Abstract
Adolescence is a developmental period characterized by significant increases in the onset of depression, but also by increases in depressive symptoms, even among psychiatrically healthy youth. Disrupted reward function has been postulated as a critical factor in the development of depression, but it is still unclear which adolescents are particularly at risk for rising depressive symptoms. We provide a conceptual stance on gender, pubertal development, and reward type as potential moderators of the association between neural response to reward and rises in depressive symptoms. In addition, we describe preliminary findings that support claims of this conceptual stance. We propose that (1) status-related rewards may be particularly salient for eliciting neural response relevant to depressive symptoms in boys, whereas social rewards may be more salient for eliciting neural response relevant to depressive symptoms in girls and (2) the pattern of reduced striatal response and enhanced medial prefrontal response to reward may be particularly predictive of depressive symptoms in pubertal adolescents. We found that greater vmPFC activation when winning rewards predicted greater increases in depressive symptoms over two years, for boys only, and less striatal activation when anticipating rewards predicted greater increases in depressive symptoms over two years, for adolescents in mid to late pubertal stages but not those in pre to early puberty. We also propose directions for future studies, including the investigation of social vs. monetary reward directly and the longitudinal assessment of parallel changes in pubertal development, neural response to reward, and depressive symptoms.
Keywords: Reward, Adolescence, Depression, Puberty
Introduction
Rates of depressive episodes rise significantly during adolescence with age 15 found to be the peak age of onset (Lewisohn, Clarke, Seeley, & Rohde, 1994). Not only do many adolescents experience their first depressive episode during this time period, many additional adolescents experience subthreshold rises in depressive symptoms that are distressing and interfere with adolescent nevertheless development (Lewinsohn, Seeley, Solomon, & Zeiss, 2000). This increase in depressive symptoms during adolescence appears normative in some ways (Sawyer, Pfeiffer, & Spence, 2009) but there are also individual differences in the increase.
Much research has been conducted to delineate risk factors that may contribute to risk for onset of clinical depression during adolescence (Cicchetti & Toth, 1998; Davey, Yucel, & Allen, 2008; Forbes & Dahl, 2005). Depression is characterized by a number of symptoms including elevated negative affect and disturbances in sleep and appetite, but it is consistently distinguished from other affective disorders by dysregulation in positive affect and low reward responding (for review, see Forbes & Dahl, 2005). Accordingly, adolescents with Major Depressive Disorder (MDD) have less striatal response and more ventral medial prefrontal cortex (vmPFC) response to monetary reward (Forbes et al., 2006, 2009). Similarly, adolescents at risk for MDD (via familial history) also have less striatal response to pleasant stimuli (Monk et al., 2008) and monetary reward (Gotlib et al., 2010). These findings suggest that reward-related changes may be related to rises in depressive symptoms in youth during adolescence prior to onset of clinical levels of depression.
According to Davey et al.’s (2008) developmental model of depression, social (e.g., greater peer affliation) and neural (e.g., reward circuitry maturation) developmental changes in adolescence result in reward-related changes that may increase risk for depression during this time period. These concurrent neural and social changes during adolescence make pursuit of rewards not only more valued but also more purposeful, leading to increased use of executive function to set and work toward abstract goals involving social rewards (Davey et al., 2008). As a result, challenges or failures in obtaining dearly-valued social goals (e.g., becoming romantically involved with someone, joining a high-status peer group) can put vulnerable adolescents on a trajectory toward disrupted reward responding and, eventually, depression. Feedback to the developing dopamine system at this sensitive time in development might then lead to disrupted neural reward function and, then, depressive symptoms (Davey et al., 2008).
What is not explained by Davey et al.’s (2008) model is why some adolescents show clinically significant increases in depressive symptoms during adolescence, whereas others do not, despite these normative social and neural developmental changes. The current paper postulates that individual differences in reward function early in adolescence could predict increases in depressive symptoms—placing some adolescents at greater risk for developing clinical levels of the disorder in the future—and that the association between reward function and depression may be moderated by gender, pubertal development, and reward type. Specifically, we postulate that less striatal response and more vmPFC response to anticipation and gain of rewards during adolescence increases adolescents’ risk for depression during this developmental time period. Moreover, we hypothesize that this brain-behavior association unfolds in the context of pubertal development and the combination of gender and reward type, with status rewards as more salient to boys and affiliative rewards as more salient to girls.
Our goal is to put forward a more detailed conceptual model of reward function in the development of adolescent depression, rather than to offer a definitive explanation for the emerging gender difference in the prevalence rate of adolescent depression or the increase in depression with pubertal changes. Therefore, addressing other important etiological issues such as the role of stressful life events or cognitive bias toward negative information on depression is beyond the scope of this paper. We offer a conceptual model for putative differing mechanisms of risk—based on disrupted reward systems in differing contexts—for depression depending on adolescent characteristics (e.g., gender, pubertal stage). We outline previous literature on reward-related regions, the striatum and vmPFC, and their association with the development of depression. In addition, to provide proof of concept for our model that could inspire future research, we describe preliminary empirical support for the role of gender and pubertal development as moderating factors of reward-depression associations, and we raise questions that may be tested empirically in future research.
Striatal Activation
The striatum is a region of the brain typically associated with positive affect and reward (Forbes, 2009; Haber & Knutson, 2010). Function of the striatum appears to be relatively specific, with activation in the striatum found in response to reward win but not reward loss in many studies (for review, see Knutson & Greer, 2008). In adolescents without diagnoses of depression, striatal response is positively associated with subjective positive affect and inversely associated with depressive symptoms (Forbes et al., 2009; Forbes et al. 2010). Previous research has demonstrated that, similar to adults with depression (Epstein et al., 2006; Smoski et al., 2009), adolescents with clinical diagnoses of depression show less striatal activation during a reward decision-making task compared to healthy adolescents (Forbes et al., 2006; Forbes et al., 2009). Indeed, a recent meta-analysis conducted by our group indicates that low striatal response to monetary reward is consistent across studies of adult and adolescent depression (Olino & Forbes, in preparation).
Adolescents may be showing reward-related differences in striatal reactivity prior to onset of depression, and these patterns of neural response, especially during periods of developmental sensitivity, may even be predictive of depression. While previous findings (Forbes et al., 2006, 2009, 2010) indicate associations between depression and neural response to reward, they do not provide us with information about when reward-related developmental changes occur in relation to rise of depressive symptoms Studies of adolescents at risk for depression (Gotlib, Hamilton, Cooney, Singh, Henry, and Joorman, 2010; Monk et al. 2008) have reported less response to reward in the striatum compared to low risk adolescents, suggesting that alterations in reward function could precede the onset of clinical levels of depression. However, because these studies did not examine changes in depressive symptoms in their samples, more research examining adolescents’ striatal reactivity is needed to clarify whether these neural differences in reward function subsequently predict later increases in depressive symptoms.
vmPFC Activation
The vmPFC plays an important role in the regulation of affect and may foster the formation of and pursuit of abstract social and emotional goals (e.g., establishing social status, initiating a romantic relationship; Davey et al., 2008). In addition to abstract reward processing, many subregions of the vmPFC (i.e., the Anterior Cingulate (ACC), Brodmann area (BA) 32, and medial BA 10) have been associated with the processing of self-performance and self-mastery in relation to others (Amodio & Frith, 2006; Haber & Knutson, 2010; Masten et al 2011). The ACC, in particular, plays a role in reward decision making and self-evaluation in social contexts and is highly interconnected with the ventral striatum, among other brain regions (Haber & Knutson, 2010). Greater activation in the ACC during social peer-rejection fMRI paradigms has been associated with depressive symptoms (Masten et al., 2011).
Depressive symptoms may putatively be associated with distorted self-perception of the ability to obtain rewards successfully, with excessive focus on the loss of status or on difficulties obtaining reward. Indeed, increased mPFC response to reward has been associated with depression in adults (Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008) and adolescents (Forbes, 2009), possibly reflecting difficulty regulating striatal response or disengaging from self-focused cognition during rewarding experiences (Forbes & Dahl, 2012). The vmPFC has reciprocal connections with the striatum, and, given the inverse relation of cortical and striatal dopamine function (Grace, 1993), is thought to serve a regulatory role for the striatum (Price & Drevets, 2010). In terms of development of symptoms, some adolescents may be at greater risk for rises in depressive symptoms because of increased activation in these self-processing areas (Forbes et al., 2010).
Other regions have also been implicated in reward processing, including the amygdala and orbitofrontal cortex (OFC; Haber & Knutson, 2004). Whereas our conceptual framework focuses on the ventral striatum (due to its role in affective experience in pleasant and rewarding stimuli) and the vmPFC (due its cognitive and regulatory role in reward seeking and attainment), research has also found that monetary and social rewards are associated with activation in the amygdala and OFC (e.g., Davey et al., 2011, Monk et al., 2008). Imaging studies that examine the association between reward-related differences and risk for depression should consider these regions as well.
Moderating Factors: Gender and Reward Type, Pubertal Development
In order to provide a more comprehensive conceptual model of reward function in adolescent risk for depression, we postulate that the association of reward-related changes and depressive symptoms is moderated by (1) gender differences in response to specific types of reward and (2) pubertal development. We provide more detail on these hypotheses below.
Gender and Reward Type
Although girls are at greater risk for depressive symptoms than boys starting at adolescence (Essau, Lewinsohn, Seeley, & Sasagawa, 2010), boys also experience an increase in depressive symptoms during this time period (Cicchetti & Toth, 1998), and depression disrupts the academic and social functioning of both male and female adolescents (Lewinsohn et al., 2000). Many conceptual models have studied the impact of negative life events and stressors in girls in order to explain the emergence of depression (Hankin et al., 2007). However, this research has originated from models of stress in the etiology of depression. From a reward function perspective, it is not only important to examine whether neural response to reward is related to depression but to elaborate on how different types of reward may be relevant to depression (Forbes, 2009), and furthermore, whether these types of reward are similarly important to the development of depression in boys and girls.
The salience of different types of rewarding goals may differ for boys and girls. Conceptual perspectives have emphasized the importance of social reward in the development of adolescent depression (Davey et al., 2008; Forbes, 2009), and more affiliative social stressors (loss of a friend, end of a romantic relationship) are postulated to be more problematic for girls, whereas more power-oriented social stressors (threat to social status, reputation) may be more critical for boys (Guyer et al., 2009; Oldehinkel et al., 2007; Stroud et al., 2002). For instance, Conger, Lorenz, Elder, Simons, and Ge (1993) found that adult men showed greater sensitivity to financial stress, while women showed greater sensitivity to familial social stressors (Conger et al., 1993). Similarly, Stroud, Salovey, and Epel (2002) found that adult men showed greater cortisol reactivity to achievement related stress, whereas adult women showed greater cortisol reactivity to social rejection stress. Similar differences in reward type based on gender have also been found with adolescents. Based on a meta-analytic review of literature on adolescent peer relationships, Rose and Rudolph (2006) suggest that adolescent boys and girls also differ in how they value and respond to social rewards, with boys valuing status-related rewards with social groups and girls placing associated greater value on deeper social connections within the group. Based on this previous literature, we are postulating about mean differences, and we note that both boys and girls value each of these social rewards (affliative bonds and social status) and that individual differences may indicate that some boys value affliative social rewards more than social status (and vice versa).
From a threat-reduction model of depression, girls and boys may minimize reward-seeking behavior in forming affiliative bonds and establishing social status, respectively, in order to decrease the risk for disappointment. Less reward-seeking behavior may increase likelihood for depressive symptoms for both genders, but in differing ways. In other words, both boys and girls may both be experiencing social changes in adolescence that explain a general increase in depressive symptoms but the types of social changes that occur may be different. It is possible that the social rewards that girls seek (e.g. affliative social bonds) may be more tenuous and easily thwarted (Hankin et al., 2007) than those sought by boys (e.g., social status).
Few researchers have explicitly examined gender differences in neural responses to different types of rewarding stimuli as predictors of depressive symptoms across adolescence. However, many researchers have suggested the need for such evaluation (Gotlib et al., 2010; Masten et al., 2011). At any rate, studies evaluating reward-related differences and depression in both male and female adolescents are needed to examine neural response to ecologically valid and valued rewards for both genders (both affiliative and status-related rewards).
Puberty as a Moderator, Within Reward Type
Adolescents who have reached mid-to-late puberty and exhibit a pattern of low striatal or high mPFC response to reward might be particularly likely to experience an increase in depressive symptoms. With the marked rise of depressive symptoms occurring during adolescence, pubertal changes appear to be directly related to risk for depression. Peak age of onset for depression appears to coincide with average age of pubertal completion, and depressive symptoms increase with pubertal development (Angold & Worthman, 1993). Conceptual models propose that neural changes (e.g. striatal and mPFC development) that occur during adolescent pubertal development contribute greatly to rise in depressive symptoms during this time period (Davey et al., 2008). And, as mentioned above, the pattern of low striatal response and high mPFC response to reward receipt is associated both with pubertal development and depressive symptoms in adolescents (Forbes et al., 2010). Taken together, the association between reward response, pubertal changes, and adolescent depression suggests that pubertal maturation plays a key moderating role in the influence of reward function on rises in depressive symptoms across adolescence. However, as existing studies have been cross-sectional, it is not clear whether these reward-related changes that co-occur with pubertal development affect the development of depressive symptoms over time. As indicated by findings that the association between high cortisol reactivity and depression is moderated by pubertal development (Hankin, Badanes, Abela, & Watamura, 2010) and that associations between striatal and mPFC response and depressive symptoms were much stronger in mid/late pubertal adolescents than in pre/early pubertal adolescents (Forbes et al., 2010), such a developmental stance is critical to elucidating neural mechanisms of depression.
Mechanistic Model
In sum, we propose that developmental social changes and neural changes interact to increase risk for depression during adolescence. Neurally, adolescents undergo greater maturation of the vmPFC, changes in vmPFC function, and maturation of the dopamine system (Davey et al., 2008). These neural changes appear to be influenced by pubertal maturation, rather than age. And, while biological in nature, they may be reinforced by social behaviors that are also changing during this developmental period—adolescents spend more time with peers and engage in greater reward-seeking behaviors than during childhood (Steinberg, 2008). Together, these social and neural changes make experiencing social rewards more valued during this developmental period. Despite these developmental changes that occur on average among adolescents, individual differences greatly influence the kind of social rewards that are valued and the degree to which these rewards are pursued. We suggest that gender is associated with salience of reward type, and thus with the association between depression and neural response to reward type. We examine these relations in particular in our empirical findings below.
Case Examples
To illustrate our conceptual model of reward-related mechanisms of risk for depression in adolescents, we provide two case examples drawn from clinical cases. These cases highlight reward-related changes that may be associated with depressive symptoms and illustrate how they may be moderated by gender. The first case includes changes in seeking social rewards associated with affliative bonds in a female adolescent, whereas the second case demonstrates changes in seeking status based rewards in a male adolescent. Both cases illustrate the abstract and distal nature of rewards that are common in adolescence and may be both related to vmPFC and striatal activity.
Case 1
M. K. is a 14 year-old girl brought to an outpatient clinic by her mother, who was concerned about her recent social isolation and irritability. Her mother reports that although her daughter always had a very positive and enthusiastic attitude, she now seems flat and spends most of her time alone in her room. She also reports that she suspects this is related to trouble with a boy with whom M.K. has been romantically involved over the last year. M.K. confirms numerous ups and downs in her relationship with a boy at school with whom she recently had her first sexual experience. She reports that although she loves him, feels he is her “soulmate”, and plans to marry him, she recently learned that he has been unfaithful to her. She reports that this has hurt her very badly because she trusted him and imagined a future together. This experience has led her to question the “goodness” and loyalty of people around her. She reports spending most of her free time in her room by herself, because she has lost interest in spending time with others—even her best friend. She also reports that although she used to participate in dance and band, where she had several friendships, she no longer enjoys those activities, and has stopped participating in recent months.
Case 2
T. R. is a 16 year old boy who presented to the clinic after making suicidal statements to an older cousin. Upon presenting to the clinic, he stated that he had been a popular and well-liked teen in his school in past years. Recently, however, he had begun to withdraw from interactions with his peers. He noted that in addition to feeling sad and frustrated much of the time, he felt unmotivated to pursue many of his interests including writing and playing music. He indicated that he had enjoyed being known as a good rapper and musician among his peers and he had often been asked to perform some of his newest music. He had planned to pursue a professional career in music following graduating from high school. He mentioned liking many aspects of music culture, in particular hip hop culture. He wanted to achieve the recognition that many of his favorite artists had achieved. He had experienced a taste of that type of recognition in the past within his peer group. However, recently his parents had greatly limited the time he spent on music as they wanted him to pursue a different career path. As a result, there was much parent-child conflict over him attending college, and he found himself blocked from pursuing a dearly held goal. He stated that as a result of his parents’ influence, he was struggling with losing the reputation and status among his peers as being a good musician. He noted that he felt that he would not win in life: either he would lose the respect of his parents or of his friends.
Preliminary Empirical Support for Conceptual Model
Here, we provide empirical data that show initial support for our conceptual model that differences in reward-related functioning (both in anticipating and obtaining rewards) explain the increases in depressive symptoms observed during adolescence. This study serves as a starting point for more comprehensive measurement of reward-related risk for depression in adolescents.
Hypotheses
We evaluated the role of striatal and vmPFC activation during anticipation and win of monetary reward as predictors of increases in depressive symptoms across a two year period of adolescence in a sample of psychiatrically healthy adolescents. Adolescents in our sample were part of a larger study of reward function in normal adolescent brain development, and results on puberty and neural response to reward from this study have previously been reported (Forbes et al., 2010). However, the contribution of individual differences in reward function (as influenced by gender and pubertal maturation) to rises in depressive symptoms across adolescence—or the moderation of this association by gender or pubertal development—was not examined by Forbes et al., 2010, nor has it been studied by other researchers, to our knowledge. Thus, we evaluated the proposed hypotheses in this sample in order to examine the association of reward-related differences (moderated by gender and reward type and by puberty) with depressive symptoms in healthy adolescents. While this sample was not recruited for clinical-level depression, it is a typical community sample, which makes it suitable for evaluating changes in depressive symptoms occurring before the usual age of onset of depression. Depressive symptoms were measured longitudinally in this sample, providing an opportunity to examine these data as a preliminary test of our hypotheses.
Based on our conceptual model, we hypothesized that 1) less striatal activation and greater vmPFC activation during reward anticipation and reward win would predict greater increases in depressive symptoms; 2) because monetary reward reflects status (Izuma, Saito, & Sadato, 2008) the association between striatal and vmPFC activation and increases in depressive symptoms would be stronger for boys rather than girls; and 3) the association between striatal and vmPFC activation and increases in depressive symptoms would be stronger for adolescents in mid to late puberty rather than pre/early puberty.
Materials and methods
Participants and Procedure
The final sample included 72 adolescents—40 girls, 32 boys. Sixty-three of these adolescents provided pubertal stage data. Consistent with our previous approach to examining affective development (Forbes et al., 2006), we classified adolescents as pre/early pubertal if they were Tanner breast/genital stage 1 or 2 (n = 23) and as mid/late pubertal if they were stage 3, 4, or 5 (n =40).
Adolescents were recruited from the community through advertisements, flyers, and demographically targeted phone lists. Adolescents were recruited to be in a narrow age range—11-13 years—but to vary in pubertal development. More specifically, girls were 11-12 years old (M=11.49, SD =.60), and boys were 12-13 years old (M=12.39, SD=.59), based on findings that girls in the United States undergo puberty earlier than boys. We thus intended to maximize the pubertal variability within groups of girls and boys and to avoid confounding of puberty by age. Adolescents were free of current and lifetime psychiatric disorders; history of head injury, serious medical illness, psychotropic medication, alcohol use, or illicit drug use; and did not have braces.
The original sample included 125 adolescents. Data for 53 adolescents were excluded for excessive head movement (n=22; 10 female, 6 pre/early pubertal), having siblings in the study (n=9), possible medical or psychiatric conditions (n=3) and attrition/missing data/withdrawal from the study (n=21). As in our previous studies with adolescents (e.g., Forbes et al., 2010,), excessive head movement was defined as movement greater than 4 mm. At time 1 assessment, adolescents completed a midweek fMRI scan and the Mood and Feelings Questionnaire (MFQ;. Angold et al., 2002). Adolescents were then invited back to the study two years later to complete the MFQ a second time for time 2 assessment.
Psychiatric health
If the parent or guardian of an adolescent reported that the adolescent had received diagnosis, treatment, or medication for “any mental or behavioral health issues” during a phone screen, the adolescent was excluded from participation.
Pubertal development
Adolescents underwent physical examination by a nurse trained in scientific study of puberty to determine sexual maturation stage using criteria specified by Marshall and Tanner (1968). Because breast and genital development reflect changes in levels of gonadal steroids, which influence neural development and affect-related brain function during puberty, participants were classified based on the Tanner breast/genital scale.
Reward processing
The fMRI paradigm was a slow event-related card-guessing game that allows the probing of striatal response to the anticipation and receipt of monetary reward feedback. Participants received win, loss, or no-change feedback for each trial. Participants were told that their performance would determine a monetary reward after the scan, with $1 for each win and 50 cents deducted for each loss. Trials were presented in pseudorandom order with predetermined outcomes. Earnings totaled $6. Trials were presented in four runs, with 12 trials per run and a balanced number of trial types within runs (i.e., six possible-win and six possible-loss trials in each). During each trial, participants guessed via button press whether the value of a visually presented card was high or low (3s), learned the trial type (possible-win or possible-loss) and anticipated feedback (1s plus 11s intertribal interval). Participants were unaware of fixed outcome probabilities, and their engagement and motivation were maintained by verbal encouragement between runs. Because participants practiced the task before the scan and did not exhibit a change in reaction time across task runs, it is likely that they understood the anticipation period. During debriefing, all participants stated that they understood the task, thought that outcomes were due to chance, and found the task engaging (Forbes et al., 2010).
Depressive symptoms
Adolescents completed the MFQ (Angold et al., 2002), a psychometrically sound measure of depressive symptoms, at time 1 and at time 2. Four participants did not complete the questionnaire and were excluded from related analyses. We computed a residualized change score by regressing sex, time 1 depressive symptoms, and the interactive effect of sex and time 1 depressive symptoms on time 2 depressive symptoms to measure changes in depressive symptoms across adolescence. We included the interactive effect of sex and time 1 depressive symptoms in creating the residualized change score because changes in depressive symptoms across adolescence are likely to differ in boys and girls (Essau et al., 2010). Indeed in our sample, girls had higher levels of depressive symptoms than boys at time 2 (F = 4.05, p < .05) but not time 1. We were interested evaluating how these changes in depressive symptoms may be affected by reward type, pubertal maturation, and gender.
fMRI acquisition, processing, and analysis
Each participant was scanned using a Siemens 3T Allegra scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 34 axial slices (3mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25ms, FOV=20cm, matrix=64x64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems.
Whole-brain image analysis was completed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm) and our standard procedures for preprocessing and analysis (see Forbes et al., 2010 for greater detail). Data were realigned, spatially normalized into Montreal Neurological Institute template space, and smoothed with a 6mm full-width at half-maximum Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Preprocessed data sets were then analyzed in SPM8. As in our previous work with this version of the fMRI reward paradigm, we created 2 contrasts of interest: (a) reward anticipation > baseline and (b) reward outcome (i.e., win) > baseline, with baseline defined as the last 3s of each inter-trial interval. Based on our hypotheses and previous strategy with this task, analyses focused on the first run, since these data are less likely to reflect fatigue, boredom, frustration with task length, and habituation (Forbes et al., 2009; Forbes et al., 2010). Habituation is a particular concern because striatal response tends to diminish with repeated experience of a reward (Koob et al., 2008).
Individual contrast images were then included in region of interest (ROI) analyses to determine group-level main effects of task within striatal and mPFC ROIs using within-group t-tests, thresholded at a voxel level of p < .01 and a minimum extent of 10 contiguous voxels. Next, tests of multiple comparisons (FDR) were conducted to determine whether reward-related activation was significant at p < .05 in these regions after correcting for multiple comparisons. The striatal region of interest (ROI) was constructed using the WFU PickAtlas Tool (v1.04) and defined as a 3642-voxel sphere with 20mm radius, centered on the ventral striatum using Talairach coordinates x = 0, y = 10, and z = −10, and encompassing the head of the caudate nucleus and ventral areas. The mPFC ROI was constructed using the PickAtlas and defined as a 5393-voxel sphere including medial Brodmann Area (BA)10 and BA32, which are part of the anterior rostral mPFC region implicated in social cognition and self systems (Amodio & Frith, 2006).
Hypotheses were tested using regression models with a dummy-coded moderator variable (gender, pubertal group), residualized change in depressive symptoms, and the multiplicative interaction of these two main effects predicting striatal or mPFC response to reward anticipation or reward outcome. Models were run separately for reward anticipation and reward outcome. Hypothesis tests were then corrected for multiple comparisons by masking with the main effects of task (e.g., response to reward outcome) within each ROI and applying the false discovery rate to resulting clusters.
Results
Depressive Symptoms
The means and standard deviations for time 1, time 2, and residualized change in depressive symptoms are listed in Table 1. Girls had higher levels of depressive symptoms than boys at time 2 (F = 4.05, p < .05). Gender was unrelated to depressive symptoms at time 1 or change in symptoms from time 1 to time 2. Girls were more likely to be in pre/early puberty than boys at time 1 (χ2 = 8.05, p < .01). Pubertal development at time 1 was unassociated with depressive symptoms at time 1 or time 2 and with change in depressive symptoms from time 1 to time 2. Time 1 and time 2 depressive symptoms approached significance in correlating with one another (r = .24, p < .06). Greater activation in the striatum related to greater activation in the vmPFC during both reward anticipation (r = .80, p < .01) and reward outcome (r = .67, p < .01).
Table 1.
Means and Standard Deviations of Depressive Symptoms Scores
| Variable | All | Boys | Girls | Pre/Early | Mid/Late |
|---|---|---|---|---|---|
| T1 Depressive Symptoms | 6.27 (6.51) | 4.95 (4.86) | 7.52 (7.61) | 6.52 (6.55) | 6.17 (6.78) |
| T2 Depressive Symptoms | 7.55 (7.36) | 5.65 (5.06) | 9.19 (8.61) | 9.60(10.40) | 6.61 (5.65) |
| Residualized Depressive Symptoms | .00 (6.88) | .00 (5.00) | .00 (8.12) | .60 (9.89) | −.18 (4.65) |
Reward-Related Reactivity and Depressive Symptoms: Gender and Puberty as Moderators
As our hypotheses focus on the interactive effect of gender and changes in depressive symptoms and on the interactive effect of puberty and changes in depressive symptoms on brain reactivity, only interactive effects are described below. Main effect findings are listed in Tables 2 and 3. Exploration of outliers in SPSS identified two participants with a residualized change score for depressive symptoms more than 3 SD above the mean. Significant findings were not changed upon removal of these two participants from SPM analyses. We present here the results from our full sample of participants.
Table 2.
Reward-Related Brain Function, Gender, and Depressive Symptoms by Task Condition and Regions of Interest
| Region | x y z | t | Cluster size | pFDR |
|---|---|---|---|---|
| Reward Anticipation | ||||
| Gender Main Effect (Girls > Boys Contrast) | ||||
| Medial Frontal Gyrus, BA 10 | −6, 61, 14 | 3.18 | 50 | .01 |
| Depression Main Effect (Positive Contrast) | ||||
| N.S. | ||||
| Gender X Depression Interaction | ||||
| N.S. | ||||
| Reward Outcome | ||||
| Gender Main Effect (Girls > Boys Contrast) | ||||
| Caudate | −12, 1, 15 | 2.85 | 20 | .04 |
| Depression Main Effect | ||||
| N. S. | ||||
| Gender X Depression Interaction | ||||
| BA 10 | 20, 45, 5 | 3.27 | 21 | .01 |
| Post-Hoc Analyses (Boys Only) | ||||
| Depression Main Effect (Positive Contrast) | ||||
| Anterior Cingulate (BA 32) | −18, 39, 9 | 3.76 | 89 | .01 |
| Anterior Cingulate (BA 32 | 18, 28, 24 | 3.23 | 32 | .05 |
| Post-Hoc Analyses (Girls Only) | ||||
| Depression Main Effect | ||||
| N.S. |
Note: Talairach Coordinates
Table 3.
Reward-Related Brain Function, Puberty, and Depressive Symptoms by Task Condition and Regions of Interest
| Region | x y z | t | Cluster size | p FDR |
|---|---|---|---|---|
| Reward Anticipation | ||||
| Puberty Main Effect (Pre/Early > Mid/Late Contrast) | ||||
| BA 10 | −8, 61, 15 | 3.21 | 47 | .03 |
| Depression Main Effect | ||||
| N.S. | ||||
| Puberty X Depression Interaction | ||||
| N.S. | ||||
| Within-group Analyses (Pre/Early Puberty Only) | ||||
| Depression Main Effect | ||||
| N.S. | ||||
| Within-group Analyses (Mid/Late Puberty Only) | ||||
| Depression Main Effect (Negative Contrast) | ||||
| Caudate | 0, 18, 3 | 3.23 | 14 | .004 |
| Reward Outcome | ||||
| Puberty Main Effect (Pre/Early > Mid/Late Contrast) | ||||
| Anterior Cingulate (BA 32) | −20, 36, 11 | 3.84 | 59 | .01 |
| Anterior Cingulate (BA 32) | −2, 35, −3 | 3.09 | 66 | .05 |
| Depression Main Effect | ||||
| N. S. | ||||
| Puberty X Depression Interaction | ||||
| N.S. | ||||
| Within-group Analyses (Pre/Early Puberty Only) | ||||
| Depression Main Effect | ||||
| N.S. | ||||
| Within-group Analyses (Mid/Late Puberty Only) | ||||
| Depression Main Effect (Negative Contrast) | ||||
| N. S. |
Note: Talairach Coordinates
Gender as a Moderator of Depressive Symptoms and mPFC Reactivity
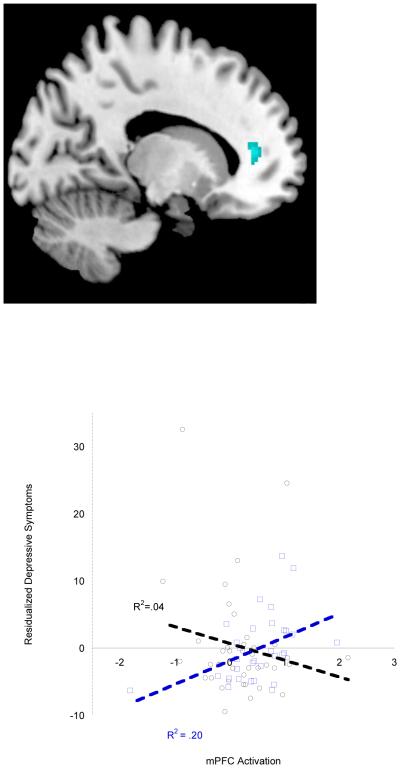
We found a significant interactive effect of gender and depressive symptoms on mPFC reactivity in medial BA 10 (21 voxels [20, 45, 5]; df = 67, t = 3.27; pFDR = .01) during Reward Outcome. Post hoc analyses within groups indicated that for boys, greater activation in the Anterior Cingulate (BA 32) (89 voxels [-18, 39, 9]; df = 29, t = 3.76, pFDR = .01; 32 voxels [18, 28, 24]; df = 29, t = 3.23; pFDR = .05) during Reward Outcome compared to baseline was associated with increasing depressive symptoms over time (Figure 1).
Figure 1.
mPFC Activation (Anterior Cingulate/BA 32/medial BA 10)during Reward Outcome predicted change in depressive symptoms over 2 years in boys. Boys are shown in blue, and girls are shown in black, with regression fit lines for each group displayed. Figure includes values (in arbitrary units) extracted from the full SPM regression model. Results did not change when outliers were excluded.
Puberty as a Moderator of Depressive Symptoms and Striatal Reactivity
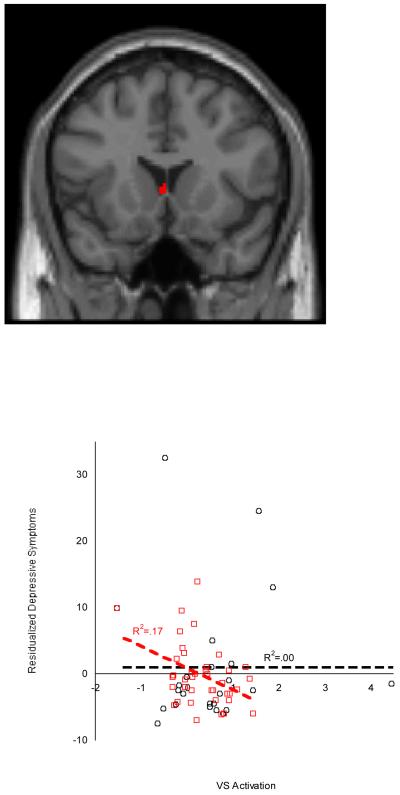
Findings from our factorial regression indicated no significant interactive effects of pubertal group and depressive symptoms on striatal or mPFC activation. However, based on our hypothesized difference in the association of neural response to reward and increasing depressive symptoms, and our previous findings that this association was particularly strong for mid/late pubertal adolescents (Forbes et al., 2010), we conducted analyses within developmental groups to evaluate the association of brain function and change in depressive symptoms. For mid/late pubertal adolescents only, less caudate activation (14 voxels [0, 18, 3]; df = 37, t = 3.23, pFDR =.004) during Reward Anticipation compared to baseline related to greater increases in depressive symptoms from time 1 to time 2 (Figure 2).
Figure 2.
Adolescents’ striatal activation (Caudate) during reward anticipation predicts change in depressive symptoms over 2 years in adolescents in mid/late puberty. Mid/Late pubertal adolescents are shown in red, and pre/early pubertal adolescents are shown in black, with corresponding regression fit lines displayed. Figure includes values (in arbitrary units) extracted from the full SPM regression model. Results did not change when outliers were excluded.
Exploratory Analyses: Other Reward-Related Reactivity
We conducted additional ROI analyses to examine associations of depression with function in the orbitofrontal cortex (OFC) and amygdala, two other critical regions in neural reward circuits. symptoms on reactivity in In particular, we found a significant interactive effect of gender and depressive the OFC (13 voxels [28, 31, −7]; df = 67, t = 3.63, pFDR =.004) and bilateral amygdala (22 voxels [28,-7, −15]; df = 67, t = 3.19, pFDR =.016; 10 voxels [-28,-7, −15]; df = 67, t = 2.81, pFDR =.04) during Reward Outcome. For boys, greater activation in the OFC related to greater increases in depressive symptoms across the two year period (10 voxels [26, 33, −18]; df = 29, t = 3.71, pFDR =.002). Post hoc analyses revealed that activation in the amygdala was not significantly associated with increases in depressive symptoms for boys or girls.
Discussion
The current conceptual model provides a framework for understanding how reward-related differences in brain function during adolescence may increase risk for depression for adolescents based on their gender and pubertal development. Previous conceptual models have focused on individual differences in environmental and social stressors and negative life events as predictors of risk (Hankin et al., 2007) or on reward-related differences that may increase risk for adolescents as a whole (Davey et al., 2008). Our model provides some information on how reward-related changes in adolescence may place youth at risk for rises in depressive symptoms depending on gender and pubertal stage. In particular, the model emphasizes that the association between reward responding and depression differs for boys and girls based on reward type (social status, affliative bonds).
We found empirical support for our conceptual claim that the relation between adolescents’ neural response to monetary reward and their increasing depressive symptoms differed based on gender and puberty. Over-regulation of positive feelings related to monetary reward may place boys at greater risk for not enjoying rewarding stimuli in real-world social experiences (Masten et al., 2011). With the ACC and BA32 postulated to play an important role in processing of self-relevant and social stimuli (Amodio & Frith, 2006), boys who show greater activation in these areas during the reward task may be more likely to think about and evaluate their performance on this task, or to compare their performance unfavorably against the imagined performance of others. Excessive self-evaluation may increase risk for rumination and other depressive symptoms (Masten et al., 2011). This pattern of response to the anticipation and outcome of reward might create a vulnerability to status challenges in some boys, influencing their development of depressive symptoms. Indeed, greater activation in the striatum was associated with greater vmPFC activation vmPFC in our sample, suggesting that certain regions of the could be coming on line to regulate the experience of positive feelings associated with receiving reward. The vmPFC may play a normative regulatory role in assessing self-performance associated with reward processing; however, over-regulation may be particularly risky for boys who may be evaluating how their mastery of status-related rewards is related to others’ performance.
Additional analyses of other reward-related regions also identified activation in other reward-related regions for boys and depressive symptoms, particularly the OFC and the amygdala. Greater activation in the OFC during this status-related reward task being associated with greater increases in depressive symptoms for boys adds further support to our claim of the salience of status-related rewards for adolescent boys relative to adolescent girls.
We also found that less striatal response during anticipation of rewarding stimuli related to a rise in depressive symptoms, but only for adolescents in mid to late puberty. For adolescents well into puberty, less response to the anticipation of monetary reward, possibly reflecting less excitement and motivation to seek rewards, may pose as a risk for increasing depressive symptoms. As the interaction between pubertal stage and striatal response was not significant, future research should examine these relations with similar-age, less developed peers to determine the relation between striatal activation and changes in depressive symptoms in these adolescents. The changes of puberty might influence brain function in response to possible rewards, possibly influencing reduced positive mood directly or through changes in behavior. Adolescents who show less striatal reactivity to reward-related activities, especially once they have entered the vulnerable period of mid-late puberty, may be at greater risk for depressive symptoms than adolescents who show more striatal activation during reward-related tasks. This lack of motivation may hinder these adolescents in pursuing rewarding experiences during a developmental time period in which reward-seeking is normative and promotes social, neural, and cognitive growth (Davey et al., 2008; Forbes & Dahl, 2005).
We note that whereas this post-hoc finding was significant, the interactive effect of puberty and depressive symptoms on reward function was not significant in our model. Based on our a priori hypotheses about the direction of effects, we pursued within-group changes in depressive symptoms as planned contrasts. Our power may be limited to detect the interaction effect and we urge future studies with larger samples to address this question.
Based on these findings, adolescent boys and girls who are vulnerable to depression may differ in the types of rewards that elicit alterations in neural response. Girls may be more likely to pursue social rewards that promote affiliative bonds, whereas boys may more likely to pursue social rewards that promote power and social status (e.g., money; Hankin et al., 2007; Rose & Rudolph, 2006). As a result, monetary rewards might elicit depression-relevant individual differences more effectively in boys than in girls. Our reward-related task focused on winning money, rather than more social rewards (e.g., being liked, making friends). Thus, we were unable to fully test the hypothesis that adolescent boys and girls differ in which kinds of rewards they value and pursue.
Accordingly, our neuroimaging study highlights a weakness in research on the affective neuroscience of depression. A substantial number of studies have examined neural response to monetary reward (i.e., status reward) in depression (see Forbes & Dahl, 2012, for a review), but, to our knowledge, only one study to date has addressed neural response to affiliative reward in depression (Davey et al., 2011). In addition, no studies to date have manipulated social status per se as a form of reward. Clearly, additional research is needed on the neural response to social rewards in depression, as research to date could potentially inform more on depression in men. In addition, to be able to examine gender differences in neural response to reward types, future research should evaluate gender differences in neural response to social vs. non-social rewards directly, and their relation to increases in depressive symptoms. In particular, studies have not assessed response to both affiliative and status rewards, or, furthermore, to personally relevant rewards such as social or status achievements (e.g., being liked by a potential romantic partner, winning a prestigious award). Some researchers have used fMRI paradigms with affective faces to measure neural response to social rewards. However, measures that include the aforementioned more personally relevant social stimuli may target reward-related differences more accurately. For example, Davey et al. (2011) found reward-related brain areas to be activated by a social reward paradigm in which adolescents are rated by imaginary peers on how much they would like to interact with them. Similarly, Nelson and colleagues used a chat room fMRI paradigm with adolescents to measure reward-related responses to peer acceptance (Guyer, Choate, Pine, & Nelson, 2011).
Because a greater proportion of girls than boys will develop depression starting at adolescence, pinpointing risk factors that delineate which girls will increase in depressive symptoms over time may be more difficult than finding such associations with boys. It may be that there is greater variability in the trajectories to depression in girls, with girls at greater risk for depressive symptoms because of other factors in addition to their responses to rewarding stimuli. On the other hand, greater variability in boys’ depressive symptoms levels during adolescence may have allowed for more detailed exploration of their risk factors. However, considering the significant rates of depression observed in adolescent girls, research identifying reward-related risk factors is sorely needed. We suggest that use of socially rewarding paradigms in fMRI research evaluating risk factors for depression is needed to identify these reward-depression associations in adolescent girls.
Pubertal maturation also influenced the way in which reward function contributed to rises in depressive symptoms. We found support for our claim that risk for depression increases with pubertal maturation, as less striatal activation when anticipating rewards predicted rises in depressive symptoms for adolescents in mid to late puberty only. This increased risk may be due to a disconnect between neural reactivity (i.e., low striatal activation to rewards) and expected social behaviors (i.e., reward-seeking with peers) that eventually contribute to greater depressive symptoms. These low reward-related responses may make them stand out among their late pubescent peers, who may be pursuing and enjoying rewards more intensely, in a way that they did not in early pubertal adolescence or in late childhood—they may seek rewards less frequently or engage in fewer social behaviors. This low reward-related reactivity may be due to individual differences in how these adolescents value social rewards or due to socialization agents that reinforce the riskiness of social rewards (Allen & Badcock, 2003).
We evaluated these reward-related differences using contrasts based both on reward anticipation and reward outcome. Both the anticipation of rewards and receipt of rewards are important aspects of reward function in adolescents. Previous literature suggests that both reward anticipation and reward outcome elicit striatal activation (in the experiencing the positive feelings associated with rewards) and vmPFC activation (in the planning and evaluating self-performance associated with rewards; citation). In our study, we found that striatal activation was associated with depressive symptoms for mid to late puberty adolescents during reward anticipation only and we found that vmPFC activation was associated with depressive symptoms for boys during reward outcome only. Our findings may reflect how alterations in “wanting” vs. “liking” rewards may be differentially associated with reward-related areas (i.e., caudate, vmPFC; Berridge, Robinson, & Aldrige, 2009). In particular, in puberty, changes in motivation for reward may be related to caudate activation. On the other hand, in boys, disruptions in regulating “liking” experience may be associated with over-regulation in the vmPFC (Forbes & Dahl, 2012).
In sum, our results indicate that the way in which adolescents anticipate and respond to positive and rewarding stimuli may contribute to the development of depressive symptoms. Our findings also highlight which adolescents are particularly vulnerable to rises in depressive symptoms in adolescence. Previous conceptual models have pinpointed adolescence as a developmental period of vulnerability because of neural and social changes related to pursuit of abstract social rewards and positive affect (Davey et al., 2008). However, not all adolescents experience problematic depressive symptoms or depressive disorders, indicating that more information is needed about which adolescents are at risk. Our findings help identify those adolescents who may be at heightened risk because of reward-related differences in early adolescence that predict subsequent rises in depressive symptoms. This information is important as it can be used to identify and target high-risk adolescents for interventions designed to prevent clinical levels of depression.
Strengths and Limitations
Strengths include the use of a neuroimaging task to identify potential biomarkers that serve as risk factors for depressive symptoms and the longitudinal assessment of adolescents in a normative population at a point in development before the typical onset of depression (Lewinsohn et al., 2000). Thus, we were able to examine the development of depressive symptoms in young people who had not yet experienced depression but are reaching the age at which symptoms—and the onset of disorders—increase. To our knowledge, this study is the only extant study to evaluate gender and pubertal development as factors in the association between reward-related brain function and rises in depressive symptoms in adolescents prior to onset of depression. However, we recognize the limitations in generalizing our results from a psychiatrically healthy sample to other populations, including adolescents with clinical diagnoses of depression. Our study may have benefitted from including an fMRI paradigm that more directly assesses social rewards (e.g., Davey, Allen, Harrison, Dwyer, & Yucel’s (2010) likeability paradigm). Inclusion of this type of task would have allowed for more detailed analyses of gender differences in reward function and depressive symptoms.
Future Directions
Whereas our empirical study has numerous strengths, they represent only a first step in evaluating reward-related differences as predictors of risk for depression. A more rigorous test of our postulated conceptual model of moderating effects of gender and puberty on reward-related differences in risk for adolescent depression remains to be conducted. The optimal approach to this is to measure neural response to both social (affliative) reward and monetary (status) reward in boys and girls early in adolescence and to follow their affective development into their late teens to understand who develops depression. Social reward paradigms that evaluate ecologically valid social relationships (e.g., blossoming romantic relationships) have yet to be created. These types of social reward paradigms could be used to sensitively evaluate reward-related gender differences in predicting risk for depression in adolescents. Additionally, social reward paradigms should aim to tease apart social rewards that are based on affliative bonds versus social status for a more sophisticated level of analysis.
Also, while this conceptual model focused on providing more detailed exploration of reward function as a risk for depression in adolescence, other factors play a role in increasing risk for depression in adolescence (e.g., negative life events and cognitive vulnerability to negative information). Future research should evaluate how neural response to social reward interacts with neural response to threat and rumination to predict depressive symptoms and onset of depression.
Finally, as neural response to reward, pubertal development, and depressive symptoms are all undergoing changes during adolescence, it will be invaluable to conduct future studies using longitudinal, prospective designs. These longitudinal designs could examine reward-related differences as they change across childhood, adolescent, and adult development. This approach will also allow the investigation of gender differences, pubertal development, and neural response to reward in the longitudinal development of clinical depressive disorders, including their clinical course as well as their onset. Ultimately, understanding the early predictors of depression can also provide the basis for developing effective prevention strategies. We hope that this conceptual model can serve as a springboard for more sophisticated and comprehensive evaluation of adolescent risk for depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychol Bull. 2003;129:887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: A developmental, epidemiologic, and neuroendocrine perspective. J Affect Disord. 1993;29:145–58. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8-17 year olds: Effects of age and gender. J Child Psychol Psychiatry. 2002;43:1052–63. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldrige JW. Dissecting components of reward: ‘liking’, ‘wanting’ and learning. Current Opinion in Pharmacology. 2009:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The development of depression in children and adolescents. Am Psychol. 1998;53:221–40. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;1000:316–36. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Conger RD, Lorenz FO, Elder GH, Simons RL, Ge Husband and wife differences in response to undesirable life events. J Health Soc Behav. 1993;34:71–88. [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapping. 2010;31:660–68. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Seven sins in the study of emotion: Correctives from affective neuroscience. Brain Cogn. 2003;52:129–32. doi: 10.1016/s0278-2626(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychol. 2006;163:1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Seeley JR, Sasagawa S. Gender differences in th developmental course of depression. J Affect Disord. 2010;127:185–90. doi: 10.1016/j.jad.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Bio Psychiatry. 2009:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Altered reward function in adolescent depression: What, when, and how? J Child Psychol Psychiatry. 2012:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Dev Psychopathology. 2005;17:827–50. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychol. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson DA, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:107–18. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adoles Psychiatry. 2010;49:162–72. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joorman J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–86. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Trans. 1993;91:111–34. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate CR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cog Affect Neurosci. 2011 doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haeffel GJ. Cognitive vulnerability-stress theories of depression: Examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cogn Therapy Research. 2004;28:309–45. [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Bio Psychiatry. 2010;68:484–90. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JB, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Bio Psychiatry. 2008;63:686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: Neural correlates and consequences for choice. Phil Trans R Soc. 2008;363:3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Psychological approaches to the treatment of depression in adolescents. In: Reynolds W, Johnston H, editors. Handbook of depression in children and adolescents. Plenum Press; New York: pp. 309–344. [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. J Abnorm Psych. 2000;109:345–51. [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealey K, Pfeifer JH, Dapretto M. Subgenual anterior cingulated response to peer rejection: A marker of adolescents’ risk for depression. Dev Psychopathology. 2011;23:283–92. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expression in children and adolescents at risk for major depression. Am J Psychol. 2008 doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Rosmalan JGM, Veenstra R, Dijsktra JK, Ormel J. Being admired or being liked: Classroom social status and depressive problems in early adolescent girls and boys. J Abnorm Child Psychol. 2007;35:417–27. doi: 10.1007/s10802-007-9100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Forbes EE. Striatal response to monetary reward in depression: A meta-analysis. In preparation. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropscyhopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psych Bull. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer MG, Pfeiffer S, Spence SH. Life events, coping and depressive symptoms among young adolescents: a one-year prospective study. J Affect Disord. 2009;117:48–54. doi: 10.1016/j.jad.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–51. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress response: Social rejection and achievement stress. Bio Psych. 2002;58:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]