Summary
Previous studies have suggested that exogenous nitric oxide (NO) and NO-dependent signalling pathways modulate intracellular pH (pHi) in different cell types, but the role of NO in pHi regulation in the heart is poorly understood. Therefore, in this study we investigated the effect of NO donors S-nitroso-N-acetyl-DL-penicillamine, Spermine and PAPA NONOate on pHi in isolated rat ventricular myocytes.
The cells were isolated from the hearts of adult Wistar rats, and pHi was monitored using a pH-sensitive fluorescent indicator 5-(and-6)-carboxy SNARF-1 with a confocal microscope. To test the effect of NO donors on sodium-hydrogen exchanger, basal pHi in Na+-free buffer and pHi recovery from intracellular acidosis after an ammonium chloride prepulse were monitored. The role of carbonic anhydrase was tested using acetazolamide. Cl−-OH− and Cl−-HCO3− exchangers were inhibited with 4,4 diisothiocyanatostilbene 2,2' disulfonic acid.
All three NO donors acutely decreased pHi. This effect lasted until NO donor was removed. In a Na+-free buffer decrease in basal pHi was increased, while inhibition of carbonic anhydrase and Cl−-OH− and Cl−-HCO3− exchangers did not change the effect of NO donors on pHi. After an ammonium preload, pHi recovery was accelerated in the presence of NO donors.
In conclusion, exogenous NO decreased the basal pHi leading to increased activity of sodium-hydrogen exchanger. Carbonic anhydrase and chloride-dependent sarcolemmal HCO3− and OH− transporters are not involved in the NO-induced pHi decrease in isolated rat ventricular myocytes.
Keywords: Nitric oxide, intracellular pH, cardiac myocytes, sodium hydrogen exchanger, carbonic anhydrase
Introduction
Intracellular pH (pHi) plays an important role in regulation of different intracellular processes in cardiac myocytes like intracellular Ca2+ homeostasis, contractility, intracellular signalling and electrical activity.1 Recent experimental work has also suggested that reducing pHi may afford some degree of cardioprotection during post-ischemic reperfusion of animal hearts and in simulated ischaemia/reperfusion of isolated cardiomyocytes and mitochondria.2 In rat cardiac myocytes, there is a complex mechanism involved in the regulation of pHi. Among H+ ions extruders from the cell sodium-hydrogen exchanger (NHE) and Na+-HCO3− cotransport (NBC) are the prominent mechanisms. On the other hand, Cl−-HCO3− (CBE) and Cl−-OH− exchangers (CHE) act as base extruders.3 In addition to sarcolemmal transporters, there is complex intracellular regulatory machinery, which consists of cytosolic proteins and buffers with the bicarbonate and phosphate buffers being the most important. Carbonic anhydrase (CA) mediates intracellular CO2 hydration and helps in the generation of HCO3− and H+ ions, leading to a fall of pHi4 CA is also believed to be bound to the HCO3− carrier protein, and to mediate fast pHi changes.4 All these mechanisms maintain the pHi about the steady state of 7.2. Multiple intracellular signalling pathways have been shown to be involved in pHi regulation in cardiac cells. Mitogen-activated protein kinase (MAP) with extracellular regulated kinase (ERK) phosphorylates NHE and stimulates pHi increase.5 Different agents, such as angiotensin II, endothelin-1, and catecholamines, appear to be coupled to ERK via protein kinase C resulting in stimulation of NHE.5–7 Hydrogen peroxide, intracellular acidosis, and elevated Ca2+ are believed to be other stimulators of NHE activity in the heart.3 Angiotensin II and aldosterone can increase pH sensitivity of the acid loader CBE.8, 9 while cyclic adenosine monophosphate (cAMP) is proposed to mediate NHE inhibition in sheep cardiac fibers and rat ventricular myocytes.10, 11
Nitric oxide (NO) is a free radical synthesized by mammalian cells from the l-arginine by a reaction catalyzed by a family of NO synthase enzymes.12 NO exerts its effects through stimulation of soluble guanylate cyclase, resulting in the production of second messenger cyclic guanosine monophosphate (cGMP).13 A significant role for NO has been demonstrated in the physiology and pathophysiology of the heart by numerous in vitro and in vivo studies.14 The effects of NO on pHi have been suggested to occur through different mechanisms depending on experimental model and conditions.3, 15–17 In the heart, sodium nitroprusside (NO donor) has been shown to decrease pHi in rat ventricular myocytes in a cGMP- and NHE-dependent manner,18 but studies so far have not investigated the effect of exogenous NO on Cl−-mediated pH regulatory proteins and CA-regulated pH changes in cardiac myocytes. In general, the role of NO in pHi regulation in the heart is poorly understood, and the reported effects of NO on pHi on other cells appear to be inconsistent.11, 15, 16, 19 Moreover, to our knowledge, the effect of other NO donors on pHi in ventricular myocytes has not been studied.
Therefore, the objective of this study was to test the effect of NO donors S-nitroso-N-acetyl-DL-penicillamine (SNAP), Spermine NONOate (Spermine) and PAPA NONOate (PAPA) on basal pHi in isolated rat ventricular myocytes, and to investigate the possible underlying mechanism of NO-induced pHi changes.
Methods
Animals
The investigation was conducted in accordance with the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, Wisconsin), and in accordance with U.S. National Institutes of Health standards (NIH Publication 95-23, revised 1996).
Cell isolation
Ventricular myocytes were isolated from the hearts of adult male Wistar rats (175–250 g) by enzymatic dissociation.20, 21 Animals were anesthetized with an intraperitoneal injection of thiobutabarbital (Inactin, 100–150 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) and heparin (1000 U) to prevent blood clotting. The hearts were extracted and perfused with 0.5 mg/mL of collagenase type II (Invitrogen, Carlsberg, CA, USA) and 0.25 mg/mL of protease type XIV (Sigma-Aldrich). Partially digested left ventricles were teased gently with forceps and shook for 5 min and then filtered through a nylon mesh (200 µm; Spectrum Laboratories, Inc. Rancho Dominguez, CA, USA). After isolation, the myocytes were stored in the 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered Tyrode solution [(in mM): 132 NaCl, 10 HEPES, 5 D-glucose, 5 KCl, 1 CaCl2, and 1.2 MgCl2] at 20°-22°C. Cells were allowed to recover for 1 h, and were used for experiments within 5 h.
Laser scanning confocal microscopy
Isolated ventricular myocytes were recorded in an open bath chamber (RC-22, Warner Instruments, Hamden, CT) mounted on the stage of a confocal microscope (Nikon Eclipse TE2000-U; Nikon, Tokyo, Japan) with a 40×/1.3 oil-immersion objective (Nikon). Data were analyzed using NIH ImageJ software 1.41 (National Institutes of Health, Bethesda, MD).
Measurement of pHi
pHi in intact myocytes was determined as previously described.20 Cells were loaded with 5-(and-6)-carboxy SNARF-1 and acetoxymethyl ester (SNARF-1; 10 µM, Molecular Probes, Eugene, OR, USA) in bicarbonate-free Tyrode solution at room temperature for 15 min.20, 22 After dye loading, the cells were superperfused with the same solution (without dye) for additional 20 min before recordings started. SNARF-1 was excited at 543 nm with a green HeNe laser, and emitted fluorescence was simultaneously collected at 590 and 650 nm. The 650 nm/ 590 nm emission ratio from each cell was calculated and converted to pHi values using nigericin calibration technique.23, 24 Calibration curve was made by exposing the cells to different external pH (6.0, 7.5, and 9.0) buffers in a depolarizing high K+ buffer (140 mM KCl, 1.0 mM MgCl2, 5 mM D-glucose, 10 mM HEPES, and in the presence of 10 µM nigericin).25 Nigericin, a H+/K+ exchanger ionophore sets [K+]o = [K+]I, and, therefore, in the presence of nigericin the pHo = pHi. The pH calibration data were obtained separately from individual cells and were averaged for more than 15 cells. NHE activity was assessed after myocytes were exposed to an acid load consisting of 10 mM ammonium-chloride (NH4Cl) for 3 min.3 The experiments were performed in the absence of bicarbonate to assure that the pHi recovery after NH4Cl readdition was because of NHE activation. In addition, experiments were conducted in sodium-free solution by replacing sodium with 132 mM N-methyl-D-glucamine to block NHE activity.3 Intracellular carbon dioxide hydration by CA results in the generation of HCO3− and H+-ions, leading to a fall of pHi. To examine the effect of NO donors on CA, a CA inhibitor acetazolamide (50 µM, Sigma-Aldrich) was dissolved in DMSO and added to superfusion solution shortly before NO donor was applied. 4 To determine whether NO induces pHi changes due to increased base efflux through Cl−-OH− and Cl−-HCO3− exchangers, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; 0.5 mM; Sigma-Aldrich), a nonselective anion transport inhibitor, was administered 5 min before superfusion with NO donor. 4
Spectrofluorometric measurements
To exclude the direct effect of NO donors on SNARF-1, were performed spectrofluorometric measurements in the presence of NO donors. Experiments were carried out in a stirred cuvette in the HEPES-buffered bicarbonate-free Tyrode solution containing 10 µm SNARF-1 at room temperature using QM-8 spectrofluorometer (Photon Technology International, Inc., Birmingham, NJ). Before recording, the SNARF-1 acetoxymethiyl ester was treated with esterase from porcine liver for 20 min to remove ester moiety and to activate the dye. The probe was then excited at 543 nm, and emission was collected at 590 and 650 nm.
Chemicals
5-(and-6)-carboxy SNARF-1, acetoxymethyl ester was purchased from Molecular Probes, Eugene, OR, USA. S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP; a half-life of several hours; No 82250), (Z)-1-[N-[3-aminopropyl]-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate (Spermine; a half-life of ~39 min, No 82150) and Propylamine Propylamine NONOate (PAPA; a half-life of ~15 min; No 82140), were obtained from Cayman Chemical, Ann Arbor, MI, USA. NO donors were prepared each day before the experiment and stored on ice. All other chemicals were purchased from Sigma. The NO donors were prepared fresh before each experiment in 10 mM NaOH, and diluted to concentrations used in the recording buffer. The levels of NO∙ generated by NO donors were determined using an NO∙-sensitive electrode using free radical analyzer (APOLLO, World Precision Instruments, Sarasota, FL). Based on our measurements, the maximal inhibitory effect occurred at concentration of 0.5 mM when using PAPA nonoate corresponding to 2.25–2.50 µM of NO∙ levels. Than, the bioequivalent concentrations of SNAP and Spermine were used for the comparison. A NO∙ scavenger, 2-phenyl-4,4,5,5-tetramethylimidaoline-1-oxyl-3-oxide (PTIO; EMD Biosciences, San Diego, CA) was used as a negative control. It was prepared in cold dionized water and used at final concentration of 25 µM. PTIO was added to the recording buffer solution shortly before NO donor was applied.26
Statistical analysis
Data were expressed as means ± SD, with the sample size (n). The GraphPad Prism 4.00 (GraphPad Software, La Jolla, CA, USA) software was used for statistical analyses. In each experimental group we used cardiac ventricular myocytes form at least eight rats. Statistical analysis was carried out with one-way ANOVA followed by the Bonferroni test for multiple comparisons of group means. The values were considered statistically significant when P < 0.05 (2-tailed).
Results
Effect of exogenous NO on pHi in isolated rat ventricular myocytes
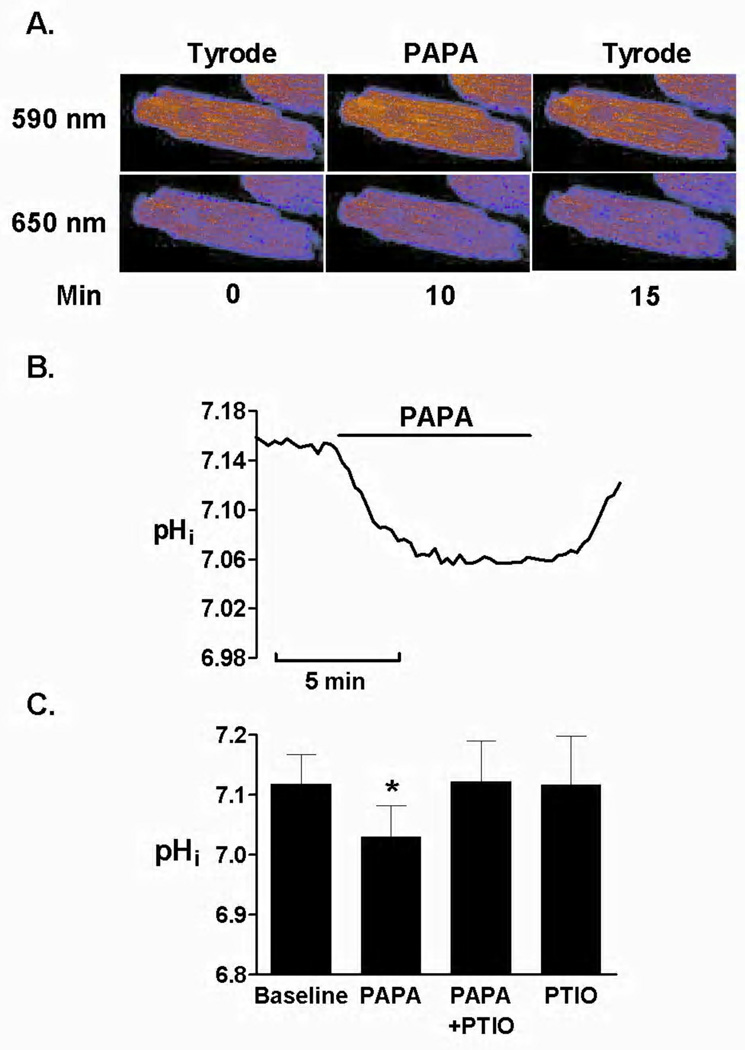
To investigate the effect of exogenous NO on pHi in the ventricular myocytes, we assessed basal pHi in a HEPES-buffered bicarbonate-free Tyrode solution at external pH of 7.4. We used NO donors with the different half-lives and stability. The half-time for SNAP is about 6 h, for Spermine it is about 39 min and PAPA has a half-life of approximately 15 min. Based on our preliminary measurements, the maximal inhibitory effect occurred at concentration of 0.5 mM when using PAPA nonoate corresponding to 2.25–2.50 µM of NO∙ levels. Than, the bioequivalent concentrations of SNAP and Spermine were used for comparison. Figure 1A shows the intracellular loading of SNARF-1 monitored at 590 nm and 650 nm and the change of SNARF-1 fluorescence intensity upon exposure to PAPA (0.5 mM) for 5 min. Myocytes' exposure to PAPA decreased the basal pHi, (7.12±0.05 versus 7.03±0.05, before and after PAPA, respectively; P<0.05, Figures 1B and 1C). After PAPA was removed from the solution the pHi returned to the basal values, indicating that NO donor induced a reversible change in pHi.
Figure 1.
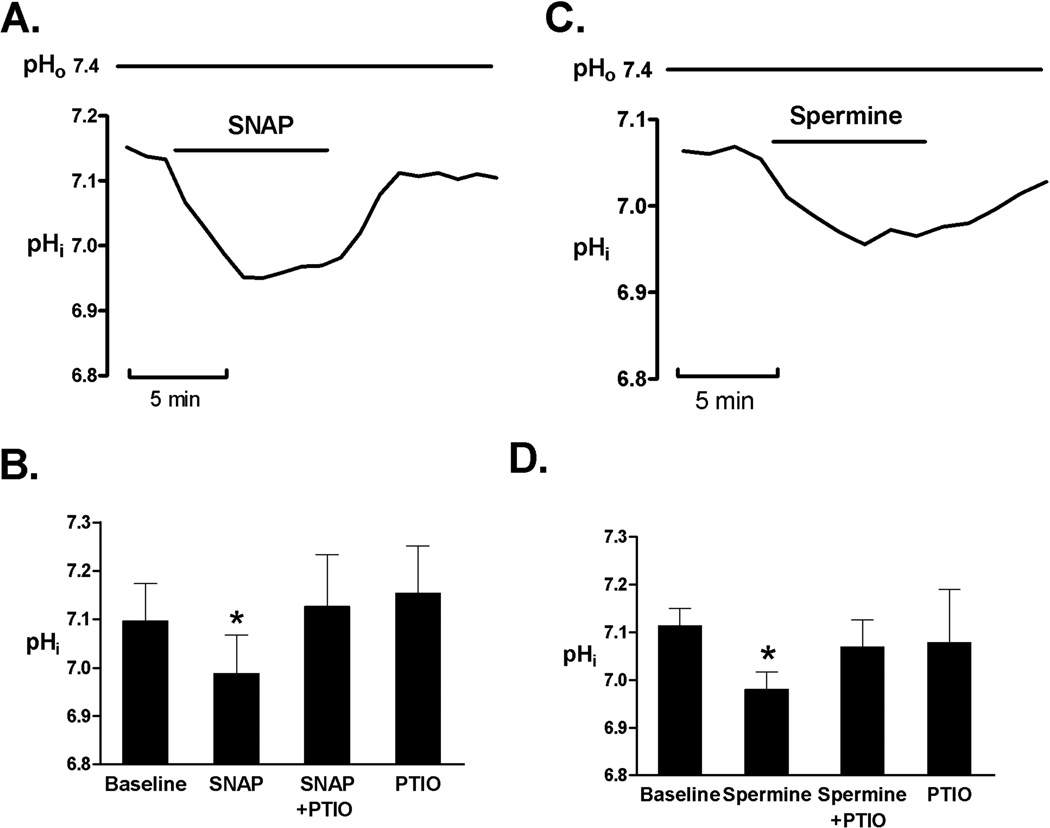
SNAP (100 µM) and Spermine (100 nM) had a similar effect (7.09±0.07 versus 6.98±0.08, before and after SNAP, respectively; P<0.05, Figures 2A and 2B) and (7.11±0.09 versus 7.01±0.08, before and after Spermine, respectively; P<0.05, Figures 2C and 2D). The similar decrease in basal pHi was obtained using three NO donors, and there was not statistical difference in the effect of NO donors on maximal pHi decrease, suggesting that common mechanism is involved. When NO scavenger (PTIO, 25 µM) was applied the NO donor-induced effect on pHi was prevented. PTIO at applied concentration had no significant effect on pHi in control cells, as summarized in Figures 1C, 2B and 2D.
Figure 2.
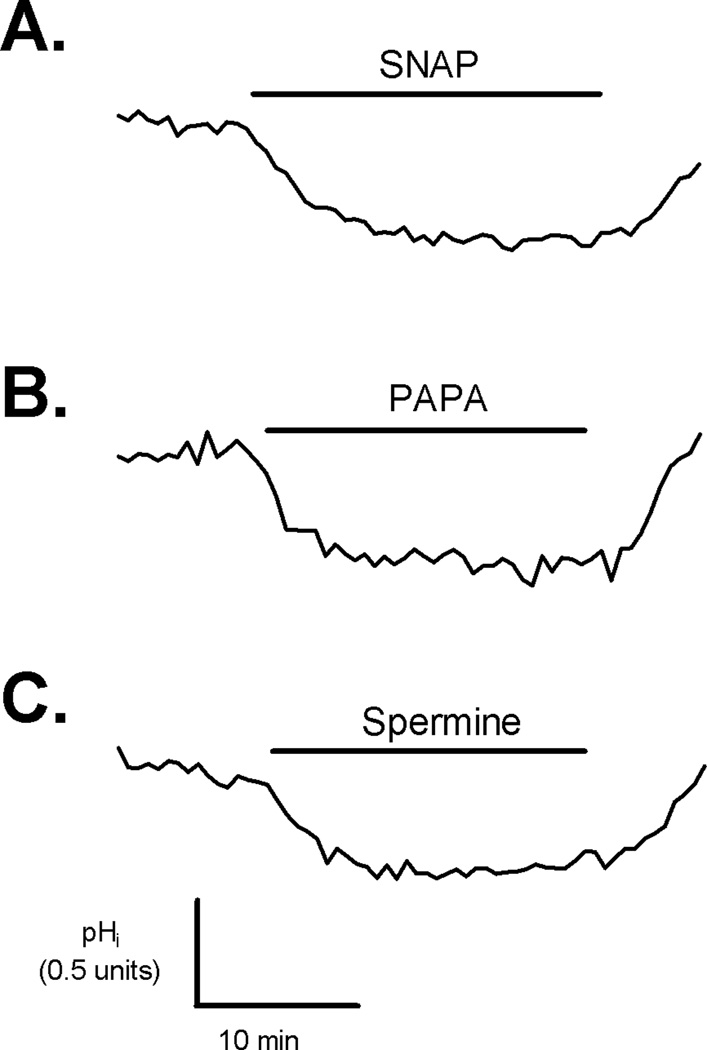
To test whether NO donors induced transient or sustained change in pHi, we exposed the myocytes to NO donors from 5 to 30 min. As shown in Figure 3A, the exposure to SNAP for 30 min caused sustained decrease in the basal pHi. This effect lasted until NO donor was removed from the superfusing solution. Similar results were obtained for PAPA and Spermine (Figure 3B–C).
Figure 3.
Sodium hydrogen exchanger activity
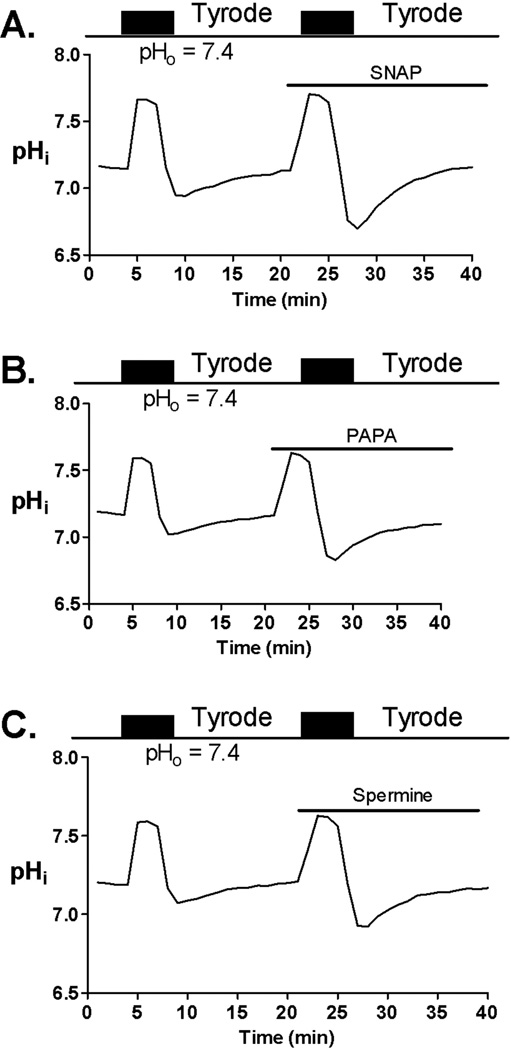
To test the effect of NO donors on NHE activity we exposed the myocytes to NH4Cl (10 mM) for 3 min. NHE activity was followed up upon NH4Cl removal.2 Addition of NH4Cl induced an increase in pHi, while upon NH4Cl removal pHi decreased sharply and then recovered toward the baseline value. This recovery of pHi in bicarbonate-free buffer is exclusively mediated by NHE.3 Interestingly, although SNAP, Spermine, and PAPA decreased basal pHi, they did not decrease the NHE activity after the acidic load by NH4Cl. Moreover, in the presence of NO donor NHE activity was increased (Figure 4A–C).
Figure 4.
Inhibition of chloride-dependent transporters and carbonic anhydrase
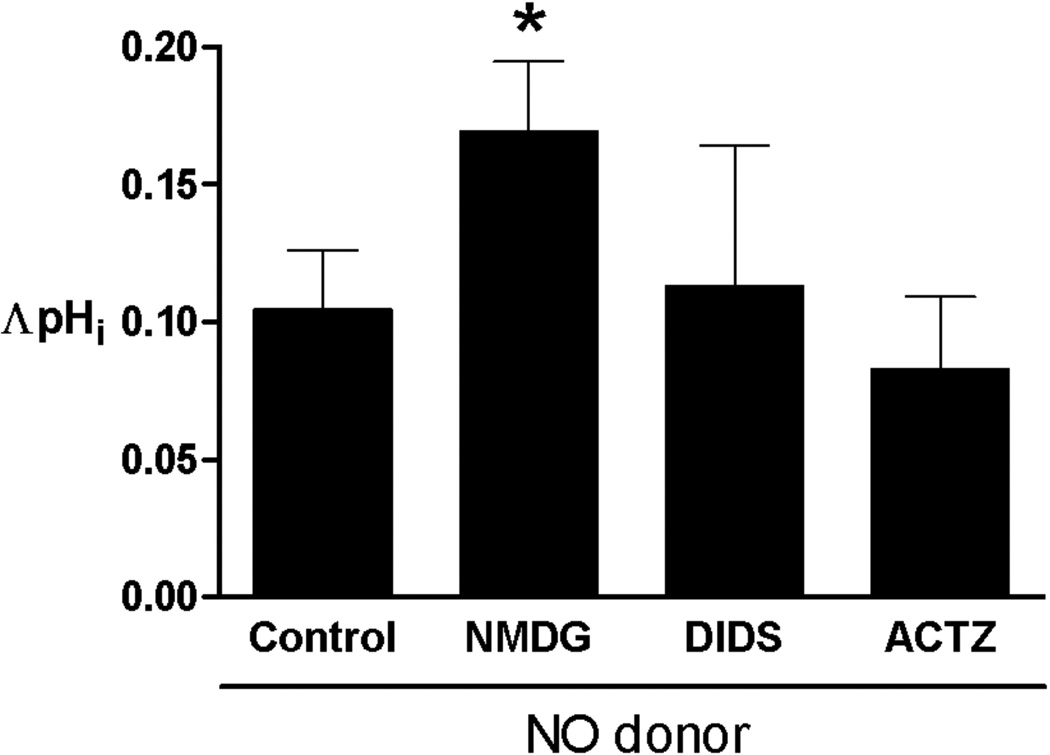
Further, we tested the role of CA and Cl−-mediated transporters in NO-induced pHi changes. Figure 5 shows the summary effects of NO donors on basal pHi when sodium was replaced by N-methyl-D-glucamine to inhibit NHE, when Cl−-dependent sarcolemmal transporters were inhibited by 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS, 0.5 mM), and when carbonic anhydrase was inhibited by acetazolamide (50 µM). Under these conditions the basal pHi decrease was found to be statistically increased only in Na+-free buffer and (0.10±0.06 versus 0.17±0.02, with and without N-methyl-D-glucamine, respectively; P<0.05). The inhibition of CA and Cl−-OH− and Cl−-HCO3− exchangers by acetazolamide and DIDS did not change the effect of NO donors on basal pHi (see Figure 5), respectively. These results suggest that basal pHi decrease is not elicited through the effect of NO on CA and Cl−-dependent sarcolemmal transporters.
Figure 5.
Discussion
In the present study, we investigated the effect of NO donors SNAP, Spermine and PAPAnonoate on basal pHi in isolated rat ventricular myocytes. Our results indicate that all three NO donors decreased basal pHi with a probable subsequent increase in NHE activity. More specifically, inhibition of NHE by Na+ replacement with N-methyl-D-glucamine enhanced the effect of NO donors, indicating that the NHE-dependent compensatory mechanism is involved. The inhibition of chloride-dependent pHi regulatory sarcolemmal transporters and CA was not effective in prevention of NO donors-induced pHi changes. Exposure of isolated cardiac myocytes to NO donors, for a time up to 30 min, induced sustained decrease in basal pHi, suggesting that a permanent change in cytosolic buffering mechanisms could play a role in pHi regulation in myocytes during their exposure to NO donor. We did not find any significant difference in a maximal decrease in pHi among NO donors used in our study, suggesting that common mechanism is involved.
Sarcolemmal H+-equivalent transport proteins such as NHE and CBE have been identified as common pHi regulatory proteins in rat ventricular myocytes and Purkinje fibers.3 These transporters can be quickly activated to remove acid or base loads from the cell. Acid extruders (NHE, NBC) are activated at low pHi when concentration of H+ ions is increased. Contrary, base extruders, CHE and CBE, are activated at high pHi.4 Different neurotransmitters, hormones and other signalling molecules have been proposed to be involved in the H+-equivalent transporters regulation. In cardiomyocytes, the number of agents like adrenalin, angiotensin II, endothelin and insulin can affect pHi and stimulate or inhibit NHE.3 β1 receptor activation by adrenaline can slow NHE activity while it stimulates NBC activity and acid extrusion.27 Extracellular pH (pHe) is involved in the control of pHi-regulatory transporters in a manner opposite to that of pHi where reduced pHe activates an acid influx by CBE and CHE, while inhibiting acid extrusion by NHE and NBC.22 In addition to sarcolemmal transporters, intracellular buffering of H+ ions by cellular proteins and CO2/HCO3− is activated within seconds and presents the first line of defence against pHi perturbations.3 pHi can also be controlled by H+ ions shuttling mechanisms with cytosolic mobile buffers. This reaction is catalysed by CA, as evidenced by attenuation of this effect by CA inhibitor acetazolamide.28
The mechanisms by which NO modulate pHi are not completely understood. Posttranslational modifications of proteins by NO have been proposed to mediate either activation or inhibition of different cellular proteins. The number of nucleophilic sites on proteins (amines, thiols, aromatic rings, alcohols, etc.) can be nitrosated by NO leading to production of the S-nitrosothiols.13 Another mechanism of NO interaction with cellular proteins includes nitration of tyrosine, tryptophan, amine, carboxylic acid, phenylalanine groups, and oxidation of thiols.12 Cardiac myocytes have been proposed to contain different mobile protein buffers involved in cytosolic pHi regulation such as alanine, ariginine, aspartate, and histidine (with histidyl dipeptides) many of which might contain NO reactive sites or residues.22 We found that upon exposure to exogenous NO, the basal pHi is decreased. Therefore, it is possible that interactions of NO with reactive sites of protein buffers can decrease the affinity of intracellular proteins for H+ ions. Previous study has demonstrated that the basal pHi and NHE activity are decreased by NO donor sodium nitroprusside in rat ventricular myocytes in a cGMP-dependent manner. In our study, NHE activity was enhanced after an ammonium preload in myocytes exposed to NO. The possible explanation for this observation is that under the conditions of intracellular acidosis, the NHE activity is increased due to stimulatory effect of increased concentration of H+-ions on NHE.3 We observed the similar effect of all three NO donors on basal pHi. In the literature, regarding the effect of NO on pHi and NHE, results appear contradictory and depend on conditions and experimental model that have been used. It has been demonstrated that NO induced rapid intracellular acidification in feeding neurons of Pleurobranchaea in a cGMP or cAMP-independent signaling pathway.17 A similar acidification response to NO donors was observed in cultured hippocampal neurons.29 NO is suggested to be involved in pHi regulation during focal cerebral ischemia, as NO synthase inhibition by L–NAME prevented brain acidosis in rabbits.19 In cultured smooth muscle cells, ANP/cGMP inhibited the activity of NHE, either in hormone- or pH-stimulated condition.30 SNAP and sodium nitroprusside decreased NHE3 activity in Caco-2 cells, but activity of NHE2 was unchanged.15 In the proximal tubule and in thick ascending limb cells, the NHE activity was decreased in response to exogenous NO.31,32 By contrast, in mouse cecum, an increase in NHE activity was observed in response to NO precursor l-arginine.16 In the heart several NHE isoforms are expressed, and the NHE1 is the most important in the pHi regulation.3 In line with this, it has been shown that phosphodiesterase 5A (an enzyme that catabolizes cGMP) inhibition by sildenafil inhibited NHE1 only after an acid load, but did not modify the basal pHi in isolated rat papillary muscles.11 In our study, the observation that basal pHi was decreased after exposure to NO, that was followed by increased NHE activity further supports the role of NO in acid-base regulation in rat ventricular myocytes.
It was unclear whether exogenous NO can affect the Cl−-mediated pHi regulatory mechanisms in rat ventricular myocytes. It has been shown that NO mediates Cl−-dependent HCO3− transporters in different cells. NO stimulates Cl− and bicarbonate secretion and inhibits Na+ and Cl− absorption in rat colon cells.33 In rat thick ascending limb cells NO inhibited HCO3− transport via a cGMP-dependent pathway.34 NO-mediated regulation of the brush-border membrane anion and Cl−/HCO3− transporters in the rabbit small intestine has also been proposed.35 To detect the effect of NO on Cl−-dependent sarcolemmal transporters we looked at basal pHi change in the presence of DIDS, an inhibitor of Cl−-dependent sarcolemmal transporters. We found that, in the presence of DIDS, NO-induced pHi decrease was not changed showing that Cl−-dependent sarcolemmal transporter was not involved in the action of the NO donor on pHi.
To determine the effect of NO on CA-mediated changes in basal pHi, we used CA inhibitor acetazolamide, which is a membrane permeable and therefore inhibits both cytosolic and membrane-bound CA.4 CA catalyzes the reversible hydration of CO2 to bicarbonate and H+-ions, and plays a crucial role in CO2 transport and in acid-base balance. Although CA mostly acts as a part of CO2/HCO3− buffer system H+ ions can be shuttled on intrinsic mobile buffers through CA-catalysed reactions with HCO3−. Inhibition of CA by acetazolamide has been shown to enhance this effect.36 It has been suggested that NO, biosynthesized from L-arginine inhibits CA, and that there is structural similarity between CA and nitrite.37 CA can generate nitric oxide from nitrite, the effect which is enhanced at low pHe.38 Moreover, CA has been suggested to be bound to sarcolemmal HCO3− transporter and is involved in transport of H+ ions.39 In the myocardium, it has been found that NHE1 is functionally linked to CA, which produces substrates for NHE1.40 Further, some agents like phenylephrine change the basal pHi through CA-mediated mechanism.41 However, our findings showed that the effect of NO donors on the basal pHi was not changed in the presence of acetazolamide. This observation suggests that shuttling of H+ ions through the cell and/or CO2/HCO3− buffering mechanism are not affected by NO, and it is unlikely that cardiac CA mediate the NO effect on pHi.
In summary, we have demonstrated that exogenous NO leads to an increased activity of NHE in isolated cardiac ventricular myocytes. This effect is preceded by the decrease in basal pHi and it is independent of CA and chloride-dependent sarcolemmal HCO3− and OH− transporters. Our findings indicate that in the cardiac myocytes NO plays a significant role in regulating cytosolic pH. However, the detailed mechanisms by which NO modulates pHi remain to be elucidated. Therefore, additional studies will be needed to confirm our observations in other species and models. Particularly, the role of other cytosolic proteins and NHE isoforms should be explored. In different clinical settings such are diabetes mellitus, acid-base disorders and cardiac ischemia-reperfusion injury NO and pHi have been shown to play a significant role in pathophysiology of those diseases. Therefore, strategies targeting No and pHi could be a novel and useful therapeutic approach.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institutes of Health Grants P01GM066730, RO1HL034708 (to ZJB) and The Federal Ministry of Science and Education grant - The effect of physical activity on muscle bioenergetics in chronic heart failure (to DP).
References
- 1.Niederer SA, Swietach P, Wilson DA, Smith NP, Vaughan-Jones RD. Measuring and modeling chloride-hydroxyl exchange in the Guinea-pig ventricular myocyte. Biophys. J. 2008;94:2385–2403. doi: 10.1529/biophysj.107.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pravdic D, Mio Y, Sedlic F, et al. Isoflurane protects cardiomyocytes and mitochondria by immediate and cytosol-independent action at reperfusion. Br. J. Pharmacol. 2010;160:220–232. doi: 10.1111/j.1476-5381.2010.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J. Mol. Cell. Cardiol. 2009;46:318–331. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Ch'en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br. J. Pharmacol. 2008;153:972–982. doi: 10.1038/sj.bjp.0707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to alpha1-adrenergic stimulation. Mol. Pharmacol. 2007;71:799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 6.Gunasegaram S, Haworth RS, Hearse DJ, Avkiran M. Regulation of sarcolemmal Na(+)/H(+) exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT(1) versus AT(2) receptors. Circ. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- 7.Wu ML, Tseng YZ. The modulatory effects of endothelin-1, carbachol and isoprenaline upon Na(+)-H+ exchange in dog cardiac Purkinje fibres. J. Physiol. 1993;471:583–597. doi: 10.1113/jphysiol.1993.sp019917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korichneva I, Puceat M, Millanvoye-Van Brussel E, Geraud G, Vassort G. Aldosterone modulates both the Na/H antiport and Cl/HCO3 exchanger in cultured neonatal rat cardiac cells. J. Mol. Cell. Cardiol. 1995;27:2521–2528. doi: 10.1006/jmcc.1995.0239. [DOI] [PubMed] [Google Scholar]
- 9.Lagadic-Gossmann D, Vaughan-Jones RD, Buckler KJ. Adrenaline and extracellular ATP switch between two modes of acid extrusion in the guinea-pig ventricular myocyte. J. Physiol. 1992;458:385–407. doi: 10.1113/jphysiol.1992.sp019423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ML, Vaughan-Jones RD. Effect of metabolic inhibitors and second messengers upon Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J. Physiol. 1994;478:301–313. doi: 10.1113/jphysiol.1994.sp020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez NG, Piaggio MR, Ennis IL, et al. Phosphodiesterase 5A inhibition induces Na+/H+ exchanger blockade and protection against myocardial infarction. Hypertension. 2007;49:1095–1103. doi: 10.1161/HYPERTENSIONAHA.107.087759. [DOI] [PubMed] [Google Scholar]
- 12.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 13.Ischiropoulos H, Gow A. Pathophysiological functions of nitric oxide-mediated protein modifications. Toxicology. 2005;208:299–303. doi: 10.1016/j.tox.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Kapil V, Webb AJ, Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96:1703–1709. doi: 10.1136/hrt.2009.180372. [DOI] [PubMed] [Google Scholar]
- 15.Gill RK, Saksena S, Syed IA, et al. Regulation of NHE3 by nitric oxide in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G747–G756. doi: 10.1152/ajpgi.00294.2001. [DOI] [PubMed] [Google Scholar]
- 16.Homaidan FR, Martello LA, Melson SJ, Burakoff R. Regulation of electrolyte transport by nitric oxide in the mouse cecum. Eur. J. Pharmacol. 1998;350:93–99. doi: 10.1016/s0014-2999(98)00221-0. [DOI] [PubMed] [Google Scholar]
- 17.Potgieter K, Hatcher NG, Gillette R, McCrohan CR. Nitric oxide potentiates cAMP-gated cation current by intracellular acidification in feeding neurons of pleurobranchaea. J. Neurophysiol. 2010;104:742–745. doi: 10.1152/jn.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95:2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- 19.Regli L, Held MC, Anderson RE, Meyer FB. Nitric oxide synthase inhibition by L-NAME prevents brain acidosis during focal cerebral ischemia in rabbits. J. Cereb. Blood Flow Metab. 1996;16:988–995. doi: 10.1097/00004647-199609000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111:267–274. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth. Analg. 2009;109:405–411. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan-Jones RD, Peercy BE, Keener JP, Spitzer KW. Intrinsic H(+) ion mobility in the rabbit ventricular myocyte. J. Physiol. 2002;541:139–158. doi: 10.1113/jphysiol.2001.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990;417:234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer KW, Bridge JH. Relationship between intracellular pH and tension development in resting ventricular muscle and myocytes. Am. J. Physiol. 1992;262:C316–C327. doi: 10.1152/ajpcell.1992.262.2.C316. [DOI] [PubMed] [Google Scholar]
- 25.Salvi A, Quillan JM, Sadee W. Monitoring intracellular pH changes in response to osmotic stress and membrane transport activity using 5-chloromethylfluorescein. AAPS PharmSci. 2002;4:E21. doi: 10.1208/ps040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Q, Sedlic F, Pravdic D, Bosnjak ZJ, Kwok WM. Biphasic effect of nitric oxide on the cardiac voltage-dependent anion channel. FEBS Lett. 2011;585:328–334. doi: 10.1016/j.febslet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagadic-Gossmann D, Vaughan-Jones RD. Coupling of dual acid extrusion in the guinea-pig isolated ventricular myocyte to alpha 1- and beta-adrenoceptors. J. Physiol. 1993;464:49–73. doi: 10.1113/jphysiol.1993.sp019624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer KW, Skolnick RL, Peercy BE, Keener JP, Vaughan-Jones RD. Facilitation of intracellular H(+) ion mobility by CO(2)/HCO(3)(−) in rabbit ventricular myocytes is regulated by carbonic anhydrase. J. Physiol. 2002;541:159–167. doi: 10.1113/jphysiol.2001.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J. Neurobiol. 1999;40:171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Caramelo C, Lopez-Farre A, Riesco A, et al. Atrial natriuretic peptide and cGMP inhibit Na+/H+ antiporter in vascular smooth muscle cells in culture. Kidney Int. 1994;45:66–75. doi: 10.1038/ki.1994.8. [DOI] [PubMed] [Google Scholar]
- 31.Roczniak A, Burns KD. Nitric oxide stimulates guanylate cyclase and regulates sodium transport in rabbit proximal tubule. Am. J. Physiol. 1996;270:F106–F115. doi: 10.1152/ajprenal.1996.270.1.F106. [DOI] [PubMed] [Google Scholar]
- 32.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am. J. Physiol. Renal Physiol. 1999;277:F377–F382. doi: 10.1152/ajprenal.1999.277.3.F377. [DOI] [PubMed] [Google Scholar]
- 33.Wilson KT, Xie Y, Musch MW, Chang EB. Sodium nitroprusside stimulates anion secretion and inhibits sodium chloride absorption in rat colon. J. Pharmacol. Exp. Ther. 1993;266:224–230. [PubMed] [Google Scholar]
- 34.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO(3)(−) transport by rat thick ascending limb. Kidney Int. 2000;58:2069–2074. doi: 10.1111/j.1523-1755.2000.00379.x. [DOI] [PubMed] [Google Scholar]
- 35.Coon S, Sundaram U. Unique regulation of anion/HCO3- exchangers by constitutive nitric oxide in rabbit small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G1084–G1090. doi: 10.1152/ajpgi.00013.2003. [DOI] [PubMed] [Google Scholar]
- 36.Swietach P, Spitzer KW, Vaughan-Jones RD. pH-Dependence of extrinsic and intrinsic H(+)-ion mobility in the rat ventricular myocyte, investigated using flash photolysis of a caged-H(+) compound. Biophys. J. 2007;92:641–653. doi: 10.1529/biophysj.106.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puscas I, Coltau M, Domuta G, Baican M, Puscas C, Pasca R. Carbonic anhydrase I inhibition by nitric oxide: implications for mediation of the hypercapnia-induced vasodilator response. Clin. Exp. Pharmacol. Physiol. 2000;27:95–99. doi: 10.1046/j.1440-1681.2000.03212.x. [DOI] [PubMed] [Google Scholar]
- 38.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 39.Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am. J. Physiol. Cell Physiol. 2007;293:C738–C748. doi: 10.1152/ajpcell.00157.2007. [DOI] [PubMed] [Google Scholar]
- 40.Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J. Enzyme Inhib. Med. Chem. 2004;19:199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez BV, Johnson DE, Sowah D, et al. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J. Physiol. 2007;579:127–145. doi: 10.1113/jphysiol.2006.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.