed] [Google Scholar]
- 28.Brenowitz E A. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- 29.MacDougall-Shackleton S A, Hulse S H, Ball G F. NeuroReport. 1998;9:3047–3052. doi: 10.1097/00001756-199809140-00024. [DOI] [PubMed] [Google Scholar]
- 30.Basham M E, Nordeen E J, Nordeen K W. Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 31.Scharff C, Nottebohm F, Cynx J. J Neurobiol. 1998;36:81–90. [PubMed] [Google Scholar]
- 32.Solis M M, Doupe A J. In: The Design of Animal Communication. Hauser M D, Konishi M, editors. Cambridge, MA: MIT Press; 1999. pp. 343–368. [Google Scholar]
- 33.Volman S. J Neurosci. 1993;13:4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solis M, Doupe A. J Neurosci. 1997;17:6447–6462. doi: 10.1523/JNEUROSCI.17-16-06447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solis M M, Doupe A J. J Neurosci. 1999b;19:4559–4584. doi: 10.1523/JNEUROSCI.19-11-04559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu A C, Margoliash D. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M F, Konishi M. Nat Neurosci. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- 38.Dave A S, Yu A C, Margoliash D. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 39.Jarvis E D, Nottebohm F. Proc Natl Acad Sci USA. 1997;94:4097–5102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimpo R R, Doupe A J. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi H, Wada K, Maekawa M, Watsuji T, Hagiwara M. J Neurosci. 1999;19:3973–3981. doi: 10.1523/JNEUROSCI.19-10-03973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doupe A J, Kuhl P K. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 43.Ojemann G A. J Neurosci. 1991;11:2281–2287. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Creutzfeldt O, Ojemann G, Lettich E. Exp Brain Res. 1989;77:451–489. doi: 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]
- 45.Stoddard P K. In: Ecology and Evolution of Acoustic Communication in Birds. Kroodsma D E, Miller E H, editors. Ithaca, NY: Comstock; 1996. pp. 356–374. [Google Scholar]
- 46.Searcy W A, Nowicki S. In: The Design of Animal Communication. Hauser M D, Konishi M, editors. Cambridge, MA: MIT Press; 1999. pp. 577–595. [Google Scholar]
- 47.Brenowitz E A. J Neurobiol. 1997;33:517–531. doi: 10.1002/(sici)1097-4695(19971105)33:5<517::aid-neu3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Duffy D L, Bentley G E, Ball G F. Brain Res. 1999;844:78–82. doi: 10.1016/s0006-8993(99)01915-0. [DOI] [PubMed] [Google Scholar]
- 49.Chew S J, Vicario D S, Nottebohm F. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stripling R, Volman S F, Clayton D F. J Neurosci. 1997;17:3883–3893. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marler P, Peters S. In: The Comparative Psychology of Audition: Perceiving Complex Sounds. Dooling R J, Hulse S H, editors. Hillsdale, NJ: Erblaum; 1989. pp. 243–273. [Google Scholar]
- 52.Sinnott J M. In: Comparative Psychology of Audition: Perceiving Complex Sounds. Dooling R J, Hulse S H, editors. Hillsdale, NJ: Erlbaum; 1989. pp. 447–464. [Google Scholar]
- 53.Dooling R J, Brown S D, Klump G M, Okanoya K. J Comp Psychol. 1992;106:20–28. doi: 10.1037/0735-7036.106.1.20. [DOI] [PubMed] [Google Scholar]
- 54.Kroodsma D E, Momose H. Condor. 1991;93:424–432. [Google Scholar]
- 55.Marler P, Sherman V. J Neurosci. 1983;3:517–531. doi: 10.1523/JNEUROSCI.03-03-00517.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marler P, Sherman V. Anim Behav. 1985;33:57–71. [Google Scholar]