Abstract
Objective
The aim of this study was to develop and test a model of depression, hippocampal changes, and cognitive decline.
Method
Participants were 248 community-dwelling, depressed patients and 147 healthy, non-depressed individuals 60 years and older. Participants received a structured interview assessing current depressive symptoms and past depressive episodes, completed cognitive testing with the MMSE, and underwent structural MRI of the brain. For up to ten years, assessment of depressive symptoms and MMSE administration was repeated at least annually, and MRI was repeated every two years.
Results
Regression analyses demonstrated that depression diagnosis at baseline predicted decrease in right (but not left) hippocampal volume over a four-year period. Analyses using structural equation modeling demonstrated that a decrease in left and right hippocampal volume predicted decrease in MMSE score over four years.
Conclusion
Results provide some evidence for relationships between depression and decrease in right hippocampal volume, and between hippocampal volume and MMSE score. This would be consistent with depression as a causal factor in subsequent cognitive decline. Plausible biological mechanisms include a glucocorticoid cascade or a facilitating effect of depression on amyloid-beta plaque formation. Future studies should examine the relationship between hippocampal volume and specialized memory measures, as well as between depression diagnosis and volume of other brain structures.
Keywords: major depressive disorder, glucocorticoid cascade hypothesis, neuroimaging, structural equation modeling
Many studies demonstrate a relationship between depression and cognitive decline, but researchers are still working to understand the cause and direction of this relationship. Jorm (2001) reviewed three of the most prominent explanations for the relationship between depression and cognitive decline. One explanation is that depressive symptoms may represent an early prodrome or warning sign of dementia, rather than true depression (i.e., the prodrome hypothesis). Another explanation is that depression may be a response to early cognitive losses. That is, an individual may recognize that he or she is having memory difficulties, become concerned about the possibility of dementia and loss of independence, and therefore become depressed. A third explanation, however, is that depression may have an etiological role in cognitive decline. For instance, the glucocorticoid cascade hypothesis contends that depression initiates neurological changes that in turn lead to prolonged activation of the HPA axis, and ultimately to hippocampal damage and neuronal death, which in turn places depressed individuals at greater risk for developing dementia (Sapolsky, 1996). Alternatively, prolonged HPA activation may promote amyloid-beta plaque deposition, thereby enhancing AD pathology (for review see Dong and Csernansky, 2009). It is important to note that these explanations are not necessarily mutually exclusive.
Findings that depression may occur in the absence of cognitive impairment and predicts subsequent cognitive decline provide some evidence that depression may be a contributor to dementia. Sachs-Ericsson and colleagues reported that depressive symptoms at baseline predicted subsequent cognitive decline over a three-year period, even among individuals with no cognitive impairment at baseline (Sachs-Ericsson, Joiner, Plant, & Blazer, 2005). In a series of studies, Kessing et al. found that the risk of developing dementia increased significantly with each additional lifetime episode of depression; having a more severe course with shorter amounts of time between episodes also increased the risk of developing dementia (Kessing, Mortensen, & Bolwig, 1998; Kessing & Nilsson, 2003; Kessing, & Andersen, 2004). Thus, the influence of depression on dementia may be dose-dependent, with greater severity and more episodes of depression increasing the risk of subsequently developing dementia.
Not all studies, however, have found this relationship between depression and subsequent cognitive decline (Dufoil, Fuhrer, Dartigues, & Alpérovitch, 1996; Henderson, Korten, & Jacomb, 1997; Chen, Ganguli, Mulsant, & DeKosky, 1999). Some have argued that depression only represents a prodromal phase of Alzheimer’s disease or dementia, as it tends to have its onset within one to two years of development of dementia (Berger, Fratiglioni, Forsell, Winblad, & Bäckman, 1999; Sun et al., 2008; Ganguli, Du, Dodge, Ratcliff, & Chang, 2006; Schweitzer, Tuckwell, O’Brien, & Ames, 2002; Cervilla, Prince, Joels, & Mann, 2000; Cannon-Spoor et al., 2005). Discrepancies appear to be explained, in part, by the age of onset of the depression. Green et al. found retrospectively that depression occurring within one year prior to AD diagnosis robustly predicted AD (Green et al., 2003). Importantly, depression occurring up to 25 years prior to the diagnosis still conveyed modest risk for AD. Thus, the study provided evidence that depressive symptoms may be a prodrome of dementia (when occurring close to dementia diagnosis) and that depression may also be a risk factor for dementia (when occurring many years before dementia onset).
Emerging evidence suggests that depression may have a biological, etiological role in increasing risk of dementia. One important mechanism through which depression may increase risk of dementia is the glucocorticoid cascade hypothesis. That is, many depressed individuals have high circulating levels of glucocorticoids, which can lead to neuronal death and inhibit neurogenesis in the hippocampus (Sapolsky, 1996). In addition, some research using animal models of Alzheimer’s disease suggests that increased levels of glucocorticoids can contribute to amyloid-beta deposition (Dong and Csernansky, 2009), thus also contributing to Alzheimer’s disease pathology and to hippocampal atrophy. As decreased hippocampal volumes are associated with cognitive decline in studies of humans and rodents (Laakso et al., 1998, Lupien et al., 1998), depression could increase risk of dementia by contributing to amyloid-beta deposition and to hippocampal atrophy.
In fact, imaging studies do suggest that a history of major depression and longer lifetime duration of depression is associated with smaller hippocampal volumes (Sheline, Wang, Gado, Csernansky, & Vannier, 1996; Sheline, Sanghavi, Mintun, & Gado, 1999). In particular, research with older adults has demonstrated reduced right hippocampal volumes in older adults with depression (Bell-McGinty et al 2002).
Not all studies have detected a clear relationship between depression and hippocampal volume (Rusch, Abercrombie, Oakes, Schaefer, & Davidson, 2001; Ashtari, Greenwald, Kramer-Ginsberg, Hu, Wu, Patel, Aupperle, & Pollack, 1999). Vakili et al. found no difference between the hippocampal size of depressed participants and controls, who were between 18 and 60 years old (Vakili et al., 2000). However, they did find that among the male participants, Hamilton Depression Rating Scale (HDRS) score predicted hippocampal volume, with higher scores (indicating more severe depression) predicting reduced hippocampal volumes. Likewise, among female participants, depressed participants who did not respond to treatment had decreased right hippocampal volumes. This speaks to one important factor in the relationship of depression and Alzheimer’s disease – severity of the depression. In general, studies that have used community samples of adults may have included an insufficient number of severely depressed individuals to capture the relationship between depression and hippocampal volume.
Although the literature supports relationships between severe, chronic depression and dementia; between depression and smaller hippocampal volumes; and between smaller hippocampal volumes and dementia; only one study to date has explicitly examined the mediating role of hippocampal volumes in the depression-dementia relationship (Geerlings, den Heijer, Koudstaal, Hofman, & Breteler, 2008). Unfortunately, this study used a community sample of older adults with minimal levels of depressive symptomatology; given that more severe levels of depression may be necessary to impact the hippocampus or increase risk of dementia, the low level of depressive symptoms in Geerlings and colleagues’ study must be considered a limitation.
The current study is uniquely able to address limitations of the extant research. We prospectively examined the relationship between depression, hippocampal volume change, and cognitive change in a population of older adults with Major Depressive Disorder (MDD). We used a prospective longitudinal design in which all participants were evaluated and found to be nondemented at baseline; and depressive symptoms, cognitive functioning, and hippocampal volume were objectively measured on three occasions throughout the study. This allowed for examination of change over time among depressed individuals and controls, using more sophisticated modeling techniques (i.e. structural equation modeling).
In a recent study, our group reported greater left hippocampal volume reduction in depression versus controls and that among older depressives, there was an association between change in hippocampal volume from baseline to two years and subsequent decline in MMSE score from 2 to 2.5 years (Steffens, McQuoid, Payne & Potter, 2011). Our analyses extend this study in several ways. First, our study extended across four years of data, allowing us to examine change over time across a longer timeframe. Second, as we used structural equation modeling, a sophisticated statistical technique that allowed us to model relations among all of our variables. It also allowed us to address missing data using the Full Information Maximum Likelihood (FIML) method. Lastly, we planned to test for mediation, if appropriate. We hypothesisized that 1) depression would predict hippocampal volume reduction, 2) depression would predict cognitive decline, and 3) hippocampal volume would mediate the relationship between depression and cognitive decline, such that smaller hippocampal volume would be associated with greater cognitive decline.
Methods
This study used previously collected data from the Neurocognitive Outcomes of Depression in the Elderly (NCODE) study, an NIMH-supported study that has enrolled older depressed and non-depressed adult participants since 1994. Participants were evaluated at least annually for depression and cognitive functioning, and received MRI of the brain approximately every two years. Whereas the study has been conducted over a ten-year period, there was considerable attrition of participants with time. To capitalize on greater sample size, we examined data for the initial four-year period.
Participants
Non-demented adults age 60 and older who presented at the Psychiatry Services unit of Duke University Medical Center or at the Duke General Internal Medicine Clinic, and met DSM-III-R criteria for a current episode of Major Depression, were recruited into the study. Participants were excluded if they met criteria for another major psychiatric illness (schizophrenia, schizoaffective disorder, bipolar disorder, lifetime alcohol or substance dependence, and dementia). Likewise, participants were excluded if they had neurological illnesses that could lead to structural change visible by MRI, including Parkinson’s disease, multiple sclerosis, and seizure disorder. This study included data from 248 depressed participants.
Adults over age 60 were recruited from the Duke University Center for Aging Subject Registry. They underwent a nonfocal neurological examination to rule out neurological disorder and were excluded if they had a lifetime history of depression. Four additional control participants were eliminated from the study during the follow-up period because they experienced a first major depressive episode. This study included data from 147 (non-depressed) control participants.
Baseline depression assessment
Trained interviewers administered the Duke Depression Evaluation Schedule (DDES) to all participants to assess demographic information and DSM–III-R or -IV current and lifetime Major Depression using the National Institute of Mental Health Diagnostic Interview Schedule (DIS) depression assessment (Robins, Helzer, Croughan, & Ratcliff, 1981). Included in the DDES are indices of depression severity, including (if applicable) questions about number of lifetime episodes of depression, the number of DSM symptoms at baseline, and the number of DSM symptoms at worst episode.
Baseline cognitive screening
At baseline, a geriatric psychiatrist examined each participant, reviewed the participant’s medical records, and consulted the participant’s referring physician to determine whether the participant had dementia. Additionally, all participants were administered the MMSE. Participants (depressed or control) with MMSE scores of 25 or higher, and no indications of dementia, were enrolled. Some severely depressed patients had MMSE scores below 25. These patients were followed during an eight-week course of treatment; if their MMSE score improved during this time they were enrolled, and if it did not improve they were excluded. In addition, any participants who experienced a decline in MMSE score of 4 or more points over the initial one-year follow-up period were eliminated from the analysis as likely to have been demented at baseline (Tangalos et al., 1996). This resulted in elimination of 11 participants.
Treatment
Treatment history prior to baseline was assessed using the DDES. All participants diagnosed with Major Depressive Disorder were referred for treatment at baseline. Treatment options included antidepressant medications, electroconvulsive therapy, and individual and group cognitive behavioral therapy. The dataset includes a record of medication prescribed to study participants.
Measures
Demographic information
Participants reported demographic information during administration of the DDES, including age, sex, race/ethnicity, and years of education.
Mini Mental State Examination (MMSE)
The MMSE assesses cognitive functioning in five areas: orientation, registration, attention and calculation, recall, and language; and provides an objective measure of global cognitive functioning (M. Folstein, S. Folstein, & McHugh, 1975). Scores range from 0 to 30, with higher scores indicative of better cognitive functioning, and a score below 25 generally indicative of cognitive impairment. Participants were administered the MMSE at least yearly. The MMSE has been used extensively in epidemiologic research of older adults. In the current study, reliability is acceptable (baseline α = .7, 2-year follow-up α = .6, and 4-year follow-up α = .7).
Center for Epidemiologic Studies Depression scale (CESD)
The CESD is a 20-item self-report instrument that assesses symptoms of depression experienced in the previous week (Radloff, 1977). Items are scored from 0 to 4, with a maximum possible score of 60. CESD scores for the current study have good reliability, as represented by the inter-item correlation (α = .8).
Neuroimaging
All participants underwent structural MRI of the brain at two-year intervals. The methodology of hippocampal measurement has been reported previously by Steffens et al. (2002). Briefly, participants were imaged using a 1.5-tesla whole-body MRI system (Signa, GE Medical Systems, Milwaukee, WI). Two sets of dual-echo fast spin-echo (FSE) acquisitions were obtained, one in the axial plane for morphometry of most cerebral structures, and a second in a coronal oblique plane for segmentation of the hippocampus. The pulse sequence parameters were repetition time (TR)=4,000 msec, and echo time (TE)=30, 135 msec, with 32 KHz (±16KHz) full imaging bandwidth; echo train length=16, a 256 × 256 matrix, 3-mm section thickness, 1 Nex and a 20-cm FOV (field of view). The images were acquired in two separate acquisitions, with a 3-mm gap between sections for each acquisition. The second acquisition was offset by 3 mm from the first, so that the resulting data set consisted of contiguous sections. For the near coronal acquisition, the localizer scan was used to identify the anterior commissure–posterior commissure (AC–PC) line. Oblique, near-coronal images were prescribed perpendicular to this line, covering the entire brain from just anterior of the temporal lobe to a plane posterior to the lateral ventricles. Images were archived as normal procedure on magneto-optical disks in the MR Imaging Center. The MR images were then transferred to the Neuropsychiatric Imaging Research Laboratory (NIRL), located at Duke University Medical Center, for processing on SUN workstations, and secondary archive. Thin-slice, dual-echo FSE images, consisting of proton-density and T2-weighted images, were used for all processing. Hippocampal measurements used an oblique/coronal series of slices, and all other measures were done with axial images. Two computer programs were used to make volume measurements. Hippocampal volumes were determined with the GRID Program, which was developed at NIRL. Technicians computing hippocampal volume using the GRID program demonstrated acceptable interrater reliability (left hippocampus intraclass correlation coefficient = 0.8, right hippocampus intraclass correlation coefficient = 0.7).
Analytic Model
Descriptive analyses
Descriptive analyses were performed to examine the distribution of the study variables and check for outliers, skewness, and kurtosis. Transformations were conducted to correct outliers, skew, or kurtosis.
Analysis 1
We used multiple regression analyses to test our hypothesis that Major Depression diagnosis (depressed patient or non-depressed control) at baseline would predict subsequent decrease in (a) left and (b) right hippocampal volume over a four-year follow-up period. Hippocampal volume at baseline, cerebrum (head) size, and age were controlled in each analysis. A third multiple regression model (c) tested whether depression status predicted change in MMSE score over a four-year period. Baseline MMSE score, age, minority status, and years of education were controlled in the analyses.
Analysis 2
We predicted that indices of baseline depression severity among both depressed participants and control participants would predict change in MMSE score over a four-year follow-up period, and that this relationship would be mediated by left and right hippocampal volume four years after baseline. Specifically, we predicted that smaller hippocampal volume would be associated with greater decrease in MMSE score.
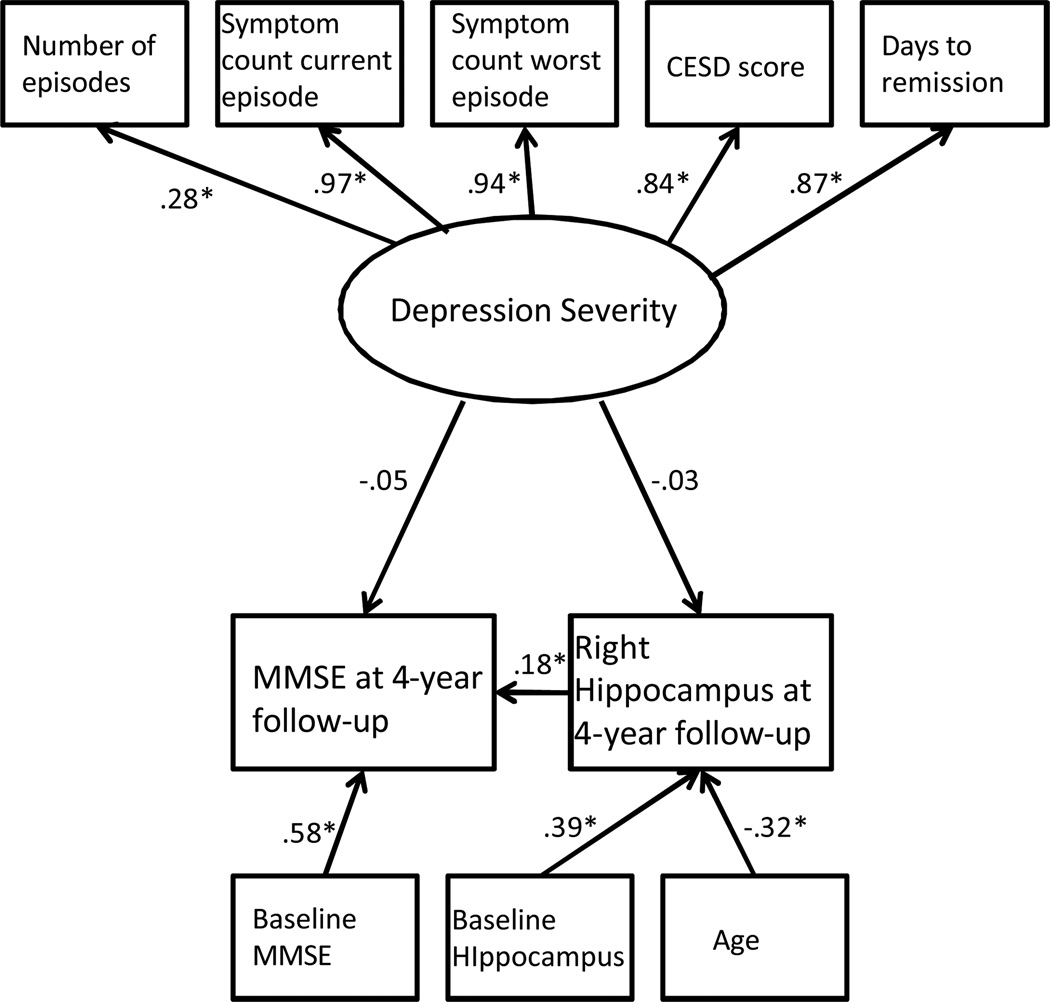
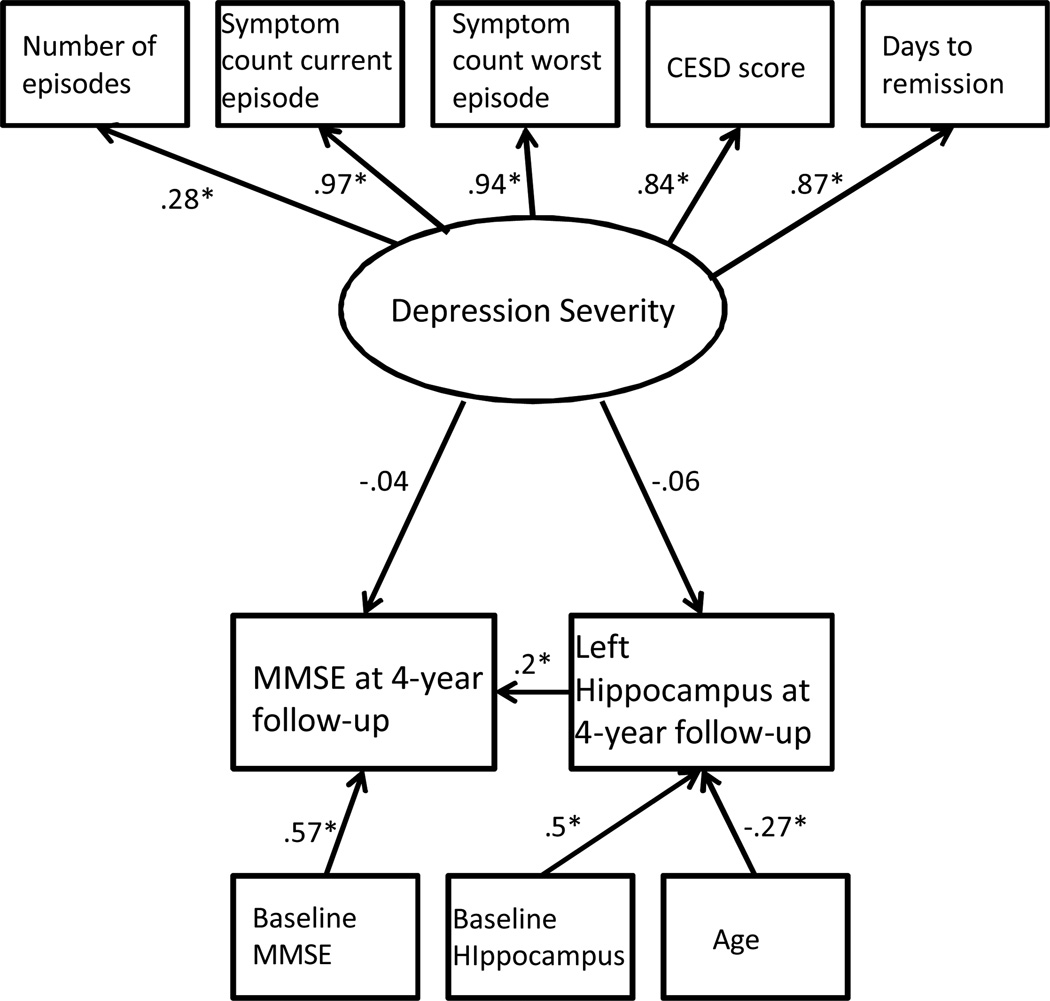
This hypothesis was tested using MPLUS version 5 (Muthén & Muthén, 2007) to evaluate two structural equation models using maximum likelihood estimation. A latent variable was created representing lifetime depression severity. The variable had five indicators: CESD score at baseline, lifetime number of depressive episodes, symptom count for the worst episode of major depression, symptom count for the current episode of major depression, and number of days to remission from the depression. Remission was defined as a Montgomery Asberg Depression Scale score below 10 (Hawley, Gale, & Sivakumaran, 2002). The latent severity of depression variable was specified as predicting reduction in right hippocampal volume over the four-year follow-up period and change in MMSE score over this four-year follow-up period. We planned to use bootstrapping to test for mediation, if appropriate (Preacher & Hayes, 2004). Additionally, the analysis considered age, gender, race, years of education, cerebrum size, baseline left or right hippocampal volume, and baseline MMSE score as control variables. We created two identical SEM models with the first including right hippocampal volume (Figure 1) and the second model replacing this variable with left hippocampal volume (Figure 2). We addressed missing data by using a full information maximum likelihood algorithm (Allison, 2003).
Figure 1.
Note the significant relationship between change in right hippocampal volume and change in MMSE score over the four-year follow-up period.
* significant at the .05 level
Figure 2.
Note the significant relationship between change in left hippocampal volume and change in MMSE score over the four-year follow-up period.
Results
Descriptive statistics
At baseline, participants were on average 70 years old (SD= 7.04). The sample was 69% female, and the distribution of participants’ ethnicities was as follows: 85.3% Caucasian, 10.9% African-American, 2.0% Mixed Race, 0.8% Asian, 0.3% Native American, and 0.7% Other (see Table 1). Mean MMSE score at baseline, excluding scores of the eleven participants identified as likely demented at baseline, was 28.49 (SD= 1.89), and participants had an average of 14 years of education (SD= 2.78).
Table 1.
Demographic and cognitive differences between depressed participants and controls.
| Variable | Depressed Mean(SD) N=238 |
Control Mean(SD N=146 |
F or χ2 | p-value |
|---|---|---|---|---|
| Age | 70.03(7.5) | 70.43(6.26) | .281 | p =.59 |
| Gender % female | 67.7% | 70.7% | .389 | p = .31 |
| %White | 89.1% | 85.7% | 0.99 | p = .20 |
| MMSE score (baseline) | 28.31(1.7) | 28.92(1.3) | 14.17 | p < .01 |
| Years of education | 14.2(2.03) | 15.41(1.76) | 35.72 | p < .01 |
| Left hippocampal volume (baseline) | 2.93mL (.41) | 2.95mL (.42) | 0.131 | p =0.72 |
| Right hippocampal volume (baseline) | 3.07mL (.42) | 3.11mL (.43) | 0.505 | p =.48 |
| Cerebrum volume (baseline) | 1149.91 mL (130) | 1141.19 mL (122.22) | 0.42 | p =.52 |
Significant differences are bolded.
The eleven participants eliminated because they were identified as likely demented at baseline, were on average more likely to be depressed (90.9% versus 9.1%, χ2(N=395)=3.83, p<.05), more likely to be a minority (36.4% versus 11.5%, χ2(N=395)=6.21, p<.05), and had fewer years of education (10.73 versus 14.33 F(1,394)=18.73, p < .01).
Alternatively, the depressed participants did not significantly differ from the control participants by age, sex, or minority status but did have fewer years of education (13.55 versus 15.38 years, F(1,383)=44.42, p < .01) and a marginally lower baseline MMSE score (28.31 versus 28.92 points, F(1,383)=14.17, p < .01).
A considerable portion of participants either dropped out between the baseline and four-year follow-up assessments or had missing data on some of the variables. Specifically, 123 participants were missing baseline imaging data, 184 participants were missing imaging data at four-year follow-up, and 47 participants were missing MMSE score at four-year follow-up. No participants had missing data for baseline MMSE score, demographic variables (age, sex, ethnicity, and years of education), or depression diagnosis and severity variables (diagnosis, CESD score, symptom counts, number of past depressive episodes), but 39 participants were missing data for days to remission.
A total of 114 participants with complete neuroimaging data were included in the first regression analysis examining the relationship of depression diagnosis and change in hippocampal volume. Participants with missing data differed from participants who remained in the study in that they were more likely to be minorities (13.6% versus 6.2%, χ2(N=384)=4.23, p<.05), had marginally lower baseline MMSE scores (28.38 versus 28.96 points, F(1,383)=26.72, p < .05), and had fewer years of education (14.44 versus 15.19 years, F(1,383)=44.19, p < .05). Importantly, participants with missing imaging data were no more likely to be depressed.
For the second regression analysis, examining the relationship of depression diagnosis and change in MMSE score, complete data were available for 336 participants. Participants with missing data had marginally lower baseline MMSE scores (27.81 versus 28.65 points, F(1,383)=28.99, p < .05) and had fewer years of education (14 versus 14.75 years, F(1,383)=23.25, p < .05). Importantly, participants with missing MMSE data were no more likely to be depressed.
Analysis 1
Regression analyses demonstrated a significant relationship between baseline depression diagnosis and change in right hippocampal volume over the four-year follow-up period (F(1,113)= −0.17, p< .05); baseline cerebrum volume and age also significantly predicted change in right hippocampal volume (Table 2).
Table 2.
Depression diagnosis predicts change in right hippocampal volume over four years controlling for baseline hippocampal volume, cerebrum (head) size, and age.
| Unstandardized coefficients | |||||
|---|---|---|---|---|---|
| Variable | df | Beta | Standard Error |
F | P value |
| Constant | 2.255 | 0.652 | 3.456 | 0.001 | |
| Baseline right hippocampal volume | 1,113 | 0.275 | .114 | 2.414 | 0.017 |
| Cerebrum volume | 1, 113 | .001 | 0.00 | 3.29 | 0.001 |
| Age | 1, 113 | −0.019 | 0.007 | −2.696 | 0.008 |
| Depressed? 0 = No, 1 = Yes | 1, 113 | −0.172 | 0.079 | −2.168 | 0.032 |
Analyses were repeated for left hippocampal volume. Depression diagnosis at baseline had no relationship to change in left hippocampal volume over the four-year follow-up period (F(1,113)= −0.10, p=0.136); age did significantly predict change in left hippocampal volume (Table 3).
Table 3.
Depression diagnosis does not predict change in left hippocampal volume over four years controlling for baseline hippocampal volume, cerebrum (head) size, and age.
| Unstandardized coefficients | |||||
|---|---|---|---|---|---|
| Variable | df | Beta | Standard Error |
F | P value |
| Constant | 2.495 | 0.583 | 4.282 | 0.00 | |
| Baseline left hippocampal volume | 1,113 | 0.411 | 0.100 | 4.103 | <.001 |
| Cerebrum volume | 1, 113 | 0.001 | 0 | 1.814 | 0.072 |
| Age | 1, 113 | −0.019 | 0.006 | −3.165 | 0.002 |
| Depressed? 0 = No, 1 = Yes | 1, 113 | −0.104 | 0.070 | −1.50 | 0.136 |
For the third regression analysis, depression diagnosis at baseline had no relationship to change in MMSE score over the four-year period (F(1,335)= 0.32, p= .832); however, consistent with the literature, age, years of education, and minority group membership all significantly predicted change in MMSE score (Table 4).
Table 4.
Depression diagnosis does not predict change in MMSE score over the four-year follow-up period, controlling for age, years of education, and minority group membership.
| Unstandardized coefficients | |||||
|---|---|---|---|---|---|
| Variable | df | Beta | Standard Error |
F | P value |
| Constant | 20.73 | 1.877 | 11.044 | <.001 | |
| MMSE baseline | 1,335 | 0.360 | 0.053 | 6.76 | <.001 |
| Age | 1,335 | −0.059 | 0.011 | −5.499 | <.001 |
| Years of education | 1,335 | 0.115 | 0.037 | 3.075 | 0.002 |
| Minority status (0 = Caucasion, 1 = not Caucasian) | 1,335 | −0.870 | 0.237 | −3.667 | <.001 |
| Depressed? 0 = No, 1 = Yes | 1,335 | 0.032 | 0.152 | 0.212 | 0.832 |
Analysis 2
Overall model fit was good for the model examining right hippocampal volume. The Chi-Square test of model fit was statistically significant, indicating a poorly fitting model (χ2(df=28)=43.75, p=0.03). The Tucker-Lewis Index (TLI=0.99), Comparative Fit Index (CFI=0.99), Root Mean Square Error of Approximation (RMSEA=0.03), and Standardized Root Mean Square Residual (SRMR=0.03) all indicated a good fitting model.
In this model (Figure 1), the path between depression severity and right hippocampal volume at four-year follow-up was not significant. The path between depression severity and MMSE score at four-year follow-up was also non-significant. However, change in right hippocampal volume over the four-year follow-up period significantly predicted change in MMSE score over the four-year follow-up period.
Overall model fit for the second SEM model including left hippocampal volume (Figure 2) was good. The Chi-Square test of model fit was statistically significant, indicating a poorly-fitting model (χ2(df=28)=43.44, p=.03). However, the Tucker-Lewis Index (TLI=0.99), Comparative Fit Index (CFI=0.99), Root Mean Square Error of Approximation (RMSEA=0.04), and Standardized Root Mean Square Residual (SRMR=0.03) all indicated a good-fitting model.
The path between depression severity and left hippocampal volume at four-year follow-up was not significant. The path between depression severity and MMSE score at four-year follow-up was also non-significant; however change in hippocampal volume over the four-year follow-up period significantly predicted change in MMSE score over the four-year follow-up period.
Discussion
The current study prospectively examined the relationship between depression, hippocampal volume change, and cognitive change in a population of older adults with Major Depressive Disorder who presented for depression treatment and a population of healthy age-matched control participants.
Regression analyses revealed that depression diagnosis at baseline predicted decrease in right (but not left) hippocampal volume over a four-year follow-up period. Subsequent analyses using structural equation modeling demonstrated a significant relationship between change in hippocampal volume and change in MMSE score over the four-year follow-up period, such that decrease in both right and left hippocampal volume was associated with decrease in MMSE score.
Other studies have also demonstrated smaller hippocampal volumes in individuals with current major depression (e.g., Sheline et al., 1996; Bell-McGinty et al, 2002; McKinnon, Yucel, & Nazarov, 2009). Our findings extend the literature, demonstrating that depression diagnosis predicts decrease in right hippocampal volume over time among older adults. An additional important finding is that this decrease in hippocampal volume predicts decreased scores on the MMSE over a four year follow-up period.
This latter finding is consistent with recent published findings examining the same dataset across a two year timeframe (Steffens, McQuoid, Payne, & Potter, 2011). In that study, investigators used regression to examine concurrent change in normalized hippocampal volumes and in MMSE score over the initial 2.5 year period of the study. They found that hippocampal volume decrease from baseline to year two of the study predicted MMSE score decrease from year two to 2.5 of the study. The current analyses examined this phenomenon over a four-year timeframe. In addition, our methodology (structural equation modeling) allowed for inclusion of additional subjects that were excluded from the regression analyses of the 2011 study. The current analyses robustly demonstrate a continued effect of hippocampal volume decrease on decline in MMSE score in years two to four of the study.
The finding that depressed participants experienced greater atrophy of the right hippocampus than non-depressed control participants over a four-year period provides some support for an etiological role of depression in cognitive decline. While our study does not directly measure the biological mechanism of action, our findings are consistent with the glucocorticoid hypothesis, as well as with findings that stress can accelerate Alzheimer’s disease pathology.
One interesting finding is the asymmetry of our results. That is, we found a significant relationship between depression and change in right hippocampal volume, but not between depression and change in left hippocampal volume. This may have occurred because the hippocampus is a reliably asymmetrical structure in normal adults, with adults tending to have larger right hippocampal volumes (Pedraza, Bowers, & Gilmore, 2004). In our sample, right hippocampus was in fact larger than the left hippocampus at baseline. As the right hippocampus is a larger structure, this may have allowed for greater decrease in volume over time. This is an important area of future research.
Decrease in both left and right hippocampal volume predicted decrease in MMSE score. Again, this provides support for an etiological role of depression in dementia, in so far as hippocampal volume decrease has cognitive implications and may lead to cognitive impairment. Moreover, this is consistent with the literature, which has shown significant relationships between hippocampal volume and MMSE score cross-sectionally (Apostolova et al., 2006, Du et al., 2001). Our study extends the extant literature, demonstrating a longitudinal relationship between change in hippocampal volume and change in MMSE score.
However, it should be noted that, while there were significant relationships between depression and right hippocampal volume change over time and between hippocampal volume decrease and decreased MMSE score over time, neither depression diagnosis nor depression severity predicted change in MMSE score over time. It is highly surprising that we found no relationship between depression diagnosis and change in MMSE score over time. Consequently, we were unable to test for mediation.
This is particularly surprising, as a previous study using this data set showed increased risk for dementia among depressed participants relative to controls (Steffens et al., 2004). Importantly, among the study participants, 31 individuals developed dementia over the course of their participation in the study. In the current study, we eliminated eleven individuals, ten of whom were in the depressed group, because their MMSE score decreased by four points or more in the first year of the study, and we considered them as likely to have prodromal dementia at baseline. By eliminating these individuals from the study, we removed those with greatest cognitive decline, which may have obscured the effect of depression on cognitive decline over time. Our lack of a finding here may also be due to our use of the MMSE as the measure of cognition. Limitations regarding this instrument are discussed below.
Additionally, there was limited variability in depression severity across the participants, with all depressed participants tending to have moderate to severe depression and multiple past depressive episodes. Therefore, it may be the case that there was insufficient variability in depression severity (i.e. a restriction of range) to enable us to detect a significant effect of severity in the population, as we hoped to do in the SEM model.
It should also be noticed that in this population of older adults with depression, many had experienced prior depressive episodes. Thus, they likely had previous experience of elevated cortisol. This historical experience of hypercortisolemia may have maximally reduced hippocampal volumes in this group prior to enrollment in the study, limiting the extent to which further hippocampal atrophy could be sustained.
Finally, it is important to note that all depressed participants in the study received treatment with antidepressant medication, including regular monitoring of symptoms by a geriatric psychiatrist and medication adjustment as necessary. In fact, among the depressed participants, there is no significant relationship between depressive symptoms at baseline and depressive symptoms at two- or four-year follow-up, most likely indicating that this treatment was highly effective in treating participants’ symptoms. If their symptoms were treated effectively, it could be that the glucocorticoid cascade was interrupted or prevented, thus minimizing further hippocampal damage.
While the restriction of range in depressive symptoms, previous experiences of hypercortisolemia, or the treatment provided to depressed participants, may have acted to obscure the effect of depression on hippocampal volume, it is also possible that our lack of significant findings is due to a true lack of relationship between depression and hippocampal volume over time. Importantly, most studies that have demonstrated a relationship between depression and hippocampal volume have been cross-sectional. While we originally argued that one strength of this study is the ability to track change in hippocampal volume over time, it may be the case that decreases in hippocampal volume occur during episodes of depression but are not maintained, or do not cascade, over time.
This study has many strengths: a sample of older, clinically depressed adults and an age-matched sample of controls; regular monitoring of depressive symptoms, regular cognitive testing, and regular MRI of the brain; and a prospective longitudinal design enabling us to examine change over time. However, some limitations must be acknowledged.
First, the sample is mostly Caucasian and college-educated, with high MMSE scores at baseline. Such a sample is not representative of the general population of older adults. Moreover, some studies have shown that individuals with greater educational attainment are more likely than individuals with few years of education to experience cognitive decline after becoming depressed. Thus, it may be the case that these results are not typical of the general population of older adults.
Moreover, older adults who are minorities, have smaller household incomes, and have fewer years of education are more likely to experience cognitive decline. While the population of participants used in this study may have been more likely to demonstrate cognitive decline after becoming depressed due to their higher educational attainment, overall they are not typical of the older adults most at risk for cognitive decline. Again, therefore, caution should be exercised in applying these results to the general population of elders.
Additionally, in this study we used the MMSE as our measure of cognitive functioning. The MMSE is a broad, global measure of cognition. Only two questions directly assess memory; the remaining questions assess orientation, registration, attention, and language. Yet, the hippocampus is primarily associated with episodic and spatial memory, with the right hippocampus being implicated in memory of visual stimuli and the left hippocampus implicated in verbal memory. Thus, the MMSE may not be the best or most sensitive measure of cognitive impairment resulting from hippocampal atrophy or damage. Future studies might examine the relationship between depression, hippocampal volume change, and change in performance on these measures.
Finally, it should be noted that there was significant attrition over time in our study. Participants’ reasons for dropping out of the study (e.g. death, unable to travel to the study site) are not recorded. However, participants who dropped out of the study tended to have lower baseline MMSE scores, fewer years of education, and were more likely to be minorities. These individuals would be at greater risk for cognitive decline, and their loss from the study may have served to mask the effects of depression on hippocampal volume loss and cognitive decline.
In conclusion, depression is thought to initiate a glucocorticoid cascade that damages the hippocampus, a brain structure key to formation of new memories, and thus places depressed individuals at increased risk for cognitive decline. In the present study we used regression and structural equation modeling to prospectively examine relationships between depression diagnosis and symptoms, hippocampal volume, and MMSE score over time in a sample of older adults. Results indicated support for the glucocorticoid cascade hypothesis, with depression diagnosis predicting decrease in right, but not left, hippocampal volume, and hippocampal volume decrease predicting decrease in MMSE score. Future research may consider use of neuroendocrine markers such as measures of cortisol to test the glucocorticoid cascade hypothesis. Lastly, specialized measures of memory, as well as examination of other brain areas affected by depression, may shed additional light on the relationship between depression and cognitive decline or dementia.
Table 5.
Correlation matrix for the first SEM model.
| Number of Episodes |
Current Symptom Count |
Worst Symptom Count |
CESD score |
Days to Remission |
MMSE 3 |
Right Hippo- campus 3 |
Age at Baseline |
MMSE1 | Right Hippo- campus 1 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Episodes | 1 | |||||||||
| Current Symptom Count | 0.27 | 1 | ||||||||
| Worst Symptom Count | 0.27 | 0.93 | 1 | |||||||
| CESD score | 0.23 | 0.81 | 0.79 | 1 | ||||||
| Days to Remission | 0.26 | 0.85 | 0.81 | 0.79 | 1 | |||||
| MMSE 3 | 0.09 | −0.14 | −0.12 | −0.11 | −0.17 | 1 | ||||
| Right Hippo-campus 3 | 0.13 | 0.02 | 0.04 | −0.02 | −0.01 | 0.27 | 1 | |||
| Age at Baseline | −0.09 | −0.1 | −0.09 | −0.05 | −0.06 | −0.23 | −0.41 | 1 | ||
| MMSE1 | 0.04 | −0.16 | −0.13 | −0.17 | −0.16 | 0.63 | 0.13 | −0.26 | 1 | |
| Right Hippo-campus 1 | 0.02 | 0.04 | 0.06 | 0.03 | 0.03 | 0.16 | 0.46 | −0.21 | 0.25 | 1 |
Acknowledgments
Support: This research was supported by NIMH grants K01 MH066380, K24 MH70027, R01 MH54846 and P50 MH 60451.
Contributor Information
Kathryn Sawyer, Department of Psychology, Florida State University
Elizabeth Corsentino, Department of Psychology, Florida State University
Natalie Sachs-Ericsson, Department of Psychology, Florida State University
David C. Steffens, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
References
- Allison PD. Missing data techniques for structural equation modeling. Journal of Abnormal Psychology. 2003;112:545–557. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006;129(11):2867–2873. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychological Medicine. 1999;29(3):629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty Sandra, Butters Meryl A, Cidis Meltzer Carolyn, Greer Phil J, Reynolds Charles F, Becker James T. Brain Morphometric Abnormalities in Geriatric Depression: Long-Term Neurobiological Effects of Illness Duration. American Journal of Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- Berger A-K, Fratiglioni L, Forsell Y, Winblad B, Bäkman L. The occurrence of depressive symptoms in the preclinical phase of AD: A population based study. Neurology. 1999;53(9):1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor E, Levy J, Zubenko G, Zubenko W, Cohen R, Mirza N, Putnam K, Sunderland T. Effects of previous major depressive illness on cognition in Alzheimer disease patients. American Journal of Geriatric Psychiatry. 2005;13(4):312–318. doi: 10.1176/appi.ajgp.13.4.312. [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Joels S, Mann A. Does depression predict cognitive outcome 9 to 12 years later? Psychological Medicine. 2002;30:1017–1023. doi: 10.1017/s0033291799002779. [DOI] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant B, DeKosky S. The temporal relationship between depressive symptoms and dementia: a community based prospective study. Archives of General Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Dong Hongxin, Csernansky John G. Effects of Stress and Stress Hormones on Amyloid-beta Protein and Plaque Deposition. Journal of Alzheimer's Disease. 2009;18(2):459–469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Fuhrer R, Dartigues J, Alpérovitch A. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. American Journal of Epidemiology. 1996;144:634–641. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Du Y, Dodge H, Ratcliff G, Chang C. Depressive Symptoms and Cognitive Decline in Late Life. Archives of General Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, den Heijer T, Koudstaal MD, Hofman A, Breteler M. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadornick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Backman D, Farrer L. Depression as a risk factor for Alzheimer disease: The MIRAGE Study. Archives of Neurology. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Hawley CJ, Galea TM, Sivakumarana T. Defining remission by cut off score on the MADRS: Selecting the optimal value. Journal of Affective Disorders. 2002;72:177–184. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- Henderson A, Korten A, Jacomb P. The course of depression in the elderly: A longitudinal community-based study in Australia. Psychological Medicine. 1997;27:119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: An updated review. Australia and New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? Journal of Neurosurgery and Neuropsychiatry. 2004;75:1662–1666. doi: 10.1136/jnnp.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing LV, Mortensen PB, Bolwig TG. Clinical consequences of sensitization in affective disorder. Journal of Affective Disorders. 1998;47:41–47. doi: 10.1016/s0165-0327(97)00128-6. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. Journal of Affective Disorders. 2003;73:261–269. doi: 10.1016/s0165-0327(02)00004-6. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Lehtovirta M, Hallikainen M, Hanninen T, Helkala E-L, Vainio P, Riekkinen PJ., Sr MRI of the hippocampus in Alzheimer's disease: Sensitivity, specificity, and analysis of the incorrectly classified subjects. Neurobiology of Aging. 1998;19(1):23–31. doi: 10.1016/s0197-4580(98)00006-2. [DOI] [PubMed] [Google Scholar]
- Lupien S, de Leon M, De Santi S, Convit A, Tarshish C, Nair N, Thakur M, McEwen B, Hauger R, Meaney M. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry and Neuroscience. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Fifth Edition. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. Journal of the International Neuropsychological Society. 2004;10(05):664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Robins LN, Helzer JEJ, Croughan, Ratcliff KS. The NIMH Diagnostic Interview Schedule: Its history, characteristics and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: Relations to anxiety symptoms. Biological Psychiatry. 2001;50(12):960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Joiner T, Plant A, Blazer DG. The influence of depression on cognitive decline in community-dwelling elderly persons. American Journal of Geriatric Psychiatry. 2005;13:402–408. doi: 10.1176/appi.ajgp.13.5.402. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schweitzer I, Tuckwell V, O’Brien J, Ames D. Is late onset depression a prodrome to dementia? International Journal of Geriatric Psychiatry. 2002;17:997–1005. doi: 10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Wang P, Gado MH, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences Medical Sciences. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. The Journal of Neuroscience. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Payne ME, Potter GG. Change in hippocampal volume of magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the Neurocognitive Outcomes of Depression in the Elderly Study. American Journal of Geriatric Psychiatry. 2011;19(1):4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Payne ME, Greenberg DL, Byrum CE, Welsh-Bohmer KA, Wagner HR, MacFall JR. Hippocampal volume and incident dementia in geriatric depression. American Journal of Geriatric Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- Steffens DC, Welsh-Bohmer KA, Burke JR, Plassman BL, Beyer JL, Gersing KR, Potter GG. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. Journal of Geriatric Psychiatry and Neurology. 2004;17(4):202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- Sun X, Steffens D, Au R, Folstein M, Summergrad P, Yee J, Rosenberg I, Mwamburi M, Qiu W. Amyloid-associated depression: A prodromal phase of Alzheimer’s disease. Archives of General Psychiatry. 2008;65(5):542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangalos EG, Smith GE, Ivnik RJ, Petersen RC, Kokmen E, Kurland LT, Offord KP, Parisi JE. The Mini-Mental State Examination in general medical practice: Clinical utility and acceptance. Mayo Clinic Proceedings. 1996;71(9):829–837. doi: 10.4065/71.9.829. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biological Psychiatry. 2000;47(12):1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]