Abstract
Background
Isoflavone compounds naturally occurring in the root of the kudzu plant have been used historically to treat alcohol-related problems. A pilot study was conducted to assess the effects of one primary isoflavone - puerarin- for its ability to modify alcohol intake in humans.
Methods
Ten (10) healthy adult volunteers were administered puerarin (1200 mg daily) in a double-blind, placebo-controlled, crossover design experiment for one week prior to an afternoon drinking session lasting 1.5 hours. Participants had access to up to six bottles of their preferred brand of beer in addition to juice and water. A time course of drinking, sip volumes, and total amount consumed were recorded.
Results
Participants consumed on average 3.5 (± 0.55) beers when treated with placebo and 2.4 (± 0.41) beers when treated with puerarin. In contrast to drinking following placebo treatment when 3 participants drank 5 beers and 1 participant drank all 6 beers, none drank 5 or 6 beers when treated with puerarin. Drinking topography also changed. When treated with puerarin, participants decreased sip size, took more sips to finish a beer, and took longer to consume each beer. Additionally, after finishing a beer, latency to opening the next beer was increased.
Conclusions
This study is the first demonstration that a single isoflavone found in the kudzu root can alter alcohol drinking in humans. These results suggest that alcohol consumption patterns are influenced by puerarin administration and this botanical medication may be a useful adjunct in the treatment of excessive alcohol intake.
Keywords: isoflavone, alcohol consumption, binge drinking, humans
1. Introduction
Although three pharmacotherapies are approved in the United States for treating alcohol abuse and dependence, none are uniformly effective, and the search for additional medications continues. Historical texts indicate that extracts of the kudzu root (Pueraria lobata) have been used in traditional Chinese herbal medicines to treat drunkenness and alcohol intoxication (Lu et al., 2009). One medication (translated from the original Chinese characters as XJL) contains not only extracts of the kudzu root but also extracts from mandarin orange peel (Citrus reticulata), five flavor berry (Schisandra chinensis), and Japanese raisin tree (Hovenia dulcis), plus ginseng, vitamin C, and sugar (Li, 1590-1596; Sun, circa 600 AD). More recent studies have systematically explored the ability of three of the major isoflavones found in the kudzu root - daidzin, daidzein, and puerarin - to reduce alcohol consumption in animal models and in humans.
Early work by Overstreet and colleagues (Overstreet et al., 1998; 1996) showed that an herbal compound containing Pueraria lobata and Citrus reticulata (NPI-028) reduced acute (one session) alcohol consumption without affecting water intake in two strains of alcohol-preferring rats when administered both parenterally (doses 0.25 to 1.0 g/kg, i.p.) and orally (doses of 1 and 1.5 g/kg). Additionally, chronic treatment of 1 g/kg, i.p. reduced alcohol intake during 5 days of treatment. Similar dose-response effects were found in African vervet monkeys given intramuscular injections of 0.18 to 0.75 g/kg. The effects of single isoflavones on alcohol consumption also have been studied. Keung and Vallee (1993) showed that daidzin reduced alcohol consumption by 50% in Syrian Golden hamsters. Heyman etal. (1996) showed a dose-related decrease in alcohol-reinforced lever pressing by daidzin-treated rats. Rats drank up to 50% less following injections of 120 mg/kg of daidzin. Two studies with puerarin have shown that this isoflavone similarly reduces alcohol consumption. Lin et al. (1996) showed a 40% reduction of ethanol intake in female alcohol preferring rats given 100 mg/kg/day for 7 days (orally, mixed in food) and a 65% reduction when the dose was increased to 300 mg/kg/day. In this same study, significant reductions in alcohol consumption of 75 and 50% were observed for daidzin and daidzein, respectively, also administered at 100 mg/kg/day. Finally, Benlhabib et al. (2004) showed a 50% suppression of alcohol intake in rats treated with 50 mg/kg puerarin. In the studies where the isoflavones were given over the course of several days, maximal suppression of alcohol intake occurs in 2 to 3 days.
An isoflavone mechanism of action for reducing alcohol consumption is unknown. Although little biochemical work has been done with puerarin, work with the structurally-related isoflavone, diadzin and its analogs, has led to several possibilities including alterations in mitochondrial aldehyde dehydrogenase (ALDH2) or through alterations of mitochondrial monoamine oxidase-acetaldehyde pathways (MAO-ALDH2; Keung, 2002; 2003). Through a blockade of biogenic amine metabolic pathways, isoflavones such as puerarin may be altering central rewarding pathways critical in the subjective and rewarding effects of alcohol (Rooke etal., 2000).
Despite the strong animal literature and the lack of uniformly effective treatments in humans, the testing of these compounds in humans has been sparse. Shebek and Rindone (2000) studied the effects of kudzu extract (isoflavone percentages not reported) on self-reported sobriety levels and cravings of patients with a diagnosis of alcoholism. No significant effects were found in assessments of up to 4 months. Lukas et al. (2005) studied the effects of a kudzu root extract that contained 25% isoflavones (19% puerarin, 4% daidzin, 2% daidzein) in heavy drinkers. Three grams of the extract (750 mg total isoflavones) were administered for one week before a 1.5 hour afternoon drinking session. In comparison to drinking following the placebo treated week, they found a significant reduction in beer consumption in addition to several significant changes in drinking topography, including an increase in the number of sips taken to finish a beer and an increase in the latency to opening subsequent beers during the drinking session. A second study where the same extract was administered for four weeks in an outpatient setting, Lukas and colleagues found a significant reduction in alcohol intake during weeks 2 through 4 (Lukas et al., submitted manuscript).
Building on these encouraging results, we repeated the in-laboratory afternoon drinking session paradigm with the single isoflavone puerarin to assess its ability to reduce short term alcohol consumption. Puerarin was selected over the other major isoflavones in the kudzu root (daidzin and daidzein) for several reasons. The safety and efficacy of puerarin have already been established in humans, especially in China where it is approved for intravenous injection as a vasodilator to treat coronary heart disease, myocardial infarction, and angina. While it is the most prevalent isoflavone in kudzu, its overall potency is lower, so its side effect profile is likely to be less. Finally, it has none of the estrogenic activity of daidzin and daidzein, and therefore would not be a complication for women.
2. Methods
2.1. Participants
Participants were recruited via newspaper and internet advertisements. Ten (10) physically and mentally healthy adult volunteers (average age of 25.8 yrs ± 3.2) who drank on average 17.6 ± 9.7 drinks per week completed the study. Eight were men and two were women. All were Caucasians, with one having a Hispanic background. None were diagnosed with current or past alcohol dependence according to a structured clinical interview for DSM-IV criteria (SCID) (First et al., 2002). Four met criteria for past alcohol abuse and one met for current alcohol abuse. One smoked tobacco cigarettes (2-3/day), 5 were regular users of caffeine (less than 300 mg/day), and one was an occasional user of marihuana. The study and related materials (protocol, informed consent, advertisements) were approved by the McLean Hospital Institutional Review Board. The study is registered at ClinicalTrials.gov (#NCT00854724). Individuals were paid for their participation.
2.2. Medication and Materials
Capsules containing 300 mg of purified puerarin (NPI-031G, 98% purity) plus 100 mg of inert filler (dried sugar beet) were prepared by Natural Pharmacia International, Inc. (Burlington, MA). Participants took 2 capsules twice a day for a total puerarin dose of 1200 mg. Puerarin was administered to participants under FDA IND #102,425 granted to the lead study investigator (DMP). Placebo capsules contained only the sugar beet filler. A third capsule containing 25 mg of riboflavin (vitamin B2) was taken at the same time as the study medications. Riboflavin causes the urine to fluoresce when viewed under ultraviolet light and was used as an immediate measure of medication compliance (Del Boca, 1996). Capsules of puerarin, placebo, and riboflavin were all identical in appearance. Drinking sessions were conducted in a laboratory room furnished to simulate a small apartment room (‘natural settings’ room). It contained an overstuffed reclining chair, end tables, bookshelves, wall hangings and pictures, lamps, carpeting, a television with DVD player, and stereo equipment. The room also contained a small kitchen area with a sink and an under-the-counter refrigerator where beer, juice, and water were kept.
2.3 Procedure
Medication was administered in a double-blind, cross-over design with the medication condition (placebo or active) in counterbalanced order across participants. Women were tested only during the follicular phase of their menstrual cycle (days 5 through 14). Medication was taken for 1 week prior to the afternoon drinking session.
Participants came individually to the laboratory on four separate occasions for a drinking session. Participants had to abstain from alcohol from at least 11 pm the prior night, and could not consume caffeinated drinks beyond 9 am of the drinking session day. Drinking sessions took place in the afternoon beginning at 4:30 pm for a period of 1.5 hours. After verification of medication compliance, a negative urine test for drugs of abuse (and pregnancy for women), and negative breath alcohol sample, participants were led to the natural settings room. They were observed on closed circuit television but otherwise did not interact with research personnel during the drinking session. Once the session began, they were free to read, watch television, and listen to music. They were instructed that if they wanted a drink, they were to go to the refrigerator, open a beverage, pour it into a glass mug and then set it on the table (in which a digital scale was hidden). They were instructed to have the mug rest on the table at all times, lifting it only to take a sip, and then to immediately replace it on the table top. They had the opportunity to drink up to six beers (of their preferred brand) in addition to juice and water, but could only have one of any type of drink open at a time. They did not have to consume the entire drink. No food was permitted during the drinking session. At the end of the session, participants gave a breath sample (for alcohol), filled out questionnaires, ate dinner, and were otherwise free to continue with their activities until at least 8:30 pm. Breath alcohol samples were taken every 30 minutes. They were released to go home by taxicab after 8:30 pm when their breath alcohol level was below 0.04% and they passed a field sobriety test.
Four separate drinking sessions were conducted. The first was a familiarization session where no medication was given to allow the participant to learn the drinking requirements and become comfortable with the surroundings. Medication (either puerarin or placebo) was begun the morning after this session. One week later, a second drinking session took place. The participants then entered a ‘washout’ phase of at least 2 weeks in duration which ended with a third drinking session. The second medication treatment week began the following morning (counterbalanced for order) followed by the fourth drinking session at the end of this second treatment week.
Once during each treatment week, participants came to the laboratory for assessment of medication compliance. Urine and blood (plasma) samples were taken and assessed for puerarin levels (Ma etal., 2005). A urine sample was again taken just before the drinking sessions.
2.4. Apparatus
Parameters of consumption in a sip-by-sip fashion were recorded through use of a custom-built end table equipped with a digital scale (Ohaus model #B10P with I5S controller) below a ceramic tile insert in the table top. The scale reader was located in an adjacent room and its output was connected to a computer. Customized software sampled the scale output at 5 Hz and could detect weight changes of greater than 1 gram. Additional details can be found in Lukas et al. (2005).
2.5. Assessments
Output from the scale allowed for an analysis of drinking behavior to include number of beers consumed, weight consumed on a sip by sip analysis, latency to opening beers, and consumption time. Immediately prior to the drinking session, participants filled out the Alcohol Urge Questionnaire (Bohn etal., 1995). This questionnaire is composed of 8 questions on a 7-point Likert scale with anchors of ‘strongly disagree’ to ‘strongly agree’ to assess three domains of the urge to drink ‘right now,’ including desire to use, expectancy of positive effects, and the inability to avoid use (Drummond et al., 2002; Farren et al., 1999). The Profile of Mood States (POMS; McNair etal., 1971) was completed immediately before and after the drinking session. A series of visual analog scales to assess changes in mood were administered before the drinking session, and periodically after the drinking session until the participant was released. Rating categories were: anxious, uncomfortable, high, clumsy, muddled/confused, slurred speech, feeling the effects of alcohol, floating, dizzy, nauseated, drunk, sleepy, terrible, great, happy, stimulated, desire to use, and desire not to use alcohol. Scales were anchored with ‘not at all’ to ‘extremely.’
During all phases of the study, participants filled out a daily diary that included self-reports of alcohol and other drug use (if any), visual analog scales of mood, sleep parameters (e.g., time to bed, awake/out of bed time), and a recording of any medication side effects. Reports of side effects were discussed and recorded in more detail during the study visits. The maximal possible number of side effects is based on the number of side effect categories that were recorded (7) multiplied by the number of study days in each phase (7) multiplied by the number of subjects participating (10). Side effects categories were: headache, cold symptoms (including sinus, runny nose, sore throat), nausea/upset stomach, lower gastrointestinal symptoms (including diarrhea), fatigue, muscle/menstrual cramps, and other.
Data were analyzed using a mixed model repeated measures analysis of variance and one-tail paired t-tests (IBM SPSS v.19 for Mac OS). One-tailed mean comparisons were used as we had strong a priori evidence to suggest that a reduction in drinking behavior would be the appropriate directional hypothesis test. A significance level was set at 0.05.
3. Results
3.1 Drinking Behavior
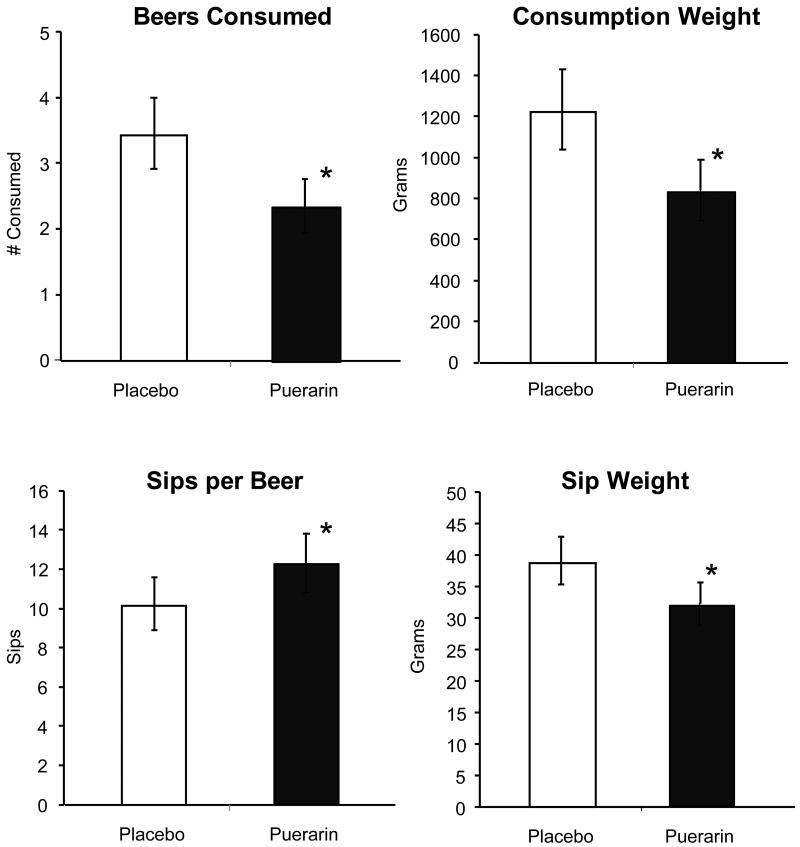
Following the placebo treatment week, participants drank an average of 3.5 ± 0.55 beers. This was significantly reduced by puerarin treatment to an average of 2.4 ±0.41 beers (t(9) = 1.93, p=.043; Figure 1). Similar significant reductions were observed in consumption weight (total weight consumed; t(9) = 1.921, p=.043). Sips taken to finish a beer increased from 10.7 ±1.5 per beer after placebo treatment to 12.4 ± 1.5 per beer after puerarin treatment (t(8) = 1.932, p=.045), and the amount (weight) of each sip decreased (37.5 ± 3.9 grams to 32.2 ± 3.5 grams; t(8) = 2.357, p=.023; Figure 1).
Figure 1.
Total number of beers consumed (upper left panel), total weight consumed (upper right panel) and 2 parameters of drinking behavior (sips taken to complete drinking a beer and the weight of each sip, (lower panels)) under placebo and puerarin treatments. Each parameter was reduced after puerarin treatment. Bars are averages ± sem.
* significantly different from placebo, p<.05.
Figure 2 (top) shows the number of subjects who drank one through six available beers. The reduction in consumption under the two medication conditions is apparent after the first beer. Following puerarin treatment, fewer participants drank the available 2 through 6 beers. No participant drank 5 or 6 beers after puerarin treatment.
Figure 2.
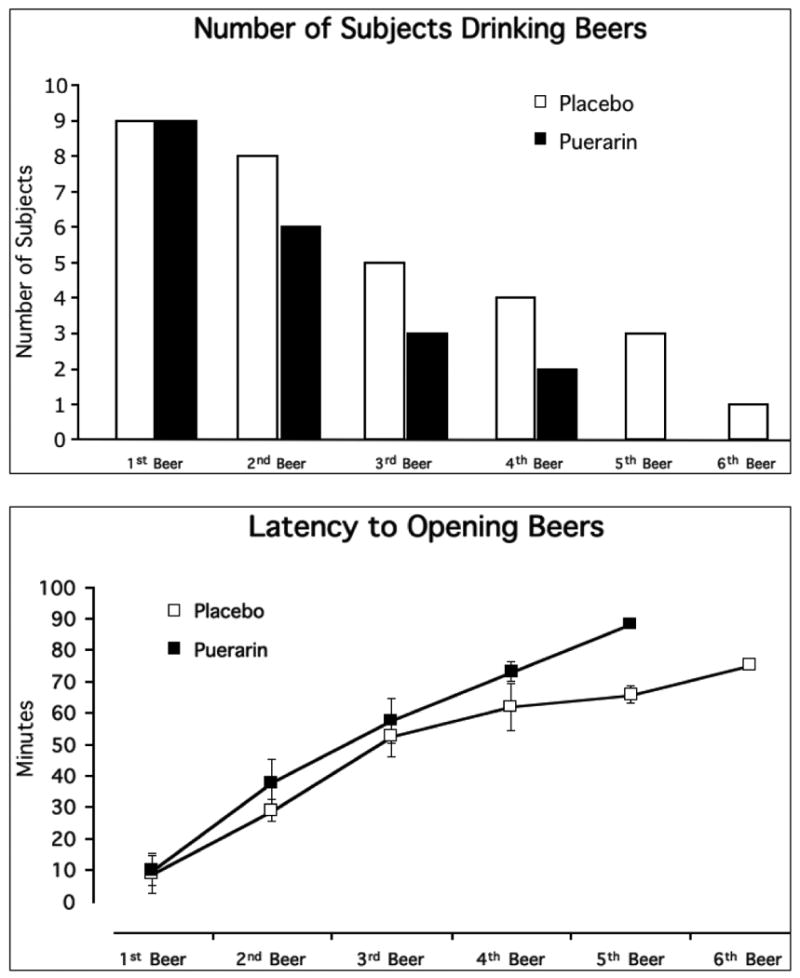
Top Panel: The number of subjects who drank each available beer during the 1.5 hour drinking session. No subject drank 5 or 6 beers following the puerarin treatment week. Bottom Panel: Latency (in minutes) to opening each available beer during the 1.5 hour drinking session. There was a trend overall for participants to take longer to open the available beers following the puerarin treatment week (p=.087).
Under puerarin treatment, participants showed a trend towards delaying the opening of each beer [F(1,51) = 3.047, p = .087] (Figure 2, bottom). This was consistent with an increase in the average time to consume an opened beer [F(1,49) = 6.170, p = .016]. Average consumption time was 18.6 ± 8.7 minutes after placebo treatment and 25.4 ± 10.4 minutes after puerarin treatment (data not shown).
Daily diary reports indicate that participants drank slightly less during the puerarin treatment week (3.0 ± 2 drinks per day) in comparison to the placebo treatment week (3.4 ±2.2 drinks per day) (t(9) = 2.112, p=.032).
3.2 Subjective Behavior
Self-reports on the daily diaries of cravings for alcohol were uniformly low (averages around 5 on a 100 point scale) and did not change throughout the study.
Assessments on the drinking session days revealed that neither total score on the Alcohol Urge Questionnaire nor responses to question #8 (specific to craving) were changed significantly by puerarin treatment. Total score under placebo conditions was 28.1 ± 10.4 and 29.3 ± 10.0 following puerarin treatment. Participants rated themselves as less fatigued (POMS subscale) overall following puerarin treatment (4.3 ± 4.1) in comparison to following placebo treat (5.8 ± 3.9) [F(1,9) = 7.232, p = .025], but there was no interaction with time (before and after drinking; data not shown). Other subscales of the POMS were not affected by puerarin treatment. No self-reports on the visual analog scales recorded just before and after the drinking sessions were affected by puerarin administration (data not shown). Several scales however did show a significant time effect (before and after drinking) indicating a direct alcohol effect. There were significant elevations after drinking in ratings of‘drunk’ [F(1,9) = 12.704, p = .006], ‘feeling the effects of alcohol’ [F(1,9) = 12.992, p = .006] and ‘floating’ [F(1,9) = 7.002, p = .027] were observed in addition to trends in reporting being more clumsy [F(1,9) = 4.8, p = .056], more uncomfortable [F(1,9) = 3.835, p = .082], and more sleepy [F(1,9) = 3.436, p = .097].
3.3. Assessment of Medication Side Effects
Mild side effects such as headache, cold symptoms, and stomach/gastrointestinal distress were reported by the participants. The incidence of these effects during the placebo phase was 10/490 (2%) and 9/490 (1.8%) during the puerarin phase of the study.
3.4. Medication Compliance and Puerarin Assays
All mid week urine samples taken during the medication weeks as well as urine samples taken just before the drinking sessions fluoresced confirming that the participants were taking their medication. All urine and plasma samples contained puerarin during th< active medication week, and none did during the placebo treatment week. Qualitative analysis of the urine samples confirmed the existence of puerarin. Quantitative analysis of plasma revealed an average level of 61.8 ± 31.1 ng/mL, with a range of 10.42 to 101.65 ng/mL during the puerarin medication week.
4. Discussion
This pilot study is believed to be the first demonstration that a single isoflavone -puerarin - found in the kudzu root can reduce human alcohol consumption and alter drinking patterns during a laboratory simulation of a binge drinking opportunity. The results extend previous work showing that isoflavones, including puerarin, found in the kudzu root are biologically active and can reduce alcohol consumption in a variety of animal models and in humans (Heyman et al., 1996; Lin et al., 1996; Lukas et al., 2005; Overstreet et al., 1996).
The measures used in this study were designed to describe not only the overall amount of beer consumed, but to allow for measures of drinking topography. For all measures analyzed, the results were consistent with both a reduction and a ‘slowing down’ of drinking. It is important to point out that there was not a cessation of drinking or a reduction to extremely low levels. Participants continued to drink moderate amounts following puerarin treatment. This is consistent with previous animal models of alcohol intake following isoflavone administration (Benlhabib etal., 2004; Overstreetetal., 1998) and with our previous work with the kudzu extract in humans (Lukas et al., 2005). Participants continued to report typical alcohol effects. Subjective reports of‘drunk,’ ‘floating,’ etc., were elevated by alcohol consumption, and these effects were not altered by puerarin treatment. There was no evidence that dysphoric alcohol effects increased (e.g., nausea, dizziness, feeling terrible). Also consistent with our previous work, subjective reports of craving or desire to drink were not altered by puerarin administration. This strengthens the hypothesis that isoflavones are not reducing drinking by altering the subjective/perceived effects of alcohol (Penetar etal., 2011).
Although this study was not specifically designed to assess the effects of puerarin on drinking as participants attend to their daily activities, analysis of the daily diary entries suggest that puerarin reduced daily drinking during the active medication week. These are encouraging results and are consistent with a 4-week outpatient study conducted with the kudzu root which found a 50% reduction of daily drinking overall, and a significant increase in abstinent days (Lukas et al., submitted manuscript). The initial results with puerarin here provide further evidence that puerarin is an active isoflavone component of the kudzu root and may be useful in reducing alcohol consumption in humans.
As mentioned, the mechanism of action for puerarin's antidipsotropic effect is unknown. Most previous work has been done using animal models with isoflavones other than puerarin and has centered on mitochondrial ALDH2 pathways or MAO-ALDH2 pathways in the liver using animal models (Gao et al., 2001; Keung, 2003). Alterations in the human mitochondrial pathway and effects on central nervous system tissues are only speculative at the present time. Accumulations of catecholamine intermediaries through MAO-ALDH2 inhibition and their effects on brain reward pathways mediating alcohol intake need to be explored.
The dose of the kudzu root extract used in our previous studies given three times a day provides 750 mg of three isoflavones: 570 mg puerarin, 120 mg daidzin, and 60 mg daidzein. We showed that this dose produced a steady state level of plasma puerarin at approximately 41 ng/mL and speculated that a therapeutic range may be 25 to 60 ng/mL (Penetar et al., 2006). Daidzin and daidzein levels were not measured. The current study using puerarin alone produced plasma puerarin levels averaging 62 ng/mL. Given that puerarin has been found in animal studies to be somewhat less potent than daidzin and daidzein in reducing alcohol consumption (Lin et al., 1996), the higher concentration of puerarin produced by the 1200 mg daily dose used here appears to add further information in determining the therapeutic range for decreasing alcohol intake in humans. Additional studies are needed to fully document puerarin's dose-response effects.
Several limitations of the study should be noted. As discussed, additional studies are needed for a dose-response assessment of puerarin's effects. Additionally, the sample size was small; however, power to detect an effect was improved through the use of a cross-over design where each of the participants acted as their own control. The subject population was restricted to moderate to heavy drinkers who did not meet diagnostic criteria for alcohol dependence. Thus the generalizability of our results to a dependent population remains an area for additional research. Finally, this study concentrated on drinking behavior in a laboratory setting and did not assess a full range of alcohol's effects.
In conclusion, this pilot study indicates that puerarin given alone suppresses alcohol consumption in humans. Given the historic herbal medicine literature of kudzu root extracts and the more recent animal and human studies, the evidence suggests that isoflavones found in the kudzu root can reduce alcohol consumption and do so without adverse effects. The further development of this botanical medication for the treatment of excessive alcohol consumption may lead to additional pharmacotherapies to treat alcohol abuse and dependence.
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIAAA SBIR grant 5R44-AA-015220 to Natural Pharmacia International, Inc., with Dr. Liu as Principal Investigator. The NIAAA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: David Penetar and Scott Lukas designed the study, wrote the protocol, analyzed the data, and wrote the manuscript.
Lindsay Toto and Stacey Farmer recruited the participants, conducted the test sessions, entered and performed the initial data analysis, and maintained the records.
Yanze Liu is the principal investigator of the SBIR grant.
Zhongze Ma performed the puerarin assays.
David Lee provided the toxicological and pharmacological analyses to obtain the IND.
All authors contributed to and approved the final manuscript.
Conflict of Interest: David Penetar holds the Investigational New Drug application #102,425 for puerarin. Yanze Liu was employed by Natural Pharmacia International, Inc. during the conduct of this study.
David Lee is a member of the scientific board at Natural Pharmacia International, Inc.
Lindsay Toto, Stacey Farmer, Zhongze Ma, and Scott Lukas have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benlhabib E, Baker JI, Keyler DE, Singh AK. Effects of purified puerarin on voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J Med Food. 2004;7:180–186. doi: 10.1089/1096620041224102. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Phillips TS. Alcohol urges in alcohol-dependent drinkers: further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction. 2002;97:1465–1472. doi: 10.1046/j.1360-0443.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- Farren CK, O'Malley S, Grebski G, Maniar S, Porter M, Kreek MJ. Variable dose naltrexone-induced hypothalamic-pituitary-adrenal stimulation in abstinent alcoholics: a preliminary study. Alcohol Clin Exp Res. 1999;23:502–508. [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (research version) Biometrics Research, NY State Psychiatric Institute; New York: 2002. [Google Scholar]
- Gao GY, Li DJ, Keung WM. Synthesis of potential antidipsotropic isoflavones: inhibitors of the mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway. J Med Chem. 2001;44:3320–3328. doi: 10.1021/jm0101390. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Keung WM, Vallee BL. Daidzin decreases ethanol consumption in rats. Alcohol Clin Exp Res. 1996;20:1083–1087. doi: 10.1111/j.1530-0277.1996.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Keung WM. Preclinical Studies of Kudzu (Pueraria lobata) as a Treatment for Alcohol Abuse. In: Keung WM, editor. Pueraria: The genus Pueraria. Taylor & Francis; New York: 2002. pp. 144–158. [Google Scholar]
- Keung WM. Anti-dipsotropic isoflavones: the potential therapeutic agents for alcohol dependence. Med Res Rev. 2003;23:669–696. doi: 10.1002/med.10049. [DOI] [PubMed] [Google Scholar]
- Keung WM, Vallee BL. Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc Natl Acad Sci U S A. 1993;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S.C., 1590-1596. Ben Cho Gang Mu (1590-1596 A.D.).
- Lin RC, Guthrie S, Xie CY, Mai K, Lee DY, Lumeng L, Li TK. Isoflavonoid compounds extracted from Pueraria lobata suppress alcohol preference in a pharmacogenetic rat model of alcoholism. Alcohol Clin Exp Res. 1996;20:659–663. doi: 10.1111/j.1530-0277.1996.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Y, Zhu W, Shi J, Ling W, Kosten TR. Traditional medicine in the treatment of drug addiction. Am J Drug Alcohol Abuse. 2009;35:1–11. doi: 10.1080/00952990802455469. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Berko J, Vicens L, Palmer C, Mallya G, Macklin EA, Lee DYW. An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol Clin Exp Res. 2005;29:756–762. doi: 10.1097/01.alc.0000163499.64347.92. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Su Z, G T, Maywalt M, Tracy M, Rodolico J, Palmer C, Ma Z, Lee DYW. A standardized kudzu extract (NPI-031) reduces alcohol consumption in non treatment-seeking male heavy drinkers. doi: 10.1007/s00213-012-2884-9. submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wu Q, Lee DYW, Tracy M, Lukas SE. Determination of puerarin in human plasma by high performance liquid chromatography. J Chromatog B, Analyt Technol Biomed Life Sci. 2005;823:108–114. doi: 10.1016/j.jchromb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Overstreet DH, Lee DYW, Chen YT, Rezvani AH. The Chinese herbal medicine NPI-028 suppresses alcohol intake in alcohol-preferring rats and monkeys without inducing taste aversion. J Perfusion. 1998;11:381–389. [Google Scholar]
- Overstreet DH, Lee DYW, Rezvani AH, Pei YH, Criswell HE, Janowsky DS. Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Penetar DM, MacLean RR, McNeil JF, Lukas SE. Kudzu extract treatment does not increase the intoxicating effects of acute alcohol in human volunteers. Alcohol Clin Exp Res. 2011;35:726–734. doi: 10.1111/j.1530-0277.2010.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Teter CJ, Ma Z, Tracy M, Lee DYW, Lukas SE. Pharmacokinetic profile of the isoflavone puerarin after acute and repeated administration of a novel kudzu extract to human volunteers. J Altern Complement Med. 2006;12:543–548. doi: 10.1089/acm.2006.12.543. [DOI] [PubMed] [Google Scholar]
- Rooke N, Li DJ, Li J, Keung WM. The mitochondrial monoamine oxidase- aldehyde dehydrogenase pathway: a potential site of action of daidzin. J Med Chem. 2000;43:4169–4179. doi: 10.1021/jm990614i. [DOI] [PubMed] [Google Scholar]
- Shebek J, Rindone JP. A pilot study exploring the effect of kudzu root on the drinking habits of patients with chronic alcoholism. J Altern Complement Med. 2000;6:45–48. doi: 10.1089/acm.2000.6.45. [DOI] [PubMed] [Google Scholar]
- Sun, S. M., circa 600 AD. Beiji-Quianjin-Yaofang.