Abstract
Aim
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS), characterized by demyelination of white matter, loss of myelin forming oligodendrocytes, changes in the blood-brain-barrier (BBB), and leukocyte infiltration. Myelin basic protein (MBP) is a component of the myelin sheath. Degradation of myelin is believed to be an important step that leads to MS pathology. Transmigration of leukocytes across the vasculature, and a compromised BBB participate in the neuroinflammation of MS. We examined the expression and regulation of the chemokine CCL2 and the cytokine IL-6 in human endothelial cells (EC), a component of the BBB, after treatment with MBP.
Methods
EC were treated with full length MBP. CCL2 and IL-6 protein were determined by ELISA. Western blot analysis was used to determine signaling pathways. A BBB model was treated with MBP and permeability was assayed using albumin conjugated to Evan’s blue dye. The levels of the tight junction proteins occludin and claudin-1, and matrix metalloprotease (MMP)-2 were assayed by Western blot.
Results
MBP significantly induced CCL2 and IL-6 protein from EC. This induction was partially mediated by the p38 MAPK pathway as there was phosphorylation after MBP treatment. MBP treatment of a BBB model caused an increase in permeability that correlated with a decrease in occludin and claudin-1, and an induction of MMP-2.
Conclusion
These data demonstrate that MBP induces chemotactic and inflammatory mediators. MBP also alters BBB permeability and tight junction expression, indicating additional factors that may contribute to the BBB breakdown characteristic of MS.
Keywords: MBP, MMP2, IL-6, CCL2, EAE, blood-brain-barrier, MS
INTRODUCTION
Multiple sclerosis (MS), an autoimmune inflammatory demyelinating disease of the central nervous system (CNS), affects mainly the white matter. This demyelination characteristic of MS is a result of the breakdown of the myelin sheath [1, 2], resulting in the release of soluble myelin basic protein (MBP) [3].
MBP is the second most abundant protein in the CNS, and is essential for the formation of myelin. Immunostaining of tissue from patients with MS revealed the presence of extracellular myelin proteins, including MBP, in leptomeninges and perivascular spaces, specifically between the basement membranes of the vessels and glial limitans [4]. Also, we have previously shown that extracellular MBP can affect CNS cells by inducing their expression of cell-surface proteins [5]. In an animal model of MS, experimental autoimmune encephalomyelitis (EAE), demyelination occurred from degradation of myelin, including cleavage of MBP to smaller peptides for presentation to T cells, thereby sensitizing them to damage myelin [6-8]. In MS, the cleavage of MBP results in peptides containing the immunodominant epitope that sensitizes T cells to digest further myelin [8]. Demyelination may also result from the phagocytosis of MBP. When demyelinated areas of rats induced to develop EAE were immunostained, MBP expression was found within macrophages which demonstrated phagocytosis of myelin [9].
MS is characterized by the presence of anti-MBP autoantibodies in sera and CSF of affected individuals, with anti-MBP levels increasing with disease progression [10, 11]. Individuals with serum anti-MBP antibodies developed clinically defined MS more often and earlier than individuals without antibodies, indicating the use of these antibodies as a possible diagnostic tool for predicting early conversion to clinically defined MS [12, 13]. Also, the presence of MBP within the cerebrospinal fluid (CSF) has been used as a marker for disease severity, with increasing concentrations seen during relapse and onset of clinical symptoms ([14-16] and reviewed in [17]). When MBP was injected into the CSF of rats, it was shown to bind to the luminal surface of endothelial cells of vessel walls, indicating that after MBP release from the brain, it can be presented on vascular endothelial cells where it can then be recognized by sensitized T cells [18].
Exacerbated release of chemokines and cytokines are also important in the pathogenesis of MS and EAE. Chemokines are chemotactic cytokines that can attract and activate cells, including those of the CNS [19, 20]. Chemokine (C-C motif) ligand 2 (MCP-1; CCL2) attracts monocytes, activated T cells, and microglia, and is secreted by astrocytes, microglia, endothelial cells and macrophages. We and others showed that CCL2 is expressed by astrocytes and macrophages in acute and chronic lesions, as well as in demyelinating plaques [21-24]. Anti-CCL2 antibodies administered prior to induction of EAE prevented disease [25, 26]. In rats with EAE, treatment with 7ND, an inhibitor of CCL2, had a complete suppression of relapse of biphasic and chronic EAE [27]. These studies underscore the important role of CCL2 in MS and EAE pathogenesis.
Interleukin (IL)-6 induces leukocyte chemoattraction to the endothelium, as well as lymphocyte activation (reviewed in [28]). It is secreted by activated immune cells, microglia, astrocytes and endothelial cells [29], and is found in high concentrations in active MS lesions [30, 31]. In a rat model of EAE, IL-6 was induced in the spinal cord, with maximum expression at onset of disease [32]. Mice treated with resveratrol, an anti-inflammatory agent, were resistant to EAE induction due to diminished IL-6 production from macrophages [33]. Anti-IL-6 antibodies reduced incidence and severity of EAE [34], whereas treatment with anti-IL-6 receptor antibodies prevented EAE induction [35].
The blood-brain-barrier (BBB) separates the CNS from the periphery. It is maintained by tight junction proteins between endothelial cells, as well as by interactions with astrocytic end feet, pericytes, microglia, and some neuronal elements. The permeability of the BBB is compromised if tight junctions are disrupted (reviewed in [36]). BBB breakdown has been associated with active demyelination in rats with EAE [37]. In mice, disease severity in the acute phase of EAE was directly correlated to the degree of BBB permeability [38]. In MS, disruption of the BBB precedes formation of demyelinating lesions [39], and development of clinical signs [40]. A disrupted BBB may be one mechanism by which MBP can leave the brain and enter the periphery to interact with vascular endothelial cells [18].
As CCL2 and IL-6 are important in the pathogenesis of MS, we addressed the mechanism(s) by which these proteins are upregulated in EC, a major component of the BBB, and how this upregulation leads to BBB disruption. We show that EC secrete CCL2 and IL-6 in response to MBP. Treatment with specific inhibitors demonstrated that production of these proteins in response to MBP is partially dependent on the p38 MAPK pathway. We also demonstrate that MBP treatment of either the endothelial cells or astrocytes in our human BBB model significantly increases permeability of the BBB, and that this may be attributed to decreased occludin and claudin-1, and increased matrix metalloprotease (MMP)-2 seen in EC after MBP treatment. Thus, MBP, released during myelin breakdown, may have several effects that mediate the pathogenesis of MS. It can induce endothelial cells to elaborate factors that lead to the recruitment of autoreactive T cells and may also actively participate in BBB breakdown, thereby augmenting the inflammatory cascade characteristic of MS.
MATERIALS AND METHODS
Cell Culture and Reagents
Human umbilical vein endothelial cells (EC) were isolated as previously described [41]. Briefly, umbilical veins were rinsed with sterile saline and digested with 0.1% collagenase. EC were cultured on 0.2% gelatin-coated tissue culture dishes with M199 media (Invitrogen), supplemented with 20% newborn calf serum (Biocell, Rancho Dominguez, California), 25μg/ml heparin (Sigma, St. Louis, Missouri), 50μg/ml ascorbic acid (Sigma), 1.6 mM L-glutamine (Invitrogen), 7.5μg/ml endothelial cell growth factor (Sigma), 2.78μg/ml bovine brain extract (Clonetics/BioWittaker, Walkersville, Maryland), 1% penicillin-streptomycin (Invitrogen), 0.16% bicarbonate, and 10mM Hepes (Sigma). Confluent EC at passage 3 were used for all experiments. The purity of the EC cultures was determined by immunostaining with factor VIII (>99% positive) as previously described [42]. We have also performed comparable experiments using brain microvascular EC and found no differences in cytokine expression or in the coculture model [43]. Total MBP antigen from human brain was obtained from Sigma. EC were treated with different doses of MBP or left untreated for 24 hours, or treated with 100μg/ml of MBP or left untreated for 4, 7 and 24 hours, after which supernatants were collected and analyzed for CCL2 and IL-6 protein secretion. For inhibitor studies, EC were pretreated with 10 μM of the MEK1/2 MAPK inhibitor, U0126, 10 μM of the p38 α and β MAPK inhibitor, SB203580, or 10 μM of the phosphatidylinositol-3-kinase (PI3K) inhibitor, LY294002 for one hour. MBP at 100μg/ml was then added to the cultures without changing the media for an additional 24 hours. Supernatants were then collected and analyzed for CCL2 and IL-6 protein expression.
Human fetal CNS tissue was obtained and used as part of an ongoing research protocol approved by the Albert Einstein College of Medicine. Astrocytes were obtained as previously described [43]. Briefly, tissue was minced and digested in 0.25% trypsin (Gibco BRL) and 1% DNase1 at 37°C for 45 minutes. The tissue was then passed through a 250μm nylon mesh filter, followed by a 150μm filter, and washed. Cells were resuspended in DMEM plus 25mM Hepes, 10% fetal calf serum, 1% penicillin-streptomycin, and 1% nonessential amino acids, and plated at 9×107 per 150cm2 flask. The cultures were maintained at 5%CO2 and 37°C. After 12 days, the cells were removed from the flasks and plated in 100mm dishes (1 flask per 2 dishes), and passaged when confluent. Purity of astrocyte cultures was determined by immunostaining with glial fibrillary acidic protein (>99% positive) and HAM56 (unreactive) as previously described [42]. In early cultures, there is some neuronal contamination, but after several passages, the neuronal population dies out, therefore, cells were used at passage 4 for the BBB model to ensure the absence of contaminating cells.
Chemokine/Cytokine ELISAs
Supernatants were analyzed for CCL2 and IL-6 protein using a sandwich ELISA according to the manufacturer’s protocol. CCL2 and IL-6 ELISA antibody pairs were purchased from R&D Systems. The limits of detection for these assays are 4pg/mL and 4.7pg/ml, respectively.
Western Blot Analysis
Twenty-four hours after plating, EC were treated with 100ng/mL MBP for 120 and 240 minutes, or left untreated. For occludin, claudin-1, and MMP2 analyses, cells were treated with MBP for 24 hours. EC were washed with 1x PBS and lysed with cell lysis buffer (Cell Signaling Technologies, Beverly, Massachusetts) supplemented with 1mM PMSF. The cells were sonicated and protein was quantified using the Bio-Rad Protein Assay (Bio-Rad Laboratories). The protein was heated at 95°C for 5 minutes and 20 μg of lysate was loaded onto each lane of a 10% SDS-PAGE gel. Proteins were transferred electrophoretically to Protran nitrocellulose (Schleicher & Schuell, Keene, New Hampshire). Membranes were blocked with 5% nonfat dry milk and 3% bovine serum albumin in 0.1% Tween-20/TBS for 1 hour, and incubated with primary antibodies (phospho-p38 MAP kinase Ab, Cell Signaling Technologies, occludin Ab, Invitrogen, Carlsbad, California, claudin-1 Ab, Invitrogen, or anti-MMP2 Ab, Cell Signaling Technologies) at a concentration of 1:1000 for phospho-p38, 1:800 for occludin and claudin-1, and 1:1000 for MMP2, overnight at 4 °C. After washing, membranes were incubated with anti-rabbit-HRP (phospho-p38, claudin-1, and MMP2) or anti-mouse-HRP (occludin) secondary antibody (1:2000, Cell Signaling Technologies) for 1 hour at room temperature. Proteins were visualized using an enhanced chemiluminescence detection kit (ECL, Amersham-Pharmacia, Piscataway, New Jersey). For α-tubulin detection, membranes were shaken in stripping buffer (62.5mM tris-HCl pH 6.8, 2% SDS, and 100mM β-mercaptoethanol) for 30 minutes at 50°C, and washed repeatedly. Membranes were then blocked with 5% nonfat dry milk and 3% bovine serum albumin in 0.1% Tween-20/TBS for 1 hour, and incubated with α-tubulin (Sigma) at a concentration of 1:10000, overnight at 4 °C. After washing, membranes were incubated with anti-mouse-HRP secondary antibody (1:2000, Cell Signaling Technologies) for 1 hour at room temperature. Protein was visualized using an enhanced chemiluminescence detection kit (ECL, Amersham-Pharmacia).
Human BBB Model
The BBB was established as previously described [42, 44, 45]. Briefly, EC and astrocytes were cultured on either side of a 3μm gelatin-coated tissue culture insert (BD-Falcon, Franklin Lakes, New Jersey). The cocultures were incubated for 3 days to establish BBB properties, including EC expression of glucose transporter-1 and γ-glutamyltranspeptidase, as well as increased expression of tight junction proteins, after which, the EC or the astrocytes were treated with 100μg/ml MBP for 24 hours. The inserts were rinsed in phenol red-free DMEM and permeability was tested.
Permeability Assay
Permeability of inserts from the BBB model was assayed as previously described [44]. Briefly, the inserts were rinsed in phenol red-free DMEM and placed in clean 24-well culture plates containing 200μL albumin (0.45%) conjugated to Evan’s blue dye (EBA) in the upper chamber and 400μL phenol red-free DMEM+10% FCS in the lower chamber. The inserts were incubated for 30 minutes at 37°C, after which the media from the lower chamber was collected and read spectrophotometrically at 620nm in order to determine the amount of EBA that passed through the cells, where increased OD readings corresponded to greater disruption of the barrier.
Statistical Analysis
The Student’s t-test (two-tailed) was used to determine significance. A value of p<0.05 was considered to be significant.
RESULTS
MBP treatment of EC induces CCL2 and IL-6 secretion
EC were treated with MBP (1μg/ml, 10μg/ml, 50μg/ml or 100μg/ml) or left untreated for 24 hours. Since MBP was diluted in HCl, each experiment also included EC treated with 10mM HCl, which was the concentration corresponding to 100μg/ml of MBP. This treatment did not result in any significant protein induction (data not shown), so the untreated corresponds to no treatment at all. Additionally, prior to treatment, all MBP preparations were sterile filtered to ensure endotoxin-free conditions. MBP treatment of EC resulted in significant secretion of CCL2. MBP at 10μg/ml, 50μg/ml and 100μg/ml significantly increased CCL2 secretion compared to untreated cells (Figure 1A; *p<0.03; n=4), where 10μg/ml of MBP resulted in 15.7 ± 3.5ng/ml, 50μg/ml of MBP resulted in 27.2 ± 3.6ng/ml, and 100μg/ml resulted in 37.2 ± 3.3ng/ml of CCL2 compared to 13 ± 3ng/ml of CCL2 for untreated cells. MBP at 50μg/ml and 100μg/ml significantly increased CCL2 secretion compared to MBP at 10μg/ml (Figure 1A; **p<0.03; n=4), however there was no significant difference in CCL2 secretion between MBP at 50μg/ml or 100μg/ml (Figure 1A). MBP at 50μg/ml and 100μg/ml treatments significantly increased IL-6 compared to untreated cells (Figure 1B; #p<0.05; n=4), where 50μg/ml of MBP resulted in 4.3 ± 2.5ng/ml and 100μg/ml resulted in 6.2 ± 1.8ng/ml of IL-6 compared to 2.5 ± 2.1ng/ml of IL-6 for untreated cells. While MBP clearly increased IL-6, its induction was not significant between MBP at 50μg/ml and 100μg/ml. Since both MBP at 50μg/ml and 100μg/ml were significantly increased compared to MBP at 10μg/ml for CCL2 and these doses also induced IL-6, MBP was used at 100μg/ml for all subsequent experiments. As these are primary cells, background cytokine levels vary among cultures. To account for varying baseline levels, the experiments were repeated at least three times and the data were expressed as fold induction.
Figure 1. MBP induces CCL2 and IL-6 secretion by EC.
MBP treatment of EC induces (A) CCL2 and (B) IL-6 protein secretion. EC were treated with different concentrations of MBP or untreated for 24 hours, after which the supernatants were collected and assayed for CCL2 or IL-6 as described in Materials and Methods. Shown are the means of 4 separate experiments ±SEM. *p<0.03 compared to untreated; **p<0.03 compared to MBP at 10μg/ml; #p<0.05 compared to untreated.
Kinetics of MBP-induced CCL2 and IL-6 secretion
EC were treated for 4, 7 or 24 hours with 100μg/ml of MBP, or left untreated. Although CCL2 secretion began to increase at 4 hours and 7 hours, significant CCL2 production occurred at 24 hours of MBP treatment, with 49.1 ± 4.9ng/ml compared to 18 ± 2.7ng/ml for untreated cells (Figure 2A; *p<0.006; n=3). Additionally, MBP-induced CCL2 production was significantly increased at 24 hours compared to MBP-induced CCL2 production at 7 hours (Figure 2A; **p<0.02; n=3). After MBP treatment, IL-6 secretion was significantly increased at 4 hours (0.9 ± 0.05ng/ml), 7 hours (2 ± 0.3ng/ml), and 24 hours (5.3 ± 0.2ng/ml), as compared to untreated cells at each time point (0.2 ± 0.01ng/ml, 0.3 ± 0.08ng/ml, 1.2 ± 0.3ng/ml, respectively; Figure 2B; *p<0.006, #p<0.04, †p<0.02; n=3). IL-6 was also significantly increased at 24 hours compared to MBP-induced IL-6 production at 7 hours (Figure 2B; ††p<0.003; n=3).
Figure 2. MBP induces CCL2 and IL-6 secretion by EC in a time dependent manner.
MBP treatment of EC induces (A) CCL2 and (B) IL-6 protein secretion. EC were treated for 4 hours, 7 hours or 24 hours with 100μg/ml of MBP or were left untreated, after which the supernatants were collected and assayed for CCL2 or IL-6 as described in Materials and Methods. Shown are the means of 3 separate experiments ±SEM. *p<0.006 compared to untreated; **p<0.02 compared to MBP at 100μg/ml for 7 hours; #p<0.04 compared to untreated; †p<0.02 compared untreated; ††p<0.003 compared to MBP at 100μg/ml for 7 hours.
MBP-induced secretion of CCL2 and IL-6 are mediated, in part, through the p38MAPK pathway
The signal transduction pathways that are activated in EC by MBP treatment resulting in CCL2 and IL-6 production were examined. Treatment of EC with 100μg/ml of MBP and either 10μM of the MEK1/2 inhibitor, U0126, 10μM of the p38 inhibitor, SB203580, or 10μM of the PI3K inhibitor, LY294002, for 24 hours demonstrated that only SB203580 significantly inhibited MBP-induced CCL2 protein (reduced by 35 ± 3.3% compared to MBP treatment only; Figure 3A; **p<0.005; n=4). There was no significant difference in MBP-induced CCL2 protein after treatment with either U0126 or LY294002. Similarly, treatment of EC with 100μg/ml of MBP and any of the above described inhibitors for 24 hours demonstrated that SB203580 significantly inhibited MBP-induced IL-6 protein (reduced by 59 ± 4.9% compared to MBP treatment only; Figure 3B; **p<0.005; n=4). There was no difference in MBP-induced IL-6 protein after treatment with U0126 or LY294002. Lysates prepared from EC treated with and without MBP at different time points were then studied by Western blot analysis to examine MBP-mediated p38 phosphorylation. As demonstrated in Figure 4, MBP induced phosphorylation of p38 after 120 minutes, and this phosphorylation remained for 240 minutes (Figure 4 upper panel, lanes 6 and 8). The lower panel represents the α-tubulin loading control.
Figure 3. MBP-induced CCL2 and IL-6 are mediated, in part, by the p38 MAPK pathway.
(A) CCL2 and (B) IL-6 proteins were determined as percent difference compared to MBP (set to 100%). EC were pretreated with 10μM of the MEK1/2 MAPK inhibitor, U0126, 10μM of the p38 MAPK inhibitor, SB203580, or 10μM of the phosphatidylinositol-3-kinase (PI3K) inhibitor LY294002 for 1 hour, then treated with or without 100μg/ml MBP for an additional 24 hours Shown are the means of 4 (CCL2) and 3 (IL-6) separate experiments ±SEM. *p<0.003, #p<0.04 compared to untreated; and **p<0.005 compared to MBP. U0=U0126 at 10μM, LY=LY294002 at 10μM, SB=SB203580 at 10μM.
Figure 4. MBP treatment of EC induces phosphorylation of p38 MAPK.
EC were treated with 100μg/ml of MBP for different time points, after which cell lysate was collected and analyzed. Western blot analysis of MBP treated EC shows phosphorylated p38 (upper panel) and α-tubulin expression (lower panel). Shown is a representative blot of 2 separate experiments. Lane 1: untreated 30 minutes; lane 2: 100μg/ml of MBP 30 minutes; lane 3: untreated 60 minutes; lane 4: 100μg/ml of MBP 60 minutes; lane 5: untreated 120 minutes; lane 6: 100μg/ml of MBP 120 minutes; lane 7: untreated 240 minutes; lane 8: 100μg/ml of MBP 240 minutes.
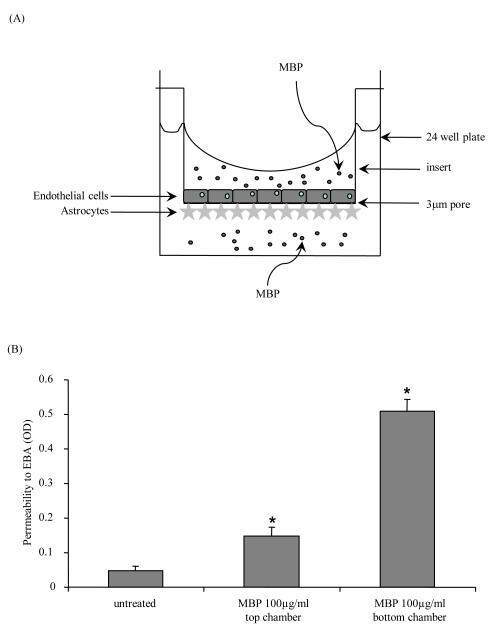
MBP treatment increases permeability of a human BBB model
In MS there is increased permeability of the BBB [46, 47]. We therefore determined whether MBP disrupted our human BBB model as assayed by an increase in permeability to Evan’s blue dye conjugated albumin. EC were plated on the upper side of a 3μm pore insert and astrocytes were plated on the under side (Figure 5A). We previously demonstrated that after three days in co-culture, the cells take on BBB properties, such as increased expression of tight junction proteins and BBB-specific proteins [42-45]. After three days in culture, the astrocyte or the EC side was treated with 100μg/ml of MBP for 24 hours, after which permeability was tested. As shown in Figure 5B, treatment of either the EC (0.147 ± 0.027 OD) or the astrocyte side (0.509 ± 0.036 OD) of the barrier resulted in significantly increased permeability as compared to the untreated barrier (0.048 ± 0.012 OD; *p<0.006; n=3). EDTA at 4mM was used as a positive control for barrier disruption (data not shown).
Figure 5. MBP treatment of the human BBB model increases permeability.
(A) EC (rectangles) were plated on the upper side of a 3 μm pore insert and astrocytes (stars) were plated on the under side of the insert, which was placed into one well of a 24 well plate. EC or astrocytes were treated with 100μg/ml of MBP (small circles) for 24 hours. (B) After the 24 hour treatment, permeability of the barrier was assayed with albumin conjugated to Evan’s blue dye (EBA). Shown are the means of 3 separate experiments ±SEM. *p<0.006 compared to untreated inserts.
MBP treatment of EC results in a decrease in the tight junction proteins occludin and claudin-1
BBB EC express increased levels of tight junction proteins. We therefore examined whether the tight junction proteins occludin and claudin-1 are altered after MBP treatment of EC. As shown in Figure 6A, Western blot analysis demonstrates that after treatment of EC with 100μg/ml of MBP, occludin is decreased as compared to untreated cells (Figure 6A, upper panel). Although occludin did not present as a distinct band, the diffuse banding pattern has been observed by others [48]. The lower panel represents the α-tubulin loading control. Densitometric analysis was also performed. Occludin was significantly reduced by 33.5 ± 7.6% after MBP treatment (Figure 6B; *p<0.05; n=2). Similar results were obtained with claudin-1 expression. As shown in Figure 6C, Western blot analysis demonstrates a reduction of claudin-1 protein expression after treatment of EC with 100μg/ml of MBP compared to untreated cells (Figure 6C, upper panel). The lower panel represents the α-tubulin loading control. Densitometric analysis demonstrated that claudin-1 was significantly reduced by 23.3 ± 5.4% after MBP treatment (Figure 6D; *p<0.05; n=2).
Figure 6. MBP treatment of EC decreases expression of the tight junction proteins occludin and claudin-1.
EC were treated for 24 hours with 100μg/ml of MBP or untreated (untx), after which the lysate was collected and analyzed. (A) Western blot analysis of MBP treated EC shows expression of occludin (upper panel) and α-tubulin (lower panel). (B) Densitometric analysis was used to determine significance in changes of occludin expression. (C) Western blot analysis of MBP treated EC shows expression of claudin-1 (upper panel) and α-tubulin (lower panel). (B) Densitometric analysis was used to determine significance in changes of claudin-1 expression. Shown is a representative blot of 2 separate experiments. *p<0.05
MBP treatment of EC results in an increase in MMP2 protein
Matrix metalloproteinases (MMPs) have been implicated in the breakdown of the BBB and in various neuroinflammatory diseases (reviewed in [49, 50]). We demonstrate that MBP increases BBB permeability of our model (see Figure 5). We therefore determined if the increase in permeability was due, at least in part, to the production of MMPs. EC were treated with 100μg/ml of MBP or were left untreated for 24 hours. Figure 7 (n=2) demonstrates that MBP treatment increases the active form of MMP2, an MMP that degrades collagen, a component of the basement membrane [51].
Figure 7. MBP treatment increases MMP2 expression by EC.

EC were treated for 24 hours with 100μg/ml of MBP or left untreated, after which the supernatants were collected and Western blots were performed. Shown is a representative blot of 2 separate experiments.
DISCUSSION
CNS inflammation, BBB breakdown, and the resulting demyelination and neuronal damage and loss are characteristics of MS [1, 52]. The breakdown of MBP, one of the major myelin proteins, is essential to the demyelination process [2]. In addition, individuals with anti-MBP antibodies in their serum have an increased number of demyelinated lesions [53]. Degradation of MBP has been hypothesized as a mechanism of generating immunodominant MBP peptides. Others showed that incubation of a charged MBP isomer from normal and MS tissue resulted in the accumulation of small peptide fragments containing the immunodominant epitope, and MBP from MS tissue generated 200 fold more peptide than MBP from normal tissue [8]. Chemokines and cytokines have also been shown to play a significant role in the pathogenesis of MS. They have been demonstrated in MS lesions and demyelinating plaques and contribute to the inflammatory cascade seen during disease (reviewed by [54]). Therefore, since MBP is present in MS tissue and chemokines and cytokines are essential to inflammation and the developing lesion, we examined the inflammatory role of MBP in mediating the pathogenesis of MS.
CCL2 is highly expressed in acute and chronic MS lesions and demyelinating plaques [21-24]. Increased expression of CCL2 by EC in vessels within demyelinating plaques has been demonstrated [55]. Brain EC, removed from demyelinating plaques of people with MS, also demonstrated and increase in CCL2 protein production [55] and mRNA expression [56] after treatment with INFγ and TNFα, two cytokines prevalent in MS brains. The CCL2 receptor, CCR2, has also been implicated in disease, as it has been detected on astrocytes, macrophages and activated microglia in active MS lesions [24, 57]. CCR2+ cells can transmigrate across an in vitro BBB model more efficiently that CCR2-cells [58] and a significantly higher percentage of CCR2+ T cells were present in individuals with secondary progressive MS (SPMS) compared to control groups [59]. EAE models have also demonstrated the importance of the CCL2-CCR2 axis in disease development, as KO mice of either CCL2 or CCR2 failed to develop disease [60-62]. In a TNFα-induced demyelinating mouse model of MS, CCL2 and CCR2 were both localized to astrocytes and appeared prior to onset of symptoms [63]. In EAE, CCL2 production by astrocytes was associated with inflammatory cell infiltrate into the CNS, but when mice were treated with a fullerene derivative that has neuroprotective effects, CCL2 expression by astrocytes was downregulated, which resulted in decreased CD11b+ cell infiltrate, and ultimately resulted in reduced disease progression, axonal loss, and demyelination [64]. Others also demonstrated that CNS production of CCL2 results in CD11b+ dendritic cells which secrete iNOS and TNF-producing macrophages, both of which are important in demyelination [65].
IL-6 has been shown to be present in active MS lesions in high concentrations [30, 31]. IL-6 levels in the CSF of individuals with relapsing-remitting MS (RRMS) were significantly higher as compared to individuals with SPMS or to controls [66]. Also, IL-6 production by peripheral blood mononuclear cells of individuals with SPMS after treatment with interferon β-1b, a therapy for MS, was decreased, and individuals with decreased IL-6 did not progress [67]. Studies in EAE confirm the importance of IL-6. Treatment of mice with a selective IL-6 inhibitor reduced symptoms of EAE [68]. Also, IL-6 KO mice are resistant to EAE due to the inability of leukocytes to enter the CNS [69].
Naïve CD4+ T cells stimulated with IL-6, can be induced to differentiate into Th17 cells [70-73], that have been shown to be important in MS and EAE. Th17 cells have been found in the MS lesion, and secrete IL-17. It has been shown that IL-17 stimulates BBB EC to produce CCL2 [70-73]. These data present an interesting mechanism demonstrating the importance of IL-6, as well as the relationship of IL-6 and CCL2 in MS.
After treatment of EC with MBP, we demonstrated that there is an increase in secreted CCL2 and IL-6. Using inhibitors to different MAPK pathways, we demonstrated that both CCL2 and IL-6 secretion by EC is dependent on the p38 MAPK pathway, and p38 MAPK is phosphorylated upon MBP treatment of EC. The p38 MAPK pathway has been demonstrated to be important in CNS disease. In the Theiler’s murine encephalomyelitis virus (TMEV) model of MS, there is an increase in cyclooxygenase (COX)-2 expression [74]. Expression of COX-2 has been found in MS tissue, where it was localized to EC [75, 76], and it has been shown to be important in inflammation, apoptosis, and regulation of BBB permeability [77-79]. Expression of COX-2 in TMEV infected EC was dependent on the p38 MAPK pathway [74]. Also, inhibition of the p38 MAPK pathway in mixed glial cells resulted in decreased inducible nitric oxide synthase and survival of oligodrendocyte progenitors [80].
BBB disruption contributes to CNS invasion by mononuclear cells that perpetuate the inflammatory cascade and myelin breakdown seen in MS and EAE [46, 47, 81, 82]. Several mediators have been hypothesized to be involved in this breakdown [83-86]. We treated our human BBB model with MBP to determine its effects on permeability. We found a significant increase in permeability of the barrier, indicating disruption, when either the EC or the astrocyte side was treated with MBP. This indicates that soluble MBP can directly interfere with the integrity of the barrier to facilitate the influx of inflammatory cells that may enhance the damage occurring in the CNS.
Tight junction proteins are a critical component in BBB integrity. Occludin and claudin-1 are important proteins that maintain the BBB [87, 88]. In MS, breakdown of the BBB is one of the earliest events that occurs in the formation of new lesions, and it precedes other abnormalities, and thereby, with subsequent inflammation, could be a critical event in the pathogenesis and development of new lesions [89]. It has been shown that there are abnormal or open tight junctions in active plaques of individuals with MS [90]. Serum from individuals with MS can decrease the expression of occludin in endothelial cells [39]. In an EAE model, it was demonstrated that occludin was dephosphorylated, and this preceded signs of disease. This decrease in phosphorylation was associated with an increase in CNS accumulation of albumin that paralleled the clinical score, implicating occludin in BBB breakdown [48]. Also, mice that expressed claudin-1 had reduced disease severity compared to wildtype mice [91].Based on these previous studies, we investigated decreases in claudin-1 and occludin proteins as a potential mechanism by which MBP increased permeability in our BBB model. Our preliminary studies demonstrated that MBP treatment of EC decreases both occludin and claudin-1 expression. This suggests that a reduction of occludin and claudin-1 mediated by interactions of MBP with EC in the CNS may therefore lead to a compromised BBB, possibly resulting in formation of new lesions.
Proteases also function in the maintenance/destruction of BBB integrity. MMP2, degrades denatured collagen as well as intact collagen type IV, a major component of the basement membrane. MMP2 has two forms, the 72 kDa pro, inactive, form, and the 64 kDa active form that results from proteolytic cleavage of the inhibitory peptide from the pro-form [51]. Immunohistochemical analysis of rats with EAE demonstrated an increase in MMP2 expression [92]. A metalloprotease inhibitor can reduce the sequelae associated with EAE [93-95]. Our preliminary results demonstrate that MBP treatment of EC increases active MMP2. A decrease of claudin-1 and occludin proteins, along with an increase in active MMP2 demonstrates the potential for a direct MBP-mediated mechanism of BBB disruption.
Although the mechanism by which MBP interacts with EC and astrocytes is unknown, it has been shown that rat astrocytes, microglia, and oligodendrocytes express the low-density lipoprotein receptor-related protein (LRP) 1 and this receptor was essential in phagocytosis of myelin vesicles from rat brain, specifically by interacting with MBP [96]. Others have demonstrated that LRP1 is expressed by human astrocytes [97] and EC [98] as well, so this may be one possible mechanism by which MBP is able to interact with these cells. Even though LRP is thought to be a receptor involved in endocytosis, whose ligands are degraded in lysosomes, it has been demonstrated that when certain ligands bind LRP, intracellular signal transduction events are initiated, and among them is permeability of the BBB [99].
Our data suggest a mechanism whereby soluble MBP, which is present in demyelinating lesions associated with MS, alters EC function. We demonstrate that MBP induces EC to elaborate inflammatory factors that participate in the pathogenesis of MS and EAE. MBP also results in disruption of the BBB, which may be due to reduction of tight junction proteins (occludin and claudin-1) and production of a proteolytic enzyme (MMP2). Once the BBB is compromised, this facilitates the influx of inflammatory cells which enhances the inflammatory cascade. This will result in further damage and demyelination of the axons of neurons, exacerbating the pathogenesis of MS.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Mental Health grants MH075679 (J. W. B.), MH070297 (J. W. B.), MH076679 (E. A. E.), MH096625 (E.A.E.), National Institutes of Health grant NS11920 (J. W. B.), the National Institutes of Health Experimental Neuropathology Training Grant NS07098 (T. G. D), and the Immunology/Pathology core of the National Institutes of Health Center for AIDS Research (CFAR) AI051519 (J.W.B. and E.A.E.).
ABBREVIATIONS
- BBB
blood-brain-barrier
- CCL-2
chemokine (C-C motif) ligand-2
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- EC
human endothelial cells
- IL-6
interleukin-6
- MBP
myelin basic protein
- MMP-2
matrix metalloprotease-2
- MS
multiple sclerosis
Footnotes
Conflict of Interest Statement: None of the authors listed above has any conflict of interest specific to this manuscript which would prevent or delay its submission to Neuropathology and Applied Neurobiology.
AUTHOR CONRIBUTIONS T.G.D. designed and performed the experiments with endothelial cells, and wrote the initial draft of the manuscript. E.A.E. and L.L. performed the blood-brain-barrier experiments as well as several Western analyses. J.W.B. aided in designing the experiments and interpreting the data. The final manuscript was edited by all authors.
REFERENCES
- 1.Pouly S, Antel JP. Multiple sclerosis and central nervous system demyelination. J Autoimmun. 1999;13:297–306. doi: 10.1006/jaut.1999.0321. [DOI] [PubMed] [Google Scholar]
- 2.Hallpike JF, Adams CW. Proteolysis and myelin breakdown: a review of recent histochemical and biochemical studies. Histochem J. 1969;1:559–78. doi: 10.1007/BF01012862. [DOI] [PubMed] [Google Scholar]
- 3.Lutton JD, Winston R, Rodman TC. Multiple sclerosis: etiological mechanisms and future directions. Exp Biol Med (Maywood) 2004;229:12–20. doi: 10.1177/153537020422900102. [DOI] [PubMed] [Google Scholar]
- 4.Kooi EJ, van Horssen J, Witte ME, Amor S, Bo L, Dijkstra CD, van der Valk P, Geurts JJ. Abundant extracellular myelin in the meninges of patients with multiple sclerosis. Neuropathology and applied neurobiology. 2009;35:283–95. doi: 10.1111/j.1365-2990.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 5.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Cross AH, Dolich S, Raine CS. Antigen processing of myelin basic protein is required prior to recognition by T cells inducing EAE. Cell Immunol. 1990;129:22–31. doi: 10.1016/0008-8749(90)90183-r. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza CA, Moscarello MA. Differences in susceptibility of MBP charge isomers to digestion by stromelysin-1 (MMP-3) and release of an immunodominant epitope. Neurochem Res. 2006;31:1045–54. doi: 10.1007/s11064-006-9116-9. [DOI] [PubMed] [Google Scholar]
- 8.Mastronardi FG, Moscarello MA. Molecules affecting myelin stability: a novel hypothesis regarding the pathogenesis of multiple sclerosis. J Neurosci Res. 2005;80:301–8. doi: 10.1002/jnr.20420. [DOI] [PubMed] [Google Scholar]
- 9.Sajad M, Zargan J, Chawla R, Umar S, Khan HA. Upregulation of CSPG3 accompanies neuronal progenitor proliferation and migration in EAE. Journal of molecular neuroscience : MN. 2011;43:531–40. doi: 10.1007/s12031-010-9476-0. [DOI] [PubMed] [Google Scholar]
- 10.Zabaleta M, Marino R, Borges J, Camargo B, Ordaz P, De Sanctis JB, Bianco NE. Activity profile in multiple sclerosis: an integrative approach. A preliminary report. Mult Scler. 2002;8:343–9. doi: 10.1191/1352458502ms803oa. [DOI] [PubMed] [Google Scholar]
- 11.Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, Berger T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;122(Pt 11):2047–56. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- 12.Belogurov AA, Jr., Kurkova IN, Friboulet A, Thomas D, Misikov VK, Zakharova MY, Suchkov SV, Kotov SV, Alehin AI, Avalle B, Souslova EA, Morse HC, 3rd, Gabibov AG, Ponomarenko NA. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. J Immunol. 2008;180:1258–67. doi: 10.4049/jimmunol.180.2.1258. [DOI] [PubMed] [Google Scholar]
- 13.Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 14.Alvord EC, Jr., Hruby S, Shaw CM, Slimp J. Myelin basic protein and its antibodies in the cerebrospinal fluid in experimental allergic encephalomyelitis, multiple sclerosis and other diseases. Progress in clinical and biological research. 1984;146:359–63. [PubMed] [Google Scholar]
- 15.Ohta M, Ohta K. Detection of myelin basic protein in cerebrospinal fluid. Expert review of molecular diagnostics. 2002;2:627–33. doi: 10.1586/14737159.2.6.627. [DOI] [PubMed] [Google Scholar]
- 16.Thomson AJ, Brazil J, Feighery C, Whelan A, Kellet J, Martin EA, Hutchinson M. CSF myelin basic protein in multiple sclerosis. Acta neurologica Scandinavica. 1985;72:577–83. doi: 10.1111/j.1600-0404.1985.tb00917.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta MK. Myelin basic protein and demyelinating diseases. Critical reviews in clinical laboratory sciences. 1987;24:287–314. doi: 10.3109/10408368609110277. [DOI] [PubMed] [Google Scholar]
- 18.Vass K, Lassmann H, Wisniewski HM, Iqbal K. Ultracytochemical distribution of myelin basic protein after injection into the cerebrospinal fluid. Evidence for transport through the blood-brain barrier and binding to the luminal surface of cerebral veins. Journal of the neurological sciences. 1984;63:423–33. doi: 10.1016/0022-510x(84)90165-5. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 20.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 21.McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol. 1998;86:20–9. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–49. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 23.Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 26.Youssef S, Wildbaum G, Karin N. Prevention of experimental autoimmune encephalomyelitis by MIP-1alpha and MCP-1 naked DNA vaccines. J Autoimmun. 1999;13:21–9. doi: 10.1006/jaut.1999.0306. [DOI] [PubMed] [Google Scholar]
- 27.Park IK, Hiraki K, Kohyama K, Matsumoto Y. Differential effects of decoy chemokine (7ND) gene therapy on acute, biphasic and chronic autoimmune encephalomyelitis: implication for pathomechanisms of lesion formation. J Neuroimmunol. 2008;194:34–43. doi: 10.1016/j.jneuroim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–84. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 29.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–94. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 30.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–35. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 31.Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–8. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 32.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 33.Imler TJ, Jr., Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(-) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–43. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- 35.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T, Takahashi T, Ripley B, Kimura A, Kishimoto T, Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9041–6. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, Wlachos A, Sobanov J, Kinnunen A, Baumruker T. FTY720 Rescue Therapy in the Dark Agouti Rat Model of Experimental Autoimmune Encephalomyelitis: Expression of Central Nervous System Genes and Reversal of Blood-Brain-Barrier Damage. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC. Loss of blood-brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci U S A. 2007;104:5656–61. doi: 10.1073/pnas.0701252104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minagar A, Ostanin D, Long AC, Jennings M, Kelley RE, Sasaki M, Alexander JS. Serum from patients with multiple sclerosis downregulates occludin and VE-cadherin expression in cultured endothelial cells. Mult Scler. 2003;9:235–8. doi: 10.1191/1352458503ms916oa. [DOI] [PubMed] [Google Scholar]
- 40.Wuerfel J, Tysiak E, Prozorovski T, Smyth M, Mueller S, Schnorr J, Taupitz M, Zipp F. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur J Neurosci. 2007;26:190–8. doi: 10.1111/j.1460-9568.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 41.Gordon PB, Sussman II, Hatcher VB. Long-term culture of human endothelial cells. In Vitro. 1983;19:661–71. doi: 10.1007/BF02628957. [DOI] [PubMed] [Google Scholar]
- 42.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–903. [PubMed] [Google Scholar]
- 43.Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–61. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- 44.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood-brain barrier specific proteins by autologous endothelial cells. Brain Res. 1993;625:238–43. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- 46.Hochmeister S, Grundtner R, Bauer J, Engelhardt B, Lyck R, Gordon G, Korosec T, Kutzelnigg A, Berger JJ, Bradl M, Bittner RE, Lassmann H. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65:855–65. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- 47.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol. 2003;201:319–27. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- 48.Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience. 2007;147:664–73. doi: 10.1016/j.neuroscience.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 49.Kieseier BC, Seifert T, Giovannoni G, Hartung HP. Matrix metalloproteinases in inflammatory demyelination: targets for treatment. Neurology. 1999;53:20–5. doi: 10.1212/wnl.53.1.20. [DOI] [PubMed] [Google Scholar]
- 50.Yong VW, Zabad RK, Agrawal S, Dasilva A Goncalves, Metz LM. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J Neurol Sci. 2007 doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–42. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 52.Pouly S, Antel JP, Ladiwala U, Nalbantoglu J, Becher B. Mechanisms of tissue injury in multiple sclerosis: opportunities for neuroprotective therapy. J Neural Transm Suppl. 2000:193–203. doi: 10.1007/978-3-7091-6284-2_16. [DOI] [PubMed] [Google Scholar]
- 53.Kuhle J, Lindberg RL, Regeniter A, Mehling M, Hoffmann F, Reindl M, Berger T, Radue EW, Leppert D, Kappos L. Antimyelin antibodies in clinically isolated syndromes correlate with inflammation in MRI and CSF. J Neurol. 2007;254:160–8. doi: 10.1007/s00415-006-0299-4. [DOI] [PubMed] [Google Scholar]
- 54.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Subileau EA, Rezaie P, Davies HA, Colyer FM, Greenwood J, Male DK, Romero IA. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:227–40. doi: 10.1097/NEN.0b013e318197eca7. [DOI] [PubMed] [Google Scholar]
- 56.Frigerio S, Gelati M, Ciusani E, Corsini E, Dufour A, Massa G, Salmaggi A. Immunocompetence of human microvascular brain endothelial cells: cytokine regulation of IL-1beta, MCP-1, IL-10, sICAM-1 and sVCAM-1. J Neurol. 1998;245:727–30. doi: 10.1007/s004150050275. [DOI] [PubMed] [Google Scholar]
- 57.Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 58.Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–23. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen TL, Sellebjerg F. Distinct chemokine receptor and cytokine expression profile in secondary progressive MS. Neurology. 2001;57:1371–6. doi: 10.1212/wnl.57.8.1371. [DOI] [PubMed] [Google Scholar]
- 60.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–26. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–80. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinones MP, Kalkonde Y, Estrada CA, Jimenez F, Ramirez R, Mahimainathan L, Mummidi S, Choudhury GG, Martinez H, Adams L, Mack M, Reddick RL, Maffi S, Haralambous S, Probert L, Ahuja SK, Ahuja SS. Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB and Akt. Mol Cell Neurosci. 2008;37:96–109. doi: 10.1016/j.mcn.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basso AS, Frenkel D, Quintana FJ, Costa-Pinto FA, Petrovic-Stojkovic S, Puckett L, Monsonego A, Bar-Shir A, Engel Y, Gozin M, Weiner HL. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J Clin Invest. 2008;118:1532–43. doi: 10.1172/JCI33464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dogan RN, Elhofy A, Karpus WJ. Production of CCL2 by Central Nervous System Cells Regulates Development of Murine Experimental Autoimmune Encephalomyelitis through the Recruitment of TNF- and iNOS-Expressing Macrophages and Myeloid Dendritic Cells. J Immunol. 2008;180:7376–84. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- 66.Malmestrom C, Andersson BA, Haghighi S, Lycke J. IL-6 and CCL2 levels in CSF are associated with the clinical course of MS: implications for their possible immunopathogenic roles. J Neuroimmunol. 2006;175:176–82. doi: 10.1016/j.jneuroim.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Angelucci F, Mirabella M, Caggiula M, Frisullo G, Patanella K, Sancricca C, Nociti V, Tonali PA, Batocchi AP. Evidence of involvement of leptin and IL-6 peptides in the action of interferon-beta in secondary progressive multiple sclerosis. Peptides. 2005;26:2289–93. doi: 10.1016/j.peptides.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 68.Wang T, Nagai H, Bouda K, Matsuura S, Takaoka Y, Niwa S, Homma T, Tanaka H, Shudo K. Effect of selective IL-6 inhibitor Am-80 on experimental autoimmune encephalomyelitis in DA rats. Acta Pharmacol Sin. 2000;21:967–76. [PubMed] [Google Scholar]
- 69.Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–8. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 70.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Mestre L, Correa F, Docagne F, Clemente D, Guaza C. The synthetic cannabinoid WIN 55,212-2 increases COX-2 expression and PGE2 release in murine brain-derived endothelial cells following Theiler’s virus infection. Biochem Pharmacol. 2006;72:869–80. doi: 10.1016/j.bcp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 75.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 76.Rose JW, Hill KE, Watt HE, Carlson NG. Inflammatory cell expression of cyclooxygenase-2 in the multiple sclerosis lesion. J Neuroimmunol. 2004;149:40–9. doi: 10.1016/j.jneuroim.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 77.Cao C, Matsumura K, Shirakawa N, Maeda M, Jikihara I, Kobayashi S, Watanabe Y. Pyrogenic cytokines injected into the rat cerebral ventricle induce cyclooxygenase-2 in brain endothelial cells and also upregulate their receptors. Eur J Neurosci. 2001;13:1781–90. doi: 10.1046/j.0953-816x.2001.01551.x. [DOI] [PubMed] [Google Scholar]
- 78.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 79.Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–8. [PubMed] [Google Scholar]
- 80.Paintlia AS, Paintlia MK, Singh I, Singh AK. IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol. 2006;176:4385–98. doi: 10.4049/jimmunol.176.7.4385. [DOI] [PubMed] [Google Scholar]
- 81.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 82.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–23. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 83.Bolton C, Paul C. Glutamate receptors in neuroinflammatory demyelinating disease. Mediators Inflamm. 2006;2006:93684. doi: 10.1155/MI/2006/93684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Encinas JM, Manganas L, Enikolopov G. Nitric oxide and multiple sclerosis. Curr Neurol Neurosci Rep. 2005;5:232–8. doi: 10.1007/s11910-005-0051-y. [DOI] [PubMed] [Google Scholar]
- 85.Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol. 2000;165:6511–8. doi: 10.4049/jimmunol.165.11.6511. [DOI] [PubMed] [Google Scholar]
- 86.Paul C, Bolton C. Modulation of blood-brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-D-aspartate receptor antagonist memantine. J Pharmacol Exp Ther. 2002;302:50–7. doi: 10.1124/jpet.302.1.50. [DOI] [PubMed] [Google Scholar]
- 87.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 89.Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113(Pt 5):1477–89. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- 90.Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12:154–69. doi: 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong M, Liu R, Guo L, Li C, Tan G. Pathological findings in rats with experimental allergic encephalomyelitis. Apmis. 2008;116:972–84. doi: 10.1111/j.1600-0463.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 93.Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, Pigott R, Stabler G, Ward GA, Gearing AJ, Miller KM. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- 94.Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994;94:2177–82. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hewson AK, Smith T, Leonard JP, Cuzner ML. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res. 1995;44:345–9. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- 96.Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, Campana WM, Gonias SL. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122:1155–62. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 98.Yu G, Rux AH, Ma P, Bdeir K, Sachais BS. Endothelial expression of E-selectin is induced by the platelet-specific chemokine platelet factor 4 through LRP in an NF-kappaB-dependent manner. Blood. 2005;105:3545–51. doi: 10.1182/blood-2004-07-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–40. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]