Abstract
Background
Pentoxifylline (PTX) improved histological features of nonalcoholic steatohepatitis (NASH) in a recent randomized placebo-controlled trial. However, the underlying mechanism responsible for the beneficial effects of PTX in NASH remains unidentified. A key role of lipid oxidation in the pathogenesis and progression of NASH has been established. PTX is known to decrease free-radical mediated oxidative stress and inhibit lipid oxidation. The primary aim of this study was to evaluate the effects of PTX on levels of lipid oxidation products in patients with NASH.
Methods
Levels of multiple structurally specific oxidized fatty acids including hydroxy-octadecadenoic acids (HODEs), oxo-octadecadenoic acids (oxoODEs), and hydroxy-eicosatetraenoic acids (HETEs) were quantified by mass spectrometry in plasma obtained at baseline and at study completion in patients who completed 1 year of therapy with PTX or placebo in a randomized controlled trial.
Results
Therapy with PTX resulted in significant decreases on 9-HODE and 13-oxoODE, oxidized lipid products of linoleic acid (LA) linked to histological severity in NAFLD. Similarly, PTX therapy was associated with significant decreases in 8-HETE, 9-HETE, and 11-HETE compared to placebo. Statistically significant correlations were demonstrated between the decrease in HODEs and oxoODEs and improved histological scores of fibrosis; and between the decrease in HETEs and improved lobular inflammation.
Conclusion
Therapy with PTX compared to placebo was associated with a significant reduction of oxidized fatty acids. This novel evidence supports that the beneficial effects of PTX in patients with NASH are likely partly mediated through decreasing lipid oxidation, largely free-radical mediated lipid oxidation. Additionally, this is the first report on the link between decreased oxidized lipid products and improved histological disease in the setting of a therapeutic trial in NASH.
Keywords: nonalcoholic fatty liver disease, pentoxifylline, clinical trials, oxidative stress
One in three adults in the United States has nonalcoholic fatty liver disease (NAFLD)(1). Nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD, is characterized by hepatocellular damage, inflammation, and liver fibrosis that can progress to cirrhosis (2, 3). The key role of oxidative stress (OS) in the injury and progression of NASH is well established (4–7). Multiple studies have documented the increase in circulating products of oxidative stress and lipid oxidation in patients with NASH (8–11). A recent study of the plasma lipidomic signature in NAFLD and NASH showed that NAFLD is associated with several alterations in the circulating lipidome, and that progression to NASH is characterized by increased lipoxygenase metabolites and specific elevation in products of nonenzymatic (free-radical mediated) oxidation of arachidonic acid (AA), indicative of enhanced OS (10). A separate study demonstrated that oxidized lipid products of free-radical oxidation of linoleic acid (LA) are also elevated in patients with NASH compared to those with NAFLD without NASH and controls (11).
In a recently published randomized controlled trial, therapy with pentoxifylline (PTX) for 1 year improved histological features of NASH compared to placebo (12). In that study, significant associations were demonstrated between PTX therapy and improvement in overall NALFD activity score (NAS), and individual scores of steatosis, lobular inflammation, and fibrosis (12). The beneficial effects of PTX in the histological features of NASH, particularly on liver fibrosis, have been further corroborated in an expanded cohort combining two randomized controlled trials (13). Although multiple potential underlying processes have been investigated, the specific mechanism mediating the beneficial effects of PTX in NASH remains unknown. No association was demonstrated between therapy with PTX and changes on insulin sensitivity measured by frequently sampled intra-venous glucose tolerance testing, on levels of tumor necrosis factor alpha (TNFa), or on liver cell apoptosis assessed by TUNEL staining (12).
PTX is a methylxanthine derivative that increases red blood cell flexibility, reduces blood viscosity, and decreases platelet aggregation (14). Additionally, PTX has anti-inflammatory properties and it is known to specifically suppress TNFa gene transcription, preventing TNFa’s synthesis (15–17). However, PTX also possesses key effects independent of TNFa. Among these, PTX is known to decrease oxidative stress (18–21). PTX has been shown to have hydroxyl and peroxyl radical scavenging effects (22, 23), and specifically inhibits lipid peroxidation (18, 23–25).
The primary aim of this study was to determine the effects of PTX compared placebo on the circulating levels of lipid oxidation products of LA and of AA, including hydroxy-octadecadenoic acids (HODEs), oxo-octadecadenoic acids (oxoODEs) and hydroxy-eicosatetraenoic acids (HETEs), in patients with NASH.
Patients and Methods
Patient population
Patients were entered in this study according to criteria followed for our double-blinded, randomized, placebo-controlled trial of pentoxifylline in NASH (12). The patient population for this study consisted of subjects with NASH who completed the one-year trial of pentoxifylline compared to placebo (12). This study was approved by the Institutional Review Boards at the involved institutions, the Cleveland Clinic and the Louis Stokes Cleveland Veterans Affairs Medical Center. Enrollment in the trial occurred between December 2006 and February 2009. All participants in that randomized controlled trial provided written informed consent for participation and for future studies in stored plasma and serum samples. The trial was completed in April 2010.
Inclusion criteria
The diagnosis of NASH was based on liver biopsy performed within the six months prior to entry into the study. Other inclusion criteria were (1) daily alcohol intake of <30 g for males and <15 g for females; (2) appropriate exclusion of other liver diseases; (3) age between 18 and 70 years and (4) the ability to provide informed consent. Patients with diabetes mellitus type 2 (DM) were included only if their therapeutic regimen was limited to oral agents including sulfonylureas (e.g. glipizide and glyburide) and/or biguanides (e.g. metformin), was stable (defined by no changes in oral agents or their dose for at least 6 months), and with relatively adequate glucose control as defined by HgbA1C < 8%.
Exclusion criteria
Patients were excluded if they had (1) history of past excessive alcohol drinking in the past 10 years; (2) evidence of any other suspected cause of liver disease by history; (3) cirrhosis defined by stage 4 fibrosis on liver biopsy or by unequivocal clinical evidence consistent with underlying cirrhosis; (4) a history of hypersensitivity to PTX or the methylxanthines; (5) a history of cerebral or retinal hemorrhage; (6) if they were taking medications known to cause steatosis; (7) taking medications showing potential benefits in previous NASH pilot studies; or (8) were taking theophylline or coumadin.
Study drug
Based on a computer-generated randomization table accessible to Research Pharmacy personnel only, patients were assigned to one of two study groups. Patients on the treatment arm received an oral dose of 400 mg three times per day of PTX for one year. In the placebo arm, identical placebo was substituted for PTX.
Assessment of liver histology
Formal review and scoring of all entry and end of study liver biopsies was performed by a dedicated liver pathologist blinded to treatment arm and who is experienced with the NAS (LY). The NAS grades NAFLD based on the unweighted sum of individual scores for steatosis, inflammation and ballooning (26). Fibrosis at entry and at the end of trial was staged 0 to 4 (0 –absent; 1 –perisinusoidal or portal/periportal only; 2 –perisinusoidal and periportal; 3 –bridging fibrosis; 4 –cirrhosis).
Plasma collection
Plasma was collected in all subjects at baseline and at study completion in the morning hours and under fasting conditions at one of the two Clinical Research Units of the Cleveland CTSA. Whole blood was drawn into a syringe and immediately transferred into a previously refrigerated EDTA tube. The tube was immediately transported on ice to the adjacent CRU Core Laboratory. Samples were centrifuged within 15 minutes of collection at 2000 × g for 10 minutes at 4 degrees Celsius. Plasma aliquots were placed into cryotubes and immediately stored at −80 degrees Celsius until analysis.
Lipid extraction from plasma
Fatty acids and oxidized fatty acids were extracted from plasma as previously described (11). Briefly, samples were thawed in ice/water bath immediately prior to sample handling. Anti-oxidant solution was added to each sample. Internal standards (synthetic 15(s)-HETE-d8; 9(s)-HODE-d4; 13(s)-HODE-d4; Linoleic Acid-d4; Arachidonic acid-d8), plasma, and potassium hydroxide were added to glass test tubes, overlaid with argon, and sealed. After hydrolysis under argon atmosphere, the released fatty acids were extracted twice into the hexane layer by liquid/liquid extraction. The combined hexane extracts were dried under nitrogen gas and re-suspended in 100 ul 85% methanol/water.
Quantification of lipid products in plasma
Levels of precursors and structurally specific fatty acid oxidation products were quantified as previously described (11, 27). Briefly, reconstituted lipid extracts were analyzed by high performance liquid chromatography (HPLC) (Cohesive Technologies Aria LX Series HPLC multiplexing system, Franklin, MA), and both the precursor fatty acids and oxidation products (HETEs, HODEs, oxoODEs) were separated through a C18 column (Phenomenex ODS (2), 2 × 150 mm, 5 um, Rancho Palos Verdes, CA) using a gradient starting from 80% Methanol (containing 2% Formic acid) for 2 minutes and then 85% Methanol for 5 minutes, followed by 100% Methanol for over 10 minutes. Quantification of precursors and oxidized fatty acids was done on a triple quadruple mass spectrometer (API 365, Applied Biosystems, Foster City, CA) with Ionics EP 10+ upgrade (Concord, Ontario, CA) using stable isotope dilution methodology and multiple reaction monitoring with characteristic parent to daughter ion transitions (27). Levels of specific hydroxy-octadecadienoic acids (HODE-9 and HODE-13), oxo-octadecadienoic acids (oxoODE-9 and 13), hydroxy-eicosatetraenoic acids (HETE-5, -8, -9, -11, -12, and -15), and the precursors LA and AA were quantified. Systematic quality control steps during testing and between batches were followed. The collaborator performing the sample processing and quantification of oxidized fatty acids by mass spectrometry was blinded to all clinical data including treatment allocation, laboratory tests results, liver histology and any other clinical information.
Assessment of insulin sensitivity
The frequently sampled intravenous glucose tolerance test (FSIVGTT)(28, 30) was completed in all subjects at baseline and at trial completion as previously described (12). After an overnight fast, 2 large bore IV catheters were placed, one in each arm. After a 30 minute rest period, dextrose 50% in a dose of 0.3 gram/kg was infused over 1 minute followed by normal saline flushes. Blood samples were drawn at -10, -5, -1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, and 19 minutes. At minute 20, a dose of 0.02 units/kg of Humulin regular insulin diluted in normal saline was administered by IV push. After the insulin infusion, blood samples were drawn at minutes 22, 24, 26, 28, 30, 33, 36, 40, 50, 60, 70, 80, 100, 120, 140, 160, and 180. The test and processing were completed at the Clinical Research Units, and the assays were run at the Human Specimen Laboratory Core of the Cleveland CTSC. YSI 2300 STAT Plus Glucose analyzer (Yellow Springs, OH) was used to measure glucose. Insulin was measured using the Human Insulin Specific assay, Millipore (Catalog #HI-14K, Billerica, MA). Acute insulin response to glucose (AIRG), insulin sensitivity index (SI), glucose effectiveness (SG), and disposition index (DI) were assessed using the MINMOD analysis program (30, 31). The AIRG represents the acute β-cell response to the glucose load calculated by the area under the curve higher than the basal insulin values. The SI measures the increase in glucose clearance rate per unit change in serum insulin concentration. The SG measures the glucose clearance rate due to the increase in glucose and independent of the insulin concentration above baseline. Finally, the DI (SI × AIRG) is an index that incorporates β-cell response and insulin sensitivity. In addition, fasting glucose and insulin were used to calculate insulin resistance according to the homeostasis model assessment technique (HOMA-IR).
Statistical Analyses
Data is presented as mean ± standard deviation (SD), median [25th, 75th percentiles] or n (%). Distribution of all continuous and ordinal variables was assessed using the Kolmogorov-Smirnov test as well as normal probability plots. The change from baseline in oxidized fatty acids was compared between treatment groups using Wilcoxon rank sum tests. Any other continuous data were analyzed by the student t test if normally distributed and by non-parametric tests otherwise. Categorical data were analyzed by the χ2 test. Any imbalances in baseline variables between groups were adjusted for in secondary analyses using analysis of covariance or logistic regression, as appropriate. Correlations between changes in oxidized fatty acids and the change in individual scores of histological parameters were evaluated using Spearman’s correlation coefficients. Multivariable modeling was used to adjust for clinically relevant variables. All statistical tests were two-sided and a p-value < 0.05 was considered statistically significant. All analyses were carried out using SAS version 9.2 (The SAS Institute, Cary, NC) and R (version 2. 13. 1, The R Institute for Statistical Computing, Vienna, Austria).
Results
Study patients
Between 2006 and 2009 a total of 55 patients with NASH were enrolled. Forty-nine patients completed the 1-year study (dropout rate 11%). Plasma for measurement of oxidized fatty acids at baseline and at the study completion was available in 47 of the 49 patients who completed the study. Characteristics at baseline of these 47 patients were similar between treatment arms including histological features of steatosis, inflammation, ballooning, and fibrosis; prevalence of relevant comorbidities including type 2 diabetes, hypertension, and hyperlipidemia; anthropomorphic measurements; Homeostatic Model Assessment (HOMA) index; and demographics (Table 1).
Table 1.
Baseline characteristics of the 47 subjects and by treatment group.
| Variable | n | Total (n=47) |
PTX (n=21) |
Placebo (n=26) |
p- value |
|---|---|---|---|---|---|
| Age at entry (yrs) | 47 | 50.1±11.2 | 50.6±12.6 | 49.8±10.1 | 0.82 |
| Male | 47 | 33(70.2) | 13(61.9) | 20(76.9) | 0.26 |
| White | 47 | 43(91.5) | 20(95.2) | 23(88.5) | 0.41 |
| BMI (kg/m2) | 47 | 33.8±5.1 | 33.1±4.7 | 34.5±5.3 | 0.36 |
| Waist (cm) | 40 | 110.7±13.6 | 109.3±15.0 | 111.8±12.7 | 0.57 |
| Diabetes Type 2 | 47 | 5(10.6) | 1(4.8) | 4(15.4) | 0.24 |
| Hypertension | 40 | 23(57.5) | 11(61.1) | 12(54.5) | 0.68 |
| Hyperlipidemia | 40 | 20(50.0) | 11(61.1) | 9(40.9) | 0.2 |
| Fasting glucose (mg/dL) | 47 | 93.0[85.0,103.0] | 90.0[84.0,95.0] | 97.0[86.0,107.0] | 0.14 |
| Acute insulin response to glucose (AIRg) | 47 | 621.2[230.7,846.2] | 455.8[196.2,899.8] | 623.3[341.7,839.4] | 0.93 |
| Disposition index (DI) | 47 | 878.7[337.1,1337.6] | 1006.4[308.9,1601.4] | 644.2[371.5,1275.1] | 0.51 |
| Insulin sensitivity index (SI) | 47 | 1.4[0.98,2.2] | 1.5[0.96,2.5] | 1.3[1.00,1.8] | 0.52 |
| Glucose effectiveness (Sg) | 47 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.7 |
| HOMA-IR | 47 | 5.7±2.8 | 5.0±2.3 | 6.3±3.1 | 0.13 |
| NAFLD activity score | 47 | 5.6±1.4 | 5.9±1.2 | 5.4±1.6 | 0.31 |
| Steatosis | 47 | 0.15 | |||
| . 1–33% | 8(17.0) | 2(9.5) | 6(23.1) | ||
| . 34–66% | 19(40.4) | 8(38.1) | 11(42.3) | ||
| . >66% | 20(42.6) | 11(52.4) | 9(34.6) | ||
| Lobular Inflammation | 47 | 0.14 | |||
| . No/minimal | 1(2.1) | 0(0.0) | 1(3.8) | ||
| . Mild | 9(19.1) | 2(9.5) | 7(26.9) | ||
| . Moderate | 31(66.0) | 16(76.2) | 15(57.7) | ||
| . Severe | 6(12.8) | 3(14.3) | 3(11.5) | ||
| Ballooning | 47 | 0.25 | |||
| . None | 1(2.1) | 0(0.0) | 1(3.8) | ||
| . Few | 23(48.9) | 13(61.9) | 10(38.5) | ||
| . Many | 23(48.9) | 8(38.1) | 15(57.7) | ||
| Fibrosis Stage | 47 | 0.9 | |||
| . 0 | 5(10.6) | 0(0.0) | 5(19.2) | ||
| . 1 | 16(34.0) | 10(47.6) | 6(23.1) | ||
| . 2 | 16(34.0) | 8(38.1) | 8(30.8) | ||
| . 3 | 10(21.3) | 3(14.3) | 7(26.9) |
n is total of subjects with nonmissing data. Data is presented as mean ± SD with t-test; median [P25, P75] with Wilcoxon rank sum test, or n (%) with Wilcoxon rank sum tests for histology scores and Pearson's chi-square test otherwise. Insulin resistance was calculated using the homeostasis model assessment for insulin resistance (HOMA-IR) according to the following formula: [glucose(mg/dL) × insulin (uU/mL)/405]. NAFLD activity score was assessed on a scale of 0 to 8 with higher scores indicating more severe disease. NAS is obtained by adding steatosis (assessed on a scale of 0 to 3), inflammation (assessed on a scale of 0 to 3) and ballooning (assessed on a scale of 0 to 2). Fibrosis stage is assessed on a scale of 0 to 4 with higher values indicating more severe disease.
Changes in oxidation products of LA with PTX treatment
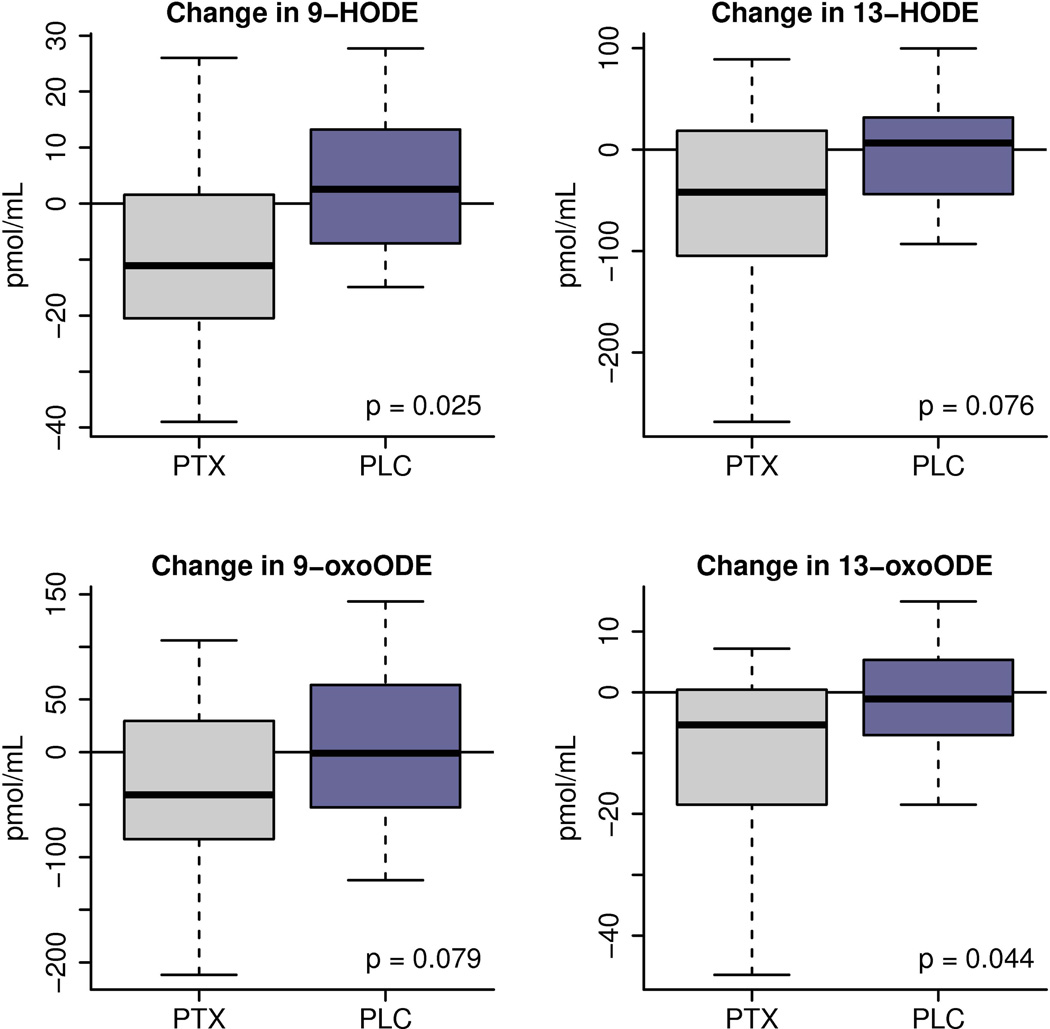
Plasma levels of structurally specific oxidation products of LA before and after treatment with PTX or placebo were quantified. At baseline, there were no statistically significant differences between treatment groups regarding oxidized lipid products levels (Table 2). Treatment of NASH patients with PTX for one year resulted in a significant decrease from baseline compared to placebo in 9-HODE (p=0.025) and 13-oxoODE (p=0.044). Decreasing trends were also noted in the additional oxidation products of LA measured, 13-HODE (p=0.076) and 9-oxoODE (p=0.079) (Figure 1).
Table 2.
Oxidation products of Linoleic Acid at baseline and change with PTX treatment
| Lipid (lipid pmol/mL) |
Baseline | Change from Baseline | ||||
|---|---|---|---|---|---|---|
| PTX (N=21) |
Placebo (N=26) |
p-value | PTX (N=21) |
Placebo (N=26) |
p-value | |
| 9-HODE | 56.8[43.4,69.3] | 44.2[39.9,57.7] | 0.18 | −11.1[−20.5,1.6] | +2.6[−7.1,13.3] | 0.025 |
| 13-HODE | 196.4[153.9,252.5] | 169.3[140.1,220.3] | 0.26 | −41.9[−104.6,18.7] | +6.6[−43.8,31.8] | 0.076 |
| 9-oxoODE | 214.7[166.2,232.8] | 187.5[132.1,251.5] | 0.29 | −40.7[−82.7,29.7] | −1.1[−52.6,63.9] | 0.079 |
| 13-oxoODE | 27.3[19.4,36.4] | 23.3[19.0,28.4] | 0.23 | −5.4[−18.5,0.418] | −1.087[−7.0,5.3] | 0.044 |
| LA (pmol/mL× 103) | 1160.0[1027.2,132] | 1014.4[944.8,1161.6] | 0.10 | −42.5[−108.70,68.3] | −4.1[−104.5,81.2] | 0.4 |
Values presented as Median [P25, P75] with Wilcoxon rank sum tests
Figure 1.
Changes from baseline after one year of therapy (PTX vs placebo) in levels of hydroxy-octadecadenoic acids (HODEs) and oxo-octadecadenoic acids (oxo-ODEs), oxidized products of the specific precursor Linoleic Acid. The box-whisker plot is represented with the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, and the upper boundary of the box indicating the 75th percentile. The whiskers extend to the most extreme data points. The p-values represent the difference between treatment groups.
Changes in oxidation products of AA with PTX treatment
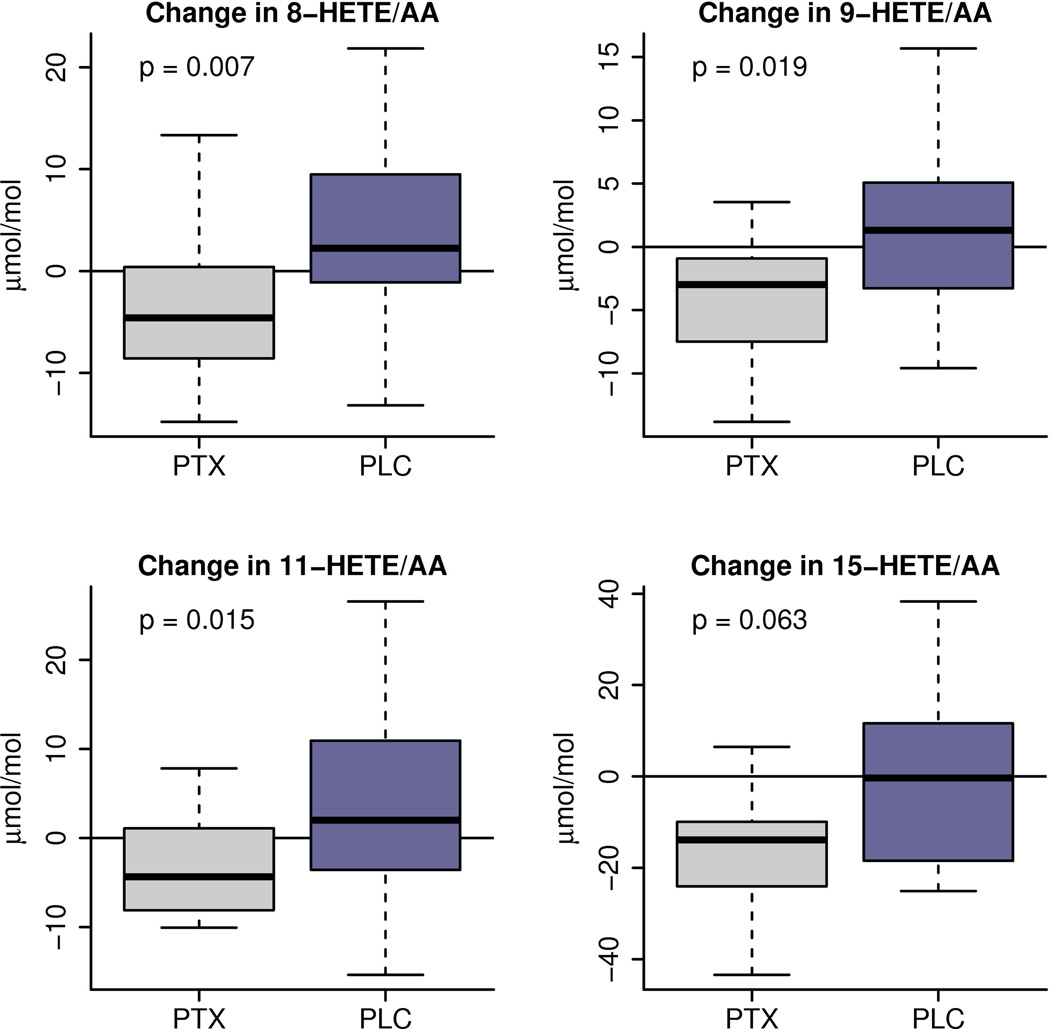
Plasma levels of structurally specific oxidation products of AA before and after treatment with PTX or placebo were also quantified. Table 3 shows the baseline values per treatment group as well as the observed changes from baseline in each group. Treatment of NASH patients with PTX for one year resulted in a significant decrease from baseline in specific oxidation products of AA including 9-HETE (p=0.019) and 11-HETE (p=0.015), both products of non-enzymatic oxidation of AA. There was also a significant decrease on 8-HETE (p=0.007), product that can arise from enzymatic or nonenzymatic oxidation of AA (Figure 2).
Table 3.
Oxidation products of Arachidonic Acid at baseline and change with PTX treatment
| Lipid (lipid:precursor µmol/mol) |
Baseline | Change from Baseline | ||||
|---|---|---|---|---|---|---|
| PTX (N=21) |
Placebo (N=26) |
p-value | PTX (N=21) |
Placebo (N=26) |
p-value | |
| 5-HETE | 36.0[25.9,44.2] | 32.9[22.6,47.8] | 0.91 | −6.0[−15.6,4.2] | +1.7[−9.6,10.1] | 0.11 |
| 8-HETE | 24.0[20.8,34.2] | 22.9[18.5,28.6] | 0.28 | −4.6[−8.6,0.402] | +2.2[−1.1,9.5] | 0.007 |
| 9-HETE | 19.1[15.7,23.3] | 16.4[13.5,21.5] | 0.16 | −3.0[−7.5,−0.917] | +1.3[−3.3,5.1] | 0.019 |
| 11-HETE | 28.5[24.1,36.7] | 24.5[19.9,33.0] | 0.13 | −4.4[−8.1,1.083] | +2.0[−3.6,10.9] | 0.015 |
| 12-HETE | 36.0[30.9,57.3] | 33.7[23.4,55.0] | 0.24 | −8.5[−17.5,−0.46] | +0.037[−15,29] | 0.066 |
| 15-HETE | 72.6[61.1,89.8] | 71.5[58.1,86.6] | 0.68 | −13.9[−24.0,−9.9] | −0.36[−18,11.6] | 0.063 |
| AA (pmol/mL× 103) | 512.7[459,579.5] | 473.5[427.7,577] | 0.28 | +7.7[−21.4,46.0] | −12.1[−77.8,28] | 0.23 |
Values presented as Median [P25, P75] with Wilcoxon rank sum tests
Figure 2.
Changes from baseline after one year of therapy (PTX vs placebo) in levels of hydroxy-eicosatetraenoic acids (HETEs), expressed as ratio to their specific precursor Arachidonic Acid. The box-whisker plot is represented with the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, and the upper boundary of the box indicating the 75th percentile. The whiskers extend to the most extreme data points. The p-values represent the difference between treatment groups.
Correlations between changes in oxidation products of LA and AA and changes in liver histopathology
Statistically significant correlations were found between plasma levels of specific oxidation products of LA and AA and changes in liver histology from baseline after PTX therapy. Table 4 shows statistically significant correlations demonstrated between improvement in fibrosis score and decreases in plasma 9-HODE (p=0.012), 13-HODE (0.043), 9-oxoODE (p=0.046), and 13-oxoODE (p=0.014). Similarly, improvement in lobular inflammation scores was correlated with decreases in 8-HETE (p=0.044), 11-HETE (p=0.021), 12-HETE (p=0.012), and 15HETE (p=0.028). Inverse correlations were demonstrated between change in plasma levels of the precursor AA and changes on liver biopsy. In this regard, an increase in plasma AA correlated with decreased lobular inflammation scores (p=0.017) and decreased fibrosis scores (p=0.029).
Table 4.
Statistically significant correlations highlight the link between decreased oxidized lipid levels and decreased liver fibrosis and inflammation scores. In contrast, the negative sign correlation coefficient for AA and the histology features indicates that improved histological severity scores were associated with increased levels of this precursor likely due to decreased oxidation.
| Change in Lipids | Change in Fibrosis | |
|---|---|---|
| Spearman’s correlation coefficient (rho) | p | |
| 9-HODE | 0.37 | 0.012 |
| 13-HODE | 0.30 | 0.043 |
| 9-oxoODE | 0.30 | 0.046 |
| 13-oxoODE | 0.36 | 0.014 |
| AA | −0.33 | 0.029 |
| Change in Lipids | Change in Inflammation | |
| Spearman’s correlation coefficient (rho) | p | |
| 8-HETE | 0.30 | 0.044 |
| 9-HETE | 0.28 | 0.067 |
| 11-HETE | 0.34 | 0.021 |
| 12-HETE | 0.37 | 0.012 |
| 15-HETE | 0.33 | 0.028 |
| AA | −0.36 | 0.017 |
Assessment of potential correlations between changes in oxidation products of LA and AA and changes in insulin sensitivity measures and other metabolic factors
Insulin sensitivity measures and HOMA were available in the 47 subjects. No changes in insulin sensitivity measures related to PTX treatment, or to the histological improvement associated with PTX, were demonstrated in the trial (12). Although a negative correlation between baseline plasma HETEs and baseline AIRg and DI was noted, there were no correlations between changes in plasma levels of HETEs, HODEs or oxoODEs and changes in AIRg, SI, SG, DI or HOMA. Furthermore, the changes in oxidized lipid products related to PTX therapy, and the correlations observed between changes in oxidized products and improvement in liver histology were independent of any changes in all measures of insulin sensitivity by IVGTT or HOMA.
Discussion
The most important finding of the present study is that therapy with PTX resulted in a decrease in plasma levels of oxidized fatty acids compared to placebo. This evidence supports that the beneficial effects observed on liver biopsy of PTX in patients with NASH compared to placebo (12) may be mediated through decrease in oxidative stress and oxidation of lipid products.
In this study, our primary aim was to investigate the effects of PTX on lipid oxidation products. The link between peripherally measured oxidized lipid products and systemic oxidative stress is well established (32), and compounds formed from oxidation of AA and of LA can be measured as reliable biomarkers of oxidative stress in vivo (33). Although potential benefits of PTX have been attributed to a number of its plausible actions, a specific mechanism of benefit in NASH had not been identified to date. The histological improvement associated with PTX in NASH has not been explained by changes in insulin sensitivity measured by IVGTT or HOMA, TNFα plasma levels, or changes on hepatocyte apoptosis measured by TUNEL (12).
In NASH, previous cross-sectional studies have shown that inflammatory oxidized lipid products of AA are elevated compared to NAFLD controls without NASH and non-NAFLD controls (10). Puri et al. reported that levels of 5-HETE, 8-HETE, 11-HETE, and 15-HETE are increased in patients with NASH (10), and remarked how the increase in 11-HETE supports a key role of nonenzymatic AA oxidation in NASH. In our study, PTX therapy resulted in reduction of 8-HETE, 9-HETE, and 11-HETE compared to placebo. Interestingly, 9-HETE and 11-HETE are specific products of nonenzymatic oxidation of AA that can result from free-radical mediated lipid oxidation. Therefore, our observations support that PTX therapy resulted in a decrease of nonenzymatic oxidation of AA in NASH. A link between histological severity of NAFLD and levels of 5-HETE, 8-HETE, 11-HETE and 15-HETE was previously reported in the cross-sectional setting by Puri et al (10). Remarkably, we observed statistically significant correlations between decreasing levels of 8-HETE, 11-HETE, 12-HETE and 15-HETE and reduction in inflammation score on liver biopsy, suggesting that the mechanism underlying PTX’s histological benefits in NASH may very well be partly related to decreased lipid peroxidation. Notably, statistically significant correlations were also observed between increased AA and decreased histological scores for inflammation and fibrosis, suggesting increased availability of this precursor due to decreased oxidation.
The role of oxidative stress and lipid peroxidation in NASH is recognized (34, 35). Data from the present study supporting that PTX decreases lipid peroxidation in NASH is in agreement with studies showing that PTX decreases lipid peroxidation-related injury in patients with other metabolic conditions (37, 38), and that it can specifically decrease oxidative stress related lipid peroxidation through hydroxyl radical scavenging properties (22, 23). Importantly, the lack of correlation between changes in oxidized lipid levels and any changes on insulin sensitivity or resistance parameters further supports that the observed changes in oxidized lipids were related to PTX’s effect on the liver disease independently of changes in insulin resistance.
Regarding oxidized lipid products of LA, elevation of HODEs and oxoODEs in NASH have also been reported previously, and levels have been correlated to histological severity including liver fibrosis (11). The data from that study supported that free radical-mediated (versus enzymatic) processes were primarily responsible for the elevated lipid oxidation products in NASH patients (11). In the present study, PTX therapy in patients with NASH resulted not only in significant decreases on 9-HODE and 13-oxoODE (and similar trends for 13-HODE and 9-oxoODE), but also significant correlations were demonstrated between the decrease in levels of 9-HODE, 13-HODE, 9-oxoODE, and 13-oxoODE and decreased histological scores of fibrosis. This is consistent with in vitro data showing that exposure of cultured human liver fat-storing cells to oxidative stress leads to lipid peroxidation and associated increase of procollagen type I mRNA and fibrogenesis (36). Furthermore, there is also experimental data showing that PTX decreases collagen deposition in mice through downregulation of oxidative stress and lipid peroxidation (18), and downregulates procollagen I and pro-fibrogenic factors in the liver of rats with chronic bile duct occlusion (39). In humans, our group previously reported a significant decrease in liver biopsy fibrosis score in patients with NASH after PTX therapy compared to placebo (12), and supporting this, decreased hepatic expression of procollagen type I gene was reported in a small subset of a separate cohort of patients with NASH treated with PTX (40). All this knowledge, along with the association of PTX-associated decrease in HODEs and oxoODEs with improved liver fibrosis newly reported here, and taken together with the association between PTX therapy and improved liver fibrosis scores on biopsy and on serum markers of fibrosis (12, 13), support that the beneficial effects of PTX on liver fibrosis in NASH are at least partly mediated through decreased oxidative stress and lipid peroxidation.
Although there were no changes from baseline with PTX therapy in any insulin sensitivity or resistance parameters, the inverse correlation observed at baseline between oxidized lipid levels and AIRg and DI was an interesting observation. This observation is consistent with established knowledge of a link between impaired glucose metabolism and the presence of NAFLD and NASH. Although the normal physiological adaptive response to decreased insulin sensitivity is a hyperbolic increase in AIRg (41), it is known that this relationship between AIRg and insulin sensitivity is shifted to the left in subjects with higher predisposition to develop progressive metabolic alterations such as overt diabetes or NAFLD (41). These subjects show evidence of decreased beta-cell compensation to emergent insulin resistance compared to normal subjects (42–44), and this is reflected in lower AIRg reflecting poorer beta-cell function even with underlying decreased insulin sensitivity (41). Furthermore, the presence of beta-cell dysfunction does not only impact glucose metabolism, but also results in less insulin to suppress lipid oxidation (45). Taken together with the fact that the majority of patients (42 of the 47 subjects) in our study were non-diabetics, our observations are in agreement with, and further corroborate, previous evidence supportive of the link between beta-cell dysfunction and increased lipid oxidation in NAFLD and NASH (45). Nevertheless, this same fact that the vast majority of the subjects in this study (42 or 47) were non-diabetics, limits the ability to extrapolate these conclusions to the specific subpopulation of NASH patients with established diabetes. Taken together with variable data on the effects of PTX in diabetic animals with NASH, this further justifies the need for future studies of PTX in NASH including a larger group of patients with diabetes.
A crucial strength of this study is the direct measurement of oxidized lipid products by mass spectrometry, which dramatically increases sensitivity, specificity and overall accuracy of systemic lipid peroxidation assessment compared to less direct and not as sophisticated methods such as measurement of thiobarbituric acid reactive substances (33). Although the subjectivity of liver biopsy interpretation is a relative limitation, the availability of liver histopathology evaluation before and after the intervention is a strength per se, akin to the use of a single pathologist (LY) with extensive experience with the NAS (26), and who interpreted and scored all liver biopsies while blinded to all clinical data and to treatment allocation. The comprehensive assessment of insulin sensitivity parameters with the FSIVGTT in addition to HOMA calculation in all subjects adds additional strength to the study, particularly since, as highlighted by other authors, the lack of assessment of underlying insulin resistant state has been a significant limitation of previous cross-sectional studies of oxidized lipids in NAFLD (10). Evidently, although our results are novel and of definite significance, future studies to corroborate and further characterize these observations in a larger number of patients are needed.
In summary, this study demonstrated that therapy with PTX in NASH results in decreased oxidized lipid products of LA and AA compared to placebo. These are novel findings. Furthermore, the oxidized lipid products modified by PTX have described associations with NASH and specifically with non-enzymatic oxidation of lipid precursors (10, 11), supporting that the beneficial effects of PTX in NASH are likely largely mediated trough decreasing free-radical mediated lipid oxidation. In addition, to our knowledge, this is the first time that decreased levels of oxidized fatty acids are shown to correlate with histological improvement in the setting of a therapeutic trial in NASH. The correlation between decreased oxidized lipid products and improved liver fibrosis scores is particularly noteworthy. Larger studies are needed for additional validation, further assessment in specific patient subpopulations such as those with diabetes, further mechanistic characterization, and definite assessment of the beneficial effects of PTX in NASH. But without a doubt, the results of this study are an important contribution in paving the way towards the future definition of clinically applicable systemic oxidative stress measures in NASH in the setting of pharmacological interventions, and perhaps towards further development of systemic antioxidant targeted therapies in NASH.
Acknowledgments
Dr. Claudia Zein is supported by Grant Number KL2 RR024990 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health, components of the NIH and NIH Roadmap for Medical Research. This project was supported by the American College of Gastroenterology Junior Faculty Career Development Award to Dr. Zein. This project was also supported by Grant Number M01 RR00080 and Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- NAFLD
Nonalcoholic Fatty Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- PTX
pentoxifylline
- AA
arachidonic acid
- LA
linoleic acid
- HETE
hydroxy-eicosatetraenoic acid
- HODE
hydroxy-octadecadenoic acid
- NAS
NAFLD activity score
- OS
oxidative stress
References
- 1.Browning JD, Szczepaniak LS, Dobbis R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 4.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 5.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37:1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. HEPATOLOGY. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 9.Horoz M, Bolukbas C, Bolukbas FF, et al. Measurement of the total antioxidant response using a novel automated method in subjects with nonalcoholic steatohepatitis. BMC Gastroenterol. 2005;5:35. doi: 10.1186/1471-230X-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri P, Wiest MM, Cheung O, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human alcoholic liver disease and nonalcoholic steatohepatitis. Journal of Lipid Research. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: A Randomized Placebo-Controlled Trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zein CO, Lopez R, Yerian LM, Anderson KA, McCullough AJ, Rinella ME. For presentation at Digestive Disease Week. San Diego, CA: 2012. May, Pentoxifylline Improves Non-invasive Serum Markers of Fibrosis: Combined Results from 2 Randomized, Placebo-Controlled Trials. [Google Scholar]
- 14.Ward A, Clissold S. Pentoxifylline. Review of its pharmacodynamic and pharmacokinetic properties. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, Larrick J. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Int J Immunopharmacol. 1996;18:739–748. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Xu UJ, Saini HK, Turan B, Liu PP, Dhalla NS. Pentoxifylline attenuates cardiac dysfunction and reduces TNF-alpha level in ischemic-reperfused heart. Am J Physiol Heart Circ Physiol. 2005;289:H832–H839. doi: 10.1152/ajpheart.00178.2005. [DOI] [PubMed] [Google Scholar]
- 17.Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–198. [PubMed] [Google Scholar]
- 18.El-lakkany N, El-Din SS, Ebeid F. The use of pentoxifylline as adjuvant therapy with praziquantel downregulates profibrogenic cytokines, collagen deposition and oxidative stress in experimental schistosomiasis mansoni. Experimental Parasitology. 2011;129:152–157. doi: 10.1016/j.exppara.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Vircheva S, Alexandrova A, Georgieva A, Mateeva P, Zamfirova R, Kubera M, Kirkova M. In vivo effects of pentoxifylline on enzyme and non-enzyme antioxidant levels in rat liver after carrageenan-induced paw inflammation. Cell Biochem Funct. 2010;28:668–672. doi: 10.1002/cbf.1705. [DOI] [PubMed] [Google Scholar]
- 20.Kozaki K, Egawa H, Bermudez L, Keefe EB, So SK, Esquivel CO. Effects of pentoxifylline pretreatment on Kupffer cells in rat liver transplantation. Hepatology. 1995;21:1079–1082. [PubMed] [Google Scholar]
- 21.Koppe SW, Sahai A, Malladi P, Whittington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Freitas JP, Filipe PM. Pentoxifylline: A hydroxyl radical scavenger. Biological Trace Element Research. 1995;47:307–311. doi: 10.1007/BF02790131. [DOI] [PubMed] [Google Scholar]
- 23.Bhat VB, Madyastha KM. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochemical and Biophysical Research Communications. 2001;288:1212–1217. doi: 10.1006/bbrc.2001.5922. [DOI] [PubMed] [Google Scholar]
- 24.Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192:313–322. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Radfar M, Larijani B, Hadjibabaie M, Rajabipour B, Mojtahedi A, Abdollahi M. Effects of pentoxifylline and oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients; a randomized, double blind placebo-controlled trial. Biomedicine & Pharmacotherapy. 2005;59:302–306. doi: 10.1016/j.biopha.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Shishehbor MH, Zhang R, Medina H, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radical Biology & Medicine. 2006;41:1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 29.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 30.Boston RC, Stefanovski D, Moate PJ, et al. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 31.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Niki E. Bio-markers of lipid peroxidation in vivo: Hydroxyoctadecadienoic acid and hydroxycholesterol. BioFactors. 2006;27:195–202. doi: 10.1002/biof.5520270117. [DOI] [PubMed] [Google Scholar]
- 33.Niki E, Yoshida Y. Biomarkers of oxidative stress: measurement, validation and application. Proceeding. J Med Invest. 2005;52(Suppl):228–230. doi: 10.2152/jmi.52.228. [DOI] [PubMed] [Google Scholar]
- 34.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 35.Cortez-Pinto H, de Moura MC, Day CP. Nonalcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat storing cells. Biochem Biophys Res Commun. 1993;194:1044–1050. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 37.Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192:313–322. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Radfar M, Larijani B, Hadjibbaie M, et al. Effects of pentoxifylline on oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients; a randomized controlled clinical trial. Biomedicine & Pharmacotherapy. 2005:302–306. doi: 10.1016/j.biopha.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Raetsch C, Jia JD, Boigk G, et al. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut. 2002;50:241–247. doi: 10.1136/gut.50.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanWagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of nonalcoholic steatohepatitis: a randomized controlled trial. Annals of Hepatology. 2011;10:277–286. [PubMed] [Google Scholar]
- 41.Utzschneider KM, Khan SE. Insulin action and beta-cell function: role in metabolic regulation. In: Eckel RH, editor. Metabolic Risk for Cardiovascular Disease, 1st Edition. American Heart Association, by Blackwell Publishing Ltd; 2011. pp. 1–17. [Google Scholar]
- 42.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cnop W, Vidal J, Hull RL, et al. Progressive loss of B-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30:677–682. doi: 10.2337/dc06-1834. [DOI] [PubMed] [Google Scholar]
- 44.Xiang AH, Wang C, Peters RK, et al. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–1079. doi: 10.2337/diabetes.55.04.06.db05-1109. [DOI] [PubMed] [Google Scholar]
- 45.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]