Abstract
Objective
To determine predictors of low birth weight (LBW) and preterm delivery (PTD) in singleton pregnancies conceived by women with and without a history of infertility.
Design
Retrospective cohort study.
Setting
Eleven infertility clinics in Northern California.
Patients
Three groups of women who carried singleton pregnancies to ≥ 20 weeks gestation: 542 infertile women who conceived after treatment, 441 infertile women who conceived spontaneously, and 1008 fertile women for comparison.
Interventions
Chart review.
Main Outcome Measures
Association of LBW or PTD with infertility treatment, maternal age, parity, obesity, or development of gestational diabetes.
Results
Infertile women who conceived with treatment were more likely to be obese, develop gestational diabetes, and have ovarian, ovulatory, or male factor infertility than infertile women who conceived spontaneously. Infertile women who conceived after treatment had 1.61 (95% CI 1.08– 2.41) times greater odds of having a LBW infant. Nulliparity was an independent predictor of LBW 1.54 (95% CI 1.09– 2.16) and PTD (OR 1.72, 95% CI 1.20–2.49) in all three groups after controlling for maternal age, history of infertility, infertility treatment, obesity, and gestational diabetes.
Conclusions
Nulliparous women and women with a history of infertility who conceive a singleton after treatment may be at increased odds for having a LBW infant. Infertile women do not appear to be at increased odds for PTD.
Keywords: infertility, obstetric, perinatal, outcomes, low birth weight, premature, preterm delivery
Introduction
Low birthweight (LBW) and preterm delivery (PTD) are of clinical concern because both are associated with increased risks of neonatal morbidity and mortality, as well as problems during childhood such as cerebral palsy, cognitive and neuromotor difficulties, and behavioral difficulties (1–7). Although an association between LBW (defined as a birthweight less than 2500 grams) and PTD (defined as delivery before 37 weeks gestation) (8) has been suggested for IVF (9), an association with infertility treatments other than ARTs has been much less studied. The goal of this retrospective cohort study is to determine risk factors for LBW and PTD by comparing three groups of women: (a) infertile women who conceived a singleton pregnancy as a result of infertility treatment, (b) infertile women who conceived a singleton pregnancy spontaneously, and (c) women without a history of infertility who conceived spontaneously.
Materials and Methods
A cohort of 51,318 women who underwent evaluation or treatment for infertility between 1965 and 1998 at 14 infertility practices in California (11 in northern California and three in southern California) was assembled for the purpose of conducting health outcomes studies. Eligible women were evaluated for infertility or received treatment between January 1, 1965 and January 1, 1998, and did not have a personal history of cancer
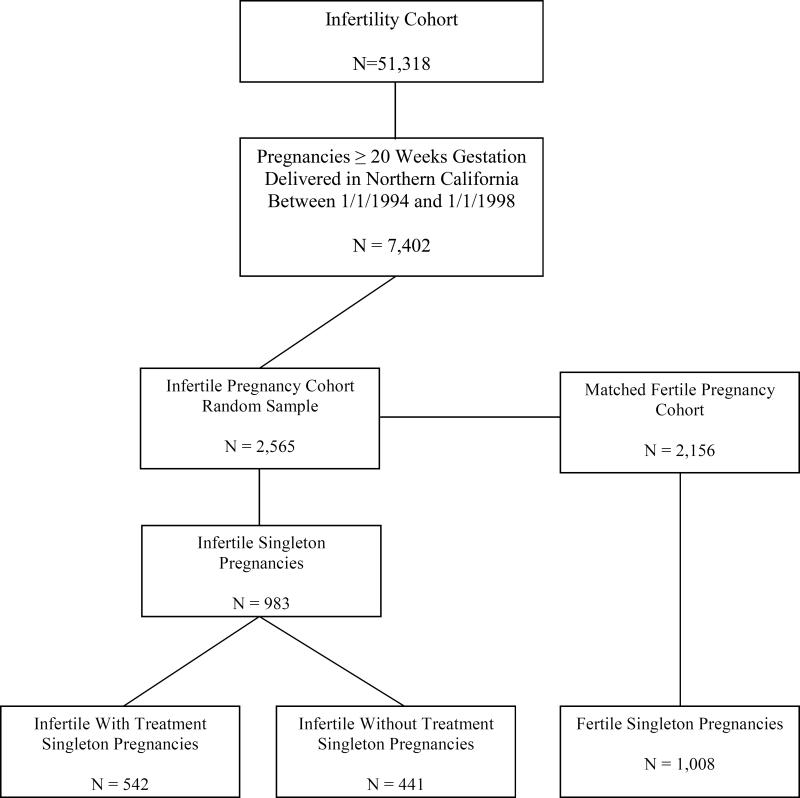
For this study, the cohort was limited to 30,448 women who underwent evaluation or treatment for infertility between January 1, 1990 and January 1, 1998 at eleven Northern California infertility practices (six private, one university-affiliated program, and four in the Kaiser Permanente Medical Care Program). These women were linked to the State of California Birth Statistical Master File and the State of California Fetal Death Statistical Master File to identify fetal deaths and births that occurred between January 1, 1994 and January 1, 1998. Through both linkages, 7,402 infertile women who had achieved a pregnancy ≥20 gestational weeks were identified. A random sample of 2,565 women was obtained of which 1,365 were Kaiser members for whom we had IRB approval to review their medical records. The remaining women were sent letters by their infertility provider inviting them to participate in the study. The final study population was comprised of 983 women who gave birth to singletons, met the eligibility criteria, and had complete medical record information. If the woman had more than one eligible pregnancy during the study period, then the index pregnancy was randomly selected using a random number generator. Institutional Review Board approvals were obtained from the Committee on Human Research at the University of California, San Francisco; The Committee for the Protection of Human Subjects of the State of California, and the Institutional Review Board of the Kaiser Foundation Research Institute.
The fertile cohort was determined by identifying pregnancies that lasted ≥ 20 weeks gestation in the State of California Birth Statistical Master File and the State of California Fetal Death Statistical Master File. For each woman identified, the next four certificates for women delivering in the same geographic area were reviewed to maximize frequency matches on date of delivery or fetal death (± 2 months), maternal age, and the exact number of gestations. Of this cohort, 2,156 women were sent letters inviting them to participate in the study. Of this group, 1,008 women who gave birth to singletons and met the eligibility criteria agreed to participate. Each participant was asked to release medical record information for herself and her child(ren) and to confirm that she was a resident of Northern California at the time of the delivery or resolution of the pregnancy and did not have a history of infertility, take longer than 12 months to conceive any pregnancy, experience ≥ 3 spontaneous abortions, or use infertility services. The study recruitment process is illustrated in Figure 1.
Figure 1.
Study Sample
All data in this study came from medical record review (Supplement). Infertility diagnoses and diagnostic tests and treatments for infertility were abstracted from medical records using standardized data collection forms. Information was also collected on health status, pregnancy conditions, and perinatal outcomes for all study subjects.
T-tests, ANOVA, and chi-square analyses were performed to compare outcomes across all three study groups. All analyses used p-values of < .05. SAS Version 9.2 (SAS Institute, Cary, NC) was used to conduct all analyses. Bonferroni post-hoc contrasts were conducted on all variables that were significantly different across the three groups. A priori power calculations conducted using the Poisson approximation, with two-sided alpha of 0.05, demonstrated 96% or greater power to detect each possible difference between the infertile and fertile cohorts.
Logistic regression analysis was used to find the best model to describe the relationship between each dependent variable (PTD, LBW) and set of independent variables. To determine potential multicollinearity between independent variables, Spearman rho correlations and variance inflation factors (VIF) were calculated. Variables that were considered for inclusion in the model were those that had associations with PTD or LBW showing p ≤ .05 in bivariate analyses. Clinically significant variables and potential confounding variables were identified through a review of the literature and included maternal age and nulliparity. These were included in all models as potential confounders or effect modifiers. In an effort to examine the incremental effect of ART, infertility treatment was further modeled by separating ART from non-ART treatment.
Results
Of the 983 women in the infertile cohort, 542 (55%) conceived with cycle-based infertility treatment and 441 (45%) conceived spontaneously without infertility treatment, (i.e., they had not undergone any infertility treatment within two menstrual cycles or 60 days prior to the estimated day of conception). Of the women who conceived after infertility treatment, 77% conceived using medications to induce or augment ovulation, 41% used IUI, 12% conceived with IVF without ICSI, and 5% conceived using IVF with ICSI. The infertility diagnoses are listed in Table 1. Infertile women who conceived with treatment were more likely to have an ovarian or ovulatory dysfunction diagnosis or to have a partner with male factor infertility as compared to infertile women who conceived without treatment. Oocyte donation was used by 22 (2%) of the women in the treatment cohort and 71 women (7%) used donor sperm.
Table 1.
Infertility Diagnoses for the Infertile Cohort
| Infertility Diagnosis | Infertile with Treatment N=542 | Infertile without Treatment N=441 | p-value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Ovarian dysfunction | 354 (66.9%) | 239 (55.1%) | <.0001 | 1.65 (1.27 – 2.15) |
| Male factor | 171 (32.3%) | 84 (19.4%) | <.0001 | 1.99 (1.48 – 2.69) |
| Tubal or pelvic damage | 186 (35.2%) | 162 (37.3%) | .49 | 1.58 (0.98 – 2.55) |
| Uterine disorders | 117 (22.1%) | 86 (19.8%) | .38 | 0.91 (0.67 – 1.19) |
| Endocrine or hormonal disorders | 56 (10.6%) | 50 (11.5%) | .65 | 1.15 (0.84 – 1.57) |
| Cervical disorders | 52 (9.8%) | 28 (6.5%) | .06 | 0.85 (0.48 – 1.49) |
| Unexplained infertility | 26 (4.9%) | 25 (5.8%) | .56 | 0.91 (0.61 – 1.36) |
Diagnosis categories are not mutually exclusive.
Women who conceived with treatment were on average 1.1 years older than women who conceived without treatment, and 3.5 years older than the fertile comparison group and were more likely to be nulliparous, nulligravid, and at greater risk for obesity and to develop gestational diabetes during the index pregnancy as compared to the other groups. Women in the fertile comparison group were more likely to smoke during the index pregnancy than either infertile group. Both the fertile comparison group and the women who conceived without treatment were more likely to consume alcohol during the index pregnancy when compared to the women who conceived with treatment. However, patients and health-care providers may have under-assessed or under-reported consumption of both smoking and alcohol use as this information was not uniformly noted and/or was missing from a high proportion of the medical records. This resulted in a loss of power to evaluate these variables; therefore they were not included in the final model.
Women who conceived with treatment were more likely to give birth to a LBW infant than women in the fertile comparison group; however, there was no difference in the rate of PTD between the three groups. These results are summarized in Table 2.
Table 2.
Characteristics and health status of infertile and fertile women who conceived a singleton pregnancy
| Variable | Infertile with treatment n=542 | Infertile without treatment n=441 | Fertile n=1008 | p-value |
|---|---|---|---|---|
| Maternal Age Mean ± SD | 36.34 ± 4.9 | 35.25 ± 4.7 | 32.87 ± 5.1 | <.0001 |
| Body Mass Index (BMI) Mean ± SD | 24.669 ± 5.4 | 24.888 ± 5.5 | 24.19 ± 5.0 | .06 |
| Nulliparous | 322 (59.7%) | 265 (39.8%) | 590 (41.5%) | <.0001 |
| Nulligravida | 157 (29.0%) | 85 (19.3%) | 226 (22.4%) | .001 |
| Chronic hypertension | 8 (1.5%) | 3 (0.7%) | 12 (1.2%) | .50 |
| Diabetes prior to pregnancy | 5 (0.9%) | 5 (1.1%) | 4 (0.4%) | .23 |
| Heart disease prior to pregnancy | 3 (0.6%) | 3 (0.7%) | 3 (0.3%) | .56 |
| Obesity prior to pregnancy | 31 (5.8%) | 10 (2.3%) | 43 (4.3%) | .03 |
| Pregnancy-induced hypertension | 35 (6.8%) | 25 (6.0%) | 63 (6.3%) | .86 |
| Gestational diabetes | 51 (9.8%) | 22 (5.1%) | 87 (8.7%) | .03 |
| Excess weight gain during pregnancy | 2 (0.4%) | 3 (0.7%) | 7 (0.7%) | .72 |
| Smoking during pregnancy | 9 (1.9%) | 8 (2.1%) | 65 (6.8%) | <.0001 |
| Alcohol during pregnancy | 35 (7.6%) | 50 (13.4%) | 113 (12.1%) | .01 |
| Intra-uterine growth restriction (IUGR) | 19 (3.5%) | 11 (2.5%) | 16 (1.6%) | .05 |
| Small for gestational age (SGA) | 14 (2.7%) | 9 (2.1%) | 11 (1.1%) | .07 |
| Sepsis | 9 (1.7%) | 6 (1.4%) | 10 (1.0%) | .49 |
| Fetal or neonatal death | 9 (2.0%) | 4 (1.1%) | 7 (0.8%) | .17 |
| Preterm (<37 weeks) | 42 (8.1%) | 29 (6.8%) | 65 (6.6%) | .54 |
| LBW(<2500 g) | 56 (10.8%) | 37 (8.6%) | 63 (6.4%) | .01 |
A logistic regression was performed to determine factors associated with increased odds of having a low birthweight singleton infant. The model contained seven independent variables including maternal age, nulliparity, fertile, infertile conceived with treatment, infertile conceived without treatment, obesity during index pregnancy, and gestational diabetes during index pregnancy. As most studies of LBW in women with a history of infertility reference nulliparity as a variable, nulliparity was included in this model although nulliparity and nulligravidity were highly correlated (r = .60).
The final logistic regression model with LBW as the outcome was statistically significant, p < .05, indicating that the model was able to distinguish between women who did and did not give birth to a LBW infant. As shown in Table 3, infertility treatment and nulliparity were independent predictors of LBW. Infertile women who conceived after treatment were 1.61 (95% CI 1.08– 2.41) odds more likely to give birth to a LBW infant than fertile women after controlling for maternal age, nulliparity, obesity, and gestational diabetes. Infertile women who conceived a pregnancy without treatment and fertile women had similar odds of LBW infants (p = .20). Nulliparous women were at 1.54 (95% CI 1.09– 2.16) greater odds to give birth to a LBW infant than parous women after controlling for maternal age, infertility treatment, obesity, and gestational diabetes. Although infertility treatment was significantly associated with LBW, conception by ART (OR=1.6: 95% CI 1.00–2.55) conferred no additional risk (p=0.88 for ART vs. non-ART treatment). Additionally, no multicollinearity was seen between nulliparity and treatment; thus, nulliparity appears to be an independent predictor of LBW.
Table 3.
Predictors of LBW in study population
| LBW Odds Ratio (95% C.I) | p-value | |
|---|---|---|
| Infertile, conceived with treatment | 1.61 (1.08– 2.41) | .02 |
|
| ||
| Infertile, conceived without treatment | 1.33 (0.86–2.06) | .20 |
| Maternal age <24 | 1.06 (0.43–2.61) | .90 |
| Maternal age 30–34 | 1.30 (0.76–2.19) | .40 |
| Maternal age 35–39 | 1.25 (0.73–2.15) | .50 |
| Maternal age 40+ | 1.06 (0.55–2.014) | .76 |
| Nulliparity | 1.54 (1.09– 2.16) | .01 |
| Obesity | 1.11 (0.50– 2.49) | .80 |
| Gestational diabetes | 0.72 (0.37– 1.41) | .33 |
Subsequent mediation analyses were conducted to assess potential causal pathways for each possible mediator. All mediators that were significant in the model were entered together and the combined effect on the infertility covariate was observed. These analyses found that cervical factors, endometriosis, and uterine anomalies together account for up to 59% of the infertility effect. However DES did not have a mediating affect (Supplement).
To test whether women who conceived with donor gametes are at greater risk for LBW as a result of any immunological vulnerability associated with that treatment approach, post-hoc analyses were performed taking the use of donor gametes into account. In addition to the base model, we modeled with separate donor sperm and donor oocyte covariates, with a single categorical infertility covariate breaking out the donor oocyte/sperm group, and a subset model excluding those that used donor gametes. In none of the models did the use of donor gametes predict LBW (p>.05)
The final model with PTD as the dependent variable was not statistically significant (p = .07), indicating that the model was not able to distinguish between women who did and did not give birth to a PTD infant. The only independent variable that made a unique, statistically significant contribution to the model of PTD was nulliparity. Nulliparous women had 1.72 (95% CI 1.2– 2.5) greater odds of giving birth to a PTD infant after controlling for maternal age, history of infertility, infertility treatment, obesity, and gestational diabetes as compared to parous women. Post-hoc power calculations indicated 80% power to detect differences in rates of PTD, IUGR, SGA, sepsis, and fetal or neonatal death of 0.7%, 2.6%, 2.3%, 2.2% and 2.1% respectively. Thus, the inability to detect even these small differences may indicate that infertile women were not at increased risk of these adverse outcomes.
Discussion
One of the most significant clinical sequelae of infertility treatment is the high frequency of multiple births and associated poor perinatal outcomes including PTD and LBW (10, 11). While some studies have described a similar increased risk in singleton pregnancies after ART (9,12,13), others have not (14,15).
By comparing perinatal outcomes between infertile women who conceived with and without a variety of infertility treatments with a comparison group of fertile women, we were able to look at the effects of both infertility and fertility treatment on PTD and LBW. Because known confounders for adverse perinatal outcomes include maternal age, parity, and multiple gestations, we evaluated only singleton gestations and adjusted for both maternal age and parity. Our final logistic regression model found that infertility treatment and nulliparity were the factors most significantly associated with an increase in the odds of infertile women delivering a LBW infant.
Our findings do not suggest that all fertility treatments necessarily place women at greater risk. Women who underwent treatment to conceive were more likely to have a diagnosis of ovarian or ovulatory dysfunction, and their male partners were more likely to have male factor infertility. Both of these diagnoses may be related to the genetic or immunologic competence of gametes. Although it has been reported that exposure to donor gametes may increase the risk of hypertensive disorders in pregnancy (16), we did not find an increased risk for LBW with the use of donor gametes. When addressing the question “Is infertility treatment associated with LBW or is LBW due to factors that result in infertility?” it is possible that subtle effects expressed at fertilization, nidation, placentation, or gestation may culminate in a LBW child.
We also identified several significant underlying differences between the groups of infertile and fertile women including maternal age, parity, and obesity. Maternal age has been associated with an increased risk of infertility as well as hypertensive disorders, gestational diabetes, antepartum hemorrhage, and adverse perinatal outcomes (17–24). Yet in ART-conceived singleton pregnancies, Suzuki and Miyake did not find age to be a factor in obstetric outcomes in nulliparous women aged 35 and older with singleton pregnancies, (25) and Schieve et al. found an elevated LBW risk that was not associated with maternal age (12). In our study maternal age did not independently predict LBW.
Nulliparity has also been associated with LBW, abnormal labor, dystocia, cesarean section delivery, and adverse perinatal outcomes (26–29). While Schieve et al. found an elevated LBW risk ART-conceived singleton infants born at ≥37 weeks gestation was not explained by parity (12), we found that infertile women who underwent treatment to conceive were more likely to be nulliparous than either fertile women or infertile women who conceived without treatment. When nulliparity was added in the final model, it did predict LBW. One explanation may be that nulliparity serves as a marker for the severity of infertility. While we found that over half of the infertility effect could be accounted for by mediators, there still remains an unexplained effect of infertility with treatment.
Gestational diabetes and obesity are associated with, pregnancy loss, preeclampsia, cesarean delivery, and increased risk of birth defects (30–34). Maman et al. reported that singleton pregnancies conceived by IVF or ovulation induction were at increased risk for maternal gestational diabetes after controlling for maternal age, gestational age, and parity (35). In our study, the infertile women who conceived with treatment were not only more likely to be obese and to develop gestational diabetes, but had an increased incidence of ovarian dysfunction including polycystic ovarian syndrome (PCOS). Because obese infertile women are more likely to have PCOS, they are more likely to require treatment in order to conceive, as well as being at increased risk of developing gestational diabetes during their pregnancy.
We acknowledge several limitations in our study. These include the fact that all study participants were residents of Northern California, the study design was retrospective and non-experimental, and infertility treatments have evolved over time.
Nonetheless, the identification of nulliparity and a history of infertility treatment as risk factors for LBW may serve to alert obstetrical care providers and prompt future research that focuses specifically on the possible mechanisms whereby women undergoing infertility treatment are at risk for adverse perinatal outcomes.
Capsule.
Nulliparous women and women with a history of infertility who conceive a singleton after infertility treatment may be at increased risk for having a low birthweight infant.
Acknowledgments (alphabetical)
Scientific Advisors: Denise Bernstein, LVN, Lauri Black, M.S., Marcelle Cedars, M.D., Nancy Chamberlain, Lisa Croen, Ph.D., Kari Danzinger, M.S., Seth Feigenbaum, M.D., Donna Ferriero, M.D., Judith Grether, Ph.D., Rebecca Jackson, M.D., Ph.D., H. Preston Nelson, M.D., Paul Turek, M.D., Yvonne Wu, M.D.
Data Collection: Sujana Bhattacharyya, Allison Boissevain, Sharyn Boissevain, Zulma Flamenco, Christine Flanders-Koenig, Jennifer Fraser, Maria Garcia, Travis Hagedorn, Kathy Homan, Stefani Machi-Harris, Jenny Magana, Wendy McDowell, Sean McGrath, Susan Murdoch, Natalie Purcell, Katie Renstrom-Whittaker, Jennifer Schwab, Sharon Tupas, Katie Willoughby
Participating Centers: Alta Bates In-Vitro Fertilization Program, Fertility Physicians of Northern California, Reproductive Science Center of the Bay Area, Kaiser Permanente of Northern California, NOVA In-Vitro Fertilization, San Francisco Center for Reproductive Medicine (now part of Pacific Fertility Center), and University of California, San Francisco.
Supported by NCI grant 1-RO1-CA69619 and NICHD grant 1- P01- HD-37074; Mary Croughan, Ph.D., Principal Investigator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147:362–9. doi: 10.1093/oxfordjournals.aje.a009458. [DOI] [PubMed] [Google Scholar]
- 2.Lems W, Hopkins B, Samson JF. Mental and motor development in preterm infants: the issue of corrected age. Early Hum Dev. 1993;34:113–23. doi: 10.1016/0378-3782(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 3.Buck GM, Msall ME, Schisterman EF, Lyon NR, Rogers BT. Extreme prematurity and school outcomes. Paediatr Perinat Epidemiol. 2000;14:324–31. doi: 10.1046/j.1365-3016.2000.00276.x. [DOI] [PubMed] [Google Scholar]
- 4.Hille ET, den Ouden AL, Saigal S, Wolke D, Lambert M, Whitaker A, et al. Behavioural problems in children who weigh 1000 g or less at birth in four countries. Lancet. 2001;357:1641–3. doi: 10.1016/S0140-6736(00)04818-2. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S. Follow-up of very low birthweight babies to adolescence. Semin Neonatol. 2000;5:107–18. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- 6.Saigal S, Rosenbaum P, Szatmari P, Campbell D. Learning disabilities and school problems in a regional cohort of extremely low birth weight (less than 1000 G) children: a comparison with term controls. J Dev Behav Pediatr. 1991;12:294–300. [PubMed] [Google Scholar]
- 7.Saigal S, Szatmari P, Rosenbaum P, Campbell D, King S. Cognitive abilities and school performance of extremely low birth weight children and matched term control children at age 8 years: a regional study. J Pediatr. 1991;118:751–60. doi: 10.1016/s0022-3476(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee Opinion Number 324 Perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2005;106:1143–6. doi: 10.1097/00006250-200511000-00061. [DOI] [PubMed] [Google Scholar]
- 9.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 10.Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, et al. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril. 2006;86:1634–41. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Keirse MJ, Helmerhorst FM. The impact of assisted reproduction on perinatal health care. Soz Praventivmed. 1995;40:343–51. doi: 10.1007/BF01325415. [DOI] [PubMed] [Google Scholar]
- 12.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 13.Tan SL, Doyle P, Campbell S, Beral V, Rizk B, Brinsden P, et al. Obstetric outcome of in vitro fertilization pregnancies compared with normally conceived pregnancies. Am J Obstet Gynecol. 1992;167:778–84. doi: 10.1016/s0002-9378(11)91589-0. [DOI] [PubMed] [Google Scholar]
- 14.Reubinoff BE, Samueloff A, Ben-Haim M, Friedler S, Schenker JG, Lewin A. Is the obstetric outcome of in vitro fertilized singleton gestations different from natural ones? A controlled study. Fertil Steril. 1997;67:1077–83. doi: 10.1016/s0015-0282(97)81442-2. [DOI] [PubMed] [Google Scholar]
- 15.Verlaenen H, Cammu H, Derde MP, Amy JJ. Singleton pregnancy after in vitro fertilization: expectations and outcome. Obstet Gynecol. 1995;86:906–10. doi: 10.1016/0029-7844(95)00322-I. [DOI] [PubMed] [Google Scholar]
- 16.Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, Philips S, Allgar V, Walker JJ. The influence of donated gametes on the incidence of hypertensive disorders in pregnancy. Hum Reprod. 1999;14:2268–2273. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- 17.Chan BC, Lao TT. Effect of parity and advanced maternal age on obstetric outcome. Int J Gynaecol Obstet. 2008;102:237–41. doi: 10.1016/j.ijgo.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Usta IM, Nassar AH. Advanced maternal age. Part I: obstetric complications. Am J Perinatol. 2008;25:521–34. doi: 10.1055/s-0028-1085620. [DOI] [PubMed] [Google Scholar]
- 19.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–94. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 20.Adamson GD, Baker VL. Subfertility: causes, treatment and outcome. Best Pract Res Clin Obstet Gynaecol. 2003;17:169–85. doi: 10.1016/s1521-6934(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 21.Bianco A, Stone J, Lynch L, Lapinski R, Berkowitz G, Berkowitz RL. Pregnancy outcome at age 40 and older. Obstet Gynecol. 1996;87:917–22. doi: 10.1016/0029-7844(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 22.Ozalp S, Tanir HM, Sener T, Yazan S, Keskin AE. Health risks for early (< or =19) and late (> or =35) childbearing. Arch Gynecol Obstet. 2003;268:172–4. doi: 10.1007/s00404-002-0359-7. [DOI] [PubMed] [Google Scholar]
- 23.Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265:30–3. doi: 10.1007/s004040000122. [DOI] [PubMed] [Google Scholar]
- 24.Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54:6–10. doi: 10.1159/000064689. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Miyake H. Obstetric outcomes in nulliparous women aged 35 and over with singleton pregnancies conceived by in vitro fertilization. Arch Gynecol Obstet. 2008;277:225–7. doi: 10.1007/s00404-007-0461-y. [DOI] [PubMed] [Google Scholar]
- 26.Blickstein I, Rhea DJ, Keith LG. Characteristics of mothers who delivered the heaviest, average-weight, and lightest triplet sets. J Perinat Med. 2005;33:113–6. doi: 10.1515/JPM.2005.021. [DOI] [PubMed] [Google Scholar]
- 27.Kus E, Nowacka A, Berner-Trabska M, Kowalska-Koprek U, Kazimierak W, Brzozowska M, et al. Influence of parity on the body mass of neonates. Ginekol Pol. 2008;79:352–7. [PubMed] [Google Scholar]
- 28.Kaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labor in term nulliparous women with spontaneous labor onset. Acta Obstet Gynecol Scand. 2009;88:402–7. doi: 10.1080/00016340902811001. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetrics and Gynecology ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445–54. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Adamson GD, Baker VL. Subfertility: causes, treatment and outcome. Best Pract Res Clin Obstet Gynaecol. 2003;17:169–85. doi: 10.1016/s1521-6934(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 31.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 32.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 33.ASRM Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90:21–9. doi: 10.1016/j.fertnstert.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19:2523–8. doi: 10.1093/humrep/deh485. [DOI] [PubMed] [Google Scholar]
- 35.Maman E, Lunenfeld E, Levy A, Vardi H, Potashnik G. Obstetric outcome of singleton pregnancies conceived by in vitro fertilization and ovulation induction compared with those conceived spontaneously. Fertil Steril. 1998;70:240–5. doi: 10.1016/s0015-0282(98)00160-5. [DOI] [PubMed] [Google Scholar]