Fibrosis is a pathologic process in which deposition of abnormal extracellular matrix by fibroblasts leads to the loss of normal tissue structure and function. In skin, fibrosis is associated with several diseases, including systemic sclerosis, localized scleroderma, nephrogenic systemic fibrosis, and keloid scars. Currently, effective targeted therapies to reverse or prevent fibrosis are lacking.
Canonical Wnt/β-catenin signaling is essential for survival and specification of dermal fibroblasts during development (Ohtola et al., 2008) and has recently been implicated as a pro-fibrotic pathway in skin (Lam and Gottardi, 2011). Central to this pathway is the nuclear translocation of stabilized β-catenin for transcriptional regulation of cell type-specific target genes (van Amerongen and Nusse, 2009). Importantly, stabilization of β-catenin depends upon inhibition of GSK3-β via canonical Wnt signaling or indirect mechanisms (Bowley et al., 2007). Evidence for aberrantly increased Wnt/β-catenin signaling activity has been observed in systemic sclerosis skin, keloid scars, and desmoid tumors as well as in the tight-skin (tsk1/+), bleomycin-induced, and GSK3-β inhibitor-induced mouse models of skin fibrosis (Akhmetshina et al., 2012; Alman et al., 1997; Bayle et al., 2008; Beyer et al., 2012; Bowley et al., 2007; Sato, 2006; Wei et al., 2012). Wnt signaling activation has also been implicated in thickening of the tsk1/+ mouse hypodermis region and in transdifferentiation of adipocytes to myofibroblasts leading to skin fibrosis (Akhmetshina et al., 2012; Bayle et al., 2008; Wei et al., 2011), but the role of hypodermal fibroblasts in skin fibrosis remains unclear. Recent studies have uncovered a potential pro-fibrotic mechanism for Wnt signaling by which a complex interplay between β-catenin and TGF-β signaling regulates type I collagen alpha-1 chain (Col1a1) mRNA expression in fibroblasts in vitro and collagen accumulation in the skin of experimental models of fibrosis (Akhmetshina et al., 2012; Wei et al., 2012). Collectively, these data demonstrate that the Wnt/β-catenin pathway can interact with TGF-β signaling to mediate fibrosis.
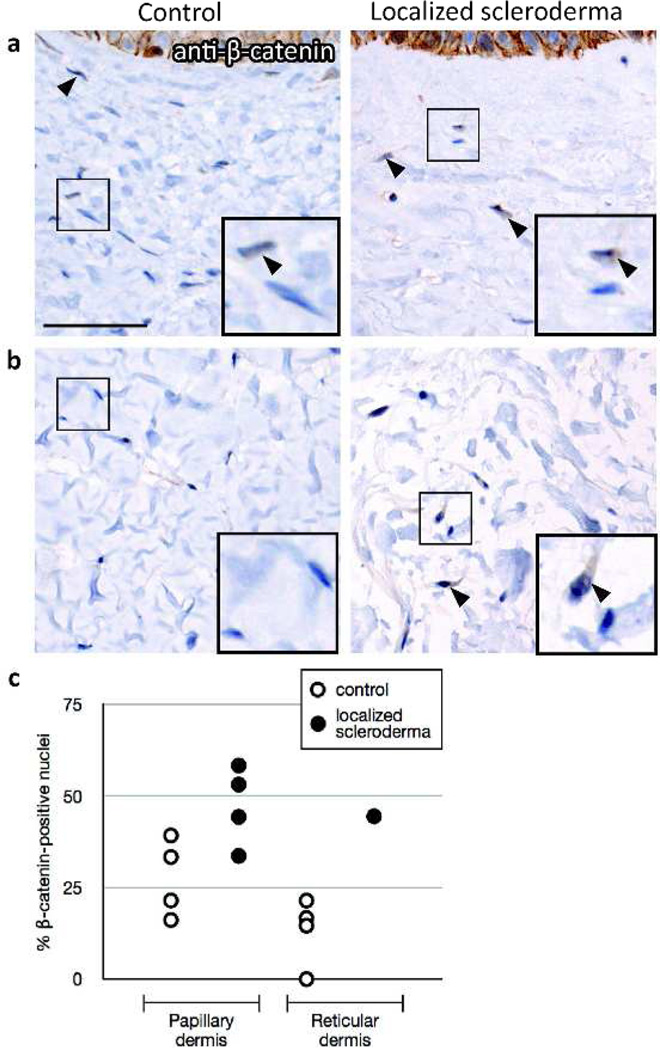
Here, we show that skin affected by localized scleroderma (morphea) has an increased percentage of nuclear β-catenin-positive dermal fibroblasts compared to healthy control skin in papillary (n=4, p=0.04; Figure 1a,c) and reticular dermis (n=1; Figure 1b,c). This finding, which to our knowledge is previously unreported, is consistent with the recent observation of increased nuclear β-catenin in dermal fibroblasts of patients with systemic sclerosis (Beyer et al., 2012; Wei et al., 2012). Thus, our data contribute to the growing body of evidence that supports a common role for β-catenin activity in dermal fibroblasts across multiple fibrotic skin diseases.
Figure 1.
Nuclear β-catenin immunoreactivity in skin affected by localized scleroderma. Brightfield immunohistochemistry using anti-β-catenin antibody (BD Bioscience, 1:100) was performed on sections of affected skin from 4 localized scleroderma patients and normal skin from 4 healthy controls. (a) Representative images with insets showing detail from papillary dermis of healthy control and localized scleroderma affected skin. β-catenin-positive nuclei indicated by filled arrowheads. (b) Reticular dermis of control (representative image) and localized scleroderma skin that had fibroblasts in reticular dermis. (c) The percentage of nuclear β-catenin-positive fibroblasts in papillary and reticular dermis was calculated from 2–3 high-power fields for each sample. Scale bar length 50 µm.
On the basis of this observation, we investigated the sufficiency of sustained dermal fibroblast beta-catenin activity for fibrogenesis. We used the tamoxifen-dependent HoxB6Cre (HoxB6Cre-ERT1) line to conditionally activate the R26R-YFP lineage tracer and remove exon 3 of endogenous β-catenin (β-cateninstab/+), resulting in the stabilization of β-catenin in ventral dermal fibroblasts (Harada et al., 1999; Srinivas et al., 2001; Nguyen et al., 2009). Pregnant mice carrying control HoxB6Cre-ERT1/+; R26R-YFP/+ and HoxB6Cre-ERT1/+; R26R-YFP/+; β-cateninstab/+ conditional mutant embryos were given one dose (3mg/40g body weight) of tamoxifen at E15.5, E16.5, or E17.5 to induce expression of stabilized β-catenin and YFP. YFP-positive recombined cells contributed to fibroblasts in the dermis and hypodermis and not to epidermis, skeletal muscle, adipose, or endothelial lineages in postnatal ventral skin (Figure 2a, data not shown). HoxB6Cre-ERT1; R26R-YFP; β-cateninstab/+ mutant cells showed a morphology consistent with fibroblasts in the dermis and the hypodermis until the latest time point analyzed (P50; Figure S1a).
Figure 2.
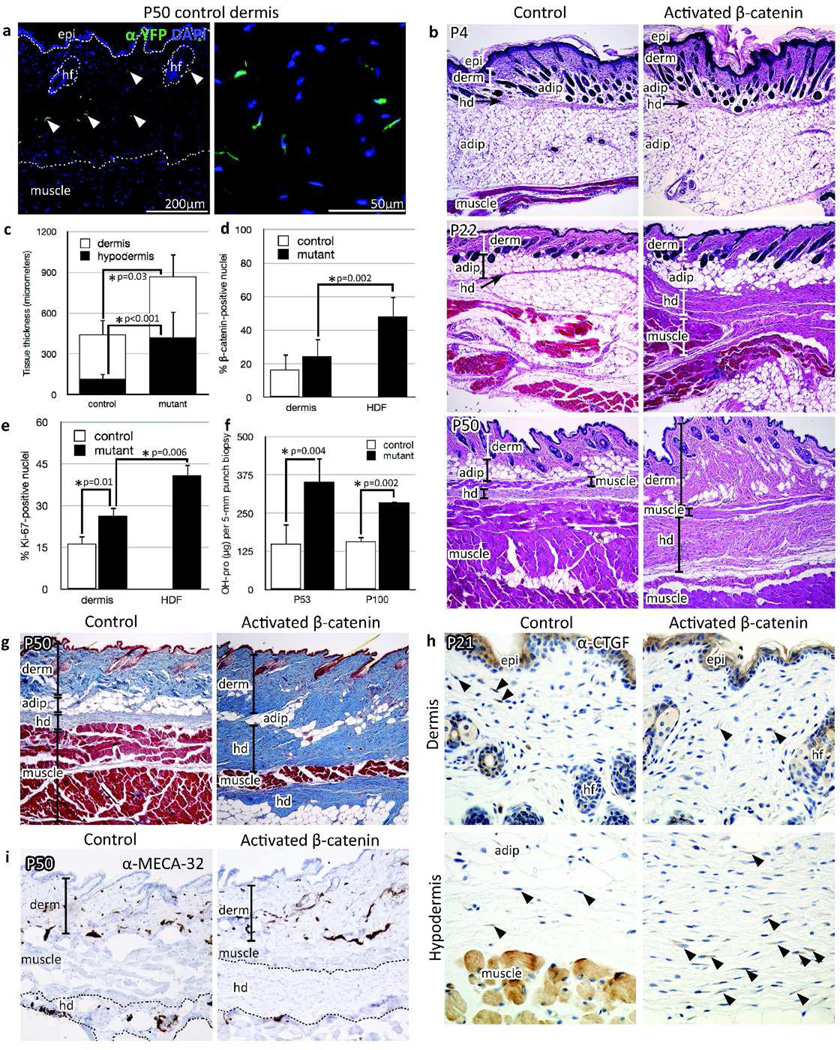
Characterization of fibrotic skin phenotype. (a) Immunofluorescence using anti-GFP antibody against YFP, P50 (Aves Labs, 1:250). epi, epidermis; hf, hair follicle. (b) H&E-stained skin at P4, P22, and P50. hd, hypodermis; adip, adipose. (c) P50 dermal and hypodermal thickness quantified by Photoshop measurement tool; n = 15 controls, 15 mutants. (d) Percentage of nuclear β-catenin-positive fibroblasts in P50 skin; n = 7 controls, 7 mutants; p=0.002. HDF, hypodermal fibrosis. (e) P22 cell proliferation assessed by anti-Ki67 immunofluorescence (Abcam 15580, 1:500); n = 3 controls, 3 mutants. (f) Hydroxyproline content per 5-mm punch biopsy of P50 and P100 control and mutant ventral skin. (g) Masson’s trichrome stain of P50 skin. (h) Anti-CTGF (Abcam 6992, 1:400) brightfield immunohistochemistry performed on P21 skin (n=5 each controls and mutants). Arrowheads indicate CTGF-positive cells. (i) Brightfield immunohistochemistry of endothelial cells in P50 skin (anti-MECA-32 antibody, Developmental Studies Hybridoma Bank, 1:10). All corresponding images were photographed at same magnification. Quantification of marker expression based on 3 high-power fields/sample.
HoxB6Cre-ERT1/+; R26YFP/+; β-cateninstab/+ mutant skin displayed a progressive increase in relative thickness of the mutant dermis and hypodermis compared to HoxB6Cre-ERT1/+; R26YFP/+ control skin. While the thickened mutant dermis appeared histologically similar to age-matched controls, the mutant hypodermal tissue was characterized by invasion of adjacent subcutaneous muscle and adipose tissue by cords of spindle-shaped cells (Figure 2b and S1b). Measurements of mutant skin sections showed 1.4-fold thicker dermis and dramatic 3.8-fold expansion of hypodermal thickness compared to littermate controls (Figure 2c). There was a significantly greater percentage of nuclear β-catenin positive fibroblasts in mutant dermis and hypodermis compared to control dermis (Figure 2d and S1c). Fibroblast proliferation was significantly increased in mutant hypodermis compared to the control and mutant dermis (Figure 2e).
We next proceeded to characterize the thickened dermis and hypodermis in the activated β-catenin mutant skin. Quantification of collagen by hydroxyproline assay demonstrated 2.3- and 1.8-fold increases in collagen content of mutant skin at P50 and P100, respectively (Figure 2f). Accordingly, collagen accumulation was apparent in the mutant dermis and hypodermis by Masson’s trichrome stain (Figure 2g). CTGF/CCN2, a marker of fibrosis (Holmes et al., 2001), was expressed by dermal and hypodermal fibroblasts of P21 control and mutant skin, and was higher in the expanded mutant hypodermis (30.9 ± 8.7% of fibroblasts) compared to the overlying dermis (17.8 ± 5.1%; p=0.01) (Figure 2h). While there was no significant difference in the quantity or appearance of blood vessels between P50 control and mutant dermis (quantification not shown), there was a pronounced absence of blood vessels in the thickened mutant hypodermis by MECA-32 immunohistochemistry (Figure 2i).
In conclusion, taken together with previous observations in other fibrotic skin diseases, increased nuclear β-catenin immunoreactivity in skin affected by localized scleroderma strongly suggests a pro-fibrotic role for β-catenin in dermal fibroblasts. Our in vivo model of activated β-catenin in skin fibroblasts holds important implications for several human diseases that involve skin fibrosis, and our findings are consistent with recent studies (Akhmetshina et al., 2012; Beyer et al., 2012; Wei et al., 2012). We have shown that restricted constitutive activation of β-catenin in a subset of mouse skin fibroblasts is sufficient for spontaneous, progressive thickening of hypodermis and dermis accompanied by increased cell proliferation. In particular, the increased percentage of proliferating and CTGF-expressing fibroblasts in the hypodermal region compared to the overlying dermis of the activated β-catenin mutant skin demonstrates that β-catenin activity in a distinct subpopulation of fibroblasts can substantially contribute to an overt skin fibrosis phenotype. This finding expands upon previous studies that have suggested a role for Wnt/β-catenin in the hypodermis of fibrotic skin (Akhmetshina et al., 2012; Bayle et al., 2008; Wei et al., 2011). Additional studies are required to determine the genetic interactions between β-catenin and other pro-fibrotic signaling pathways during the development of β-catenin-dependent skin fibrosis. We will utilize the activated β-catenin model to define interactions between β-catenin and other important mediators of fibrosis like TGF-β/Smad2/3, CTGF, PTEN, and MAPK/ERK and to identify target genes that functionally mediate the pro-fibrotic effects of β-catenin activation. Ultimately, these studies will be critical for developing novel therapeutic approaches to prevent and treat fibrotic skin diseases.
Supplementary Material
Acknowledgements
We thank David Carrino, Peggy Myung, John Varga, and members of the Atit lab for valuable discussions and critically reading the manuscript; Amad Awadallah and Andrew Jarrell for technical assistance; and Daniel Geisler, Kord Honda, Susan Mackem, Makoto M. Taketo, and Candace Matheny for their contributions. This work was supported in part by NIH-NIDCR grant R01-DE01870 (R.A.), Basil O’Connor Award from the March of Dimes (R.A.), Scleroderma Research Foundation (R.A.), NIH T32 GM07250 (E.H.), and NIH TL1 RR02499 (E.H.). All animal work was done in compliance with IACUC regulations at Case Western Reserve University. Access to archived skin biopsies from localized scleroderma patients was obtained in compliance with Case Western Reserve University Institutional Review Board for Human Studies.
Abbreviations
- P
postnatal
- E
embryonic
- CTGF
connective tissue growth factor
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Comms. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alman BA, Li C, Pajerski ME, et al. Increased β-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329. [PMC free article] [PubMed] [Google Scholar]
- Bayle J, Fitch J, Jacobsen K, et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Akhmetshina A, Dees C, et al. Inhibition of glycogen synthase kinase 3β induces dermal fibrosis by activation of the canonical Wnt pathway. Ann Rheum Dis. 2011;70:2191–2198. doi: 10.1136/ard.2010.147140. [DOI] [PubMed] [Google Scholar]
- Beyer C, Schramm A, Akhmetshina A, et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-200568. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowley E, O'Gorman DB, Gan BS. β-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141–150. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Lam AP, Gottardi CJ. β-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23:562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M-T, Zhu J, Nakamura E, et al. Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud. Dev Dyn. 2009;238:467–474. doi: 10.1002/dvdy.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtola J, Myers J, Akhtar-Zaidi B, et al. β-catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. Upregulation of the Wnt/β-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Wei J, Fang F, Lam AP, et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012 doi: 10.1002/art.34424. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: A novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.