Abstract
In areas where polyparasitism is highly prevalent, the impact of multiple parasites on the host response is underestimated. In particular, the presence of helminth infection coincident with malaria profoundly alters the production of malaria-specific IFN-γ, IL-12p70, CXCL9, CXCL10 and CXCL11, cytokines/chemokines known to be critical in mediating malaria-specific immunity. In order to elucidate the mechanisms underlying the suppression of malaria-specific cytokines/chemokines, we assessed the expression of malaria-specific IL-12Rβ1, IL-12Rβ2 and interferon regulatory factor (IRF)-1 in blood obtained from 18 filaria-infected (Fil+) and 17 filaria-uninfected (Fil−) individuals in a filaria–malaria co-endemic region of Mali. We found that Fil+ individuals had significantly lower RNA expression of IRF-1 but not IL-12Rβ1 or IL-12Rβ2 in response to malaria antigen stimulation. We also measured the frequency of IL-12-producing DCs from these subjects and found that Fil+ subjects had lower frequencies of IL-12+ mDCs after malaria antigen stimulation than did the Fil− subjects. Modeling these data in vitro, we found that mDCs pre-exposed to live microfilariae not only produced significantly lower levels of CXCL-9, CXCL-10, IL-12p35, IL-12p40, IL-12p19 and CXCL-11 following stimulation with malaria antigen but also markedly downregulated the expression of IRF-1, IRF-2 and IRF-3 compared with microfilaria-unexposed mDCs. siRNA-inhibition of irf-1 in mDCs down-regulated the production of IL-12p70 through repression of IL-12p35. Our data demonstrate that the modulation of IRFs seen in filarial (and presumably other tissue-invasive helminths) infection underlies the suppression of malaria-specific cytokines/chemokines that play a crucial role in immunity to malaria.
Keywords: Cytokines, CXCR3 ligands, DCs, Interferon regulatory factors
Introduction
In tropical and sub-tropical regions (and especially in Africa), polyparasitism is highly prevalent, and the impact of multiple parasites on the host immune system has long been underestimated [1]. Of the many parasites that co-exist within human populations in Sub-Saharan Africa, soil-transmitted and tissue-invasive helminths along with malaria are the most predominant, as this region is estimated to be home to ~85% of the malaria cases and more than 90% of world's helminth infections [2, 3].
The impact of coincident helminth and malaria parasites on the host immune system has been the focus of intense studies recently. Despite varied conclusions reached in a number of studies examining the impact of helminth infection on the outcome of clinical malaria [4–6], recent data reveal that concomitant soil-transmitted helminth (STH) or filarial infection alters quite dramatically the immune response to malaria in children [7, 8]. Most recently, we have also demonstrated that patent filarial infection profoundly altered the production of malaria-specific IL-12p70, IP-10 (CXCL-10) and IFN-γ, cytokines known to be critical in mediating immunity to malaria [8].
Chronic filarial infections are associated with a regulatory immune environment dominated by increased frequencies of IL-10-producing CD4+ T cells (11) that appear to mediate suppression of T-cell proliferation and the production of IL-2 and IFN-γ in response to filarial antigens [9, 10]. This exuberant regulation has also been shown to modulate the T-cell response to other pathogens (including malaria) through a process known as spillover or bystander suppression [11].
The induction of Th1-cell immune responses to microbial stimuli is regulated in large part by the production of IL-12 by APCs and by the expression of IL-12 receptors (IL-12Rs) by CD4+ T cells. The expression of IL-12p70 by DCs and IL-12Rs by CD4+ T cells is controlled by complex signal transduction pathways involving nuclear factor kappa B (NF-κB) and the interferon regulatory factors (IRFs), especially IRF-1 and IRF-2 [12, 13]. IRF-1, however, seems to play a dominant role in regulating IL-12p70, as its inhibition (and not that of NF-κB) abrogates the production of IL-12p70 in DCs [14]. With respect to filarial infection, exposure of DCs to microfilariae (mf) of Brugia malayi specifically suppressed the production IL-12p70 while upregulating the production of IL-8, RANTES, IL-1α, TNF-α and IL-β [15]. Thus, in the current study we sought to determine the mechanisms underlying the suppression of malaria-specific Th1-cell and pro-inflammatory responses both in vivo (in filaria-infected subjects) and in vitro. Our data demonstrate that IRF-1, in particular, plays a key role in mediating the suppression of IL-12 production in DCs that in turn influences the T-cell response to malaria antigen stimulation.
Results
Study site and patients
The study was conducted in the Kolokani district of Mali where malaria and filaria are co-endemic, with malaria having seasonal transmission. The study population has been described in detail previously [16]. The present study was conducted prior to the malaria transmission season, but all the subjects had asymptomatic malaria infection based on microscopy and HRP-2 antigen.
Filarial infection down modulates the expression of IRF-1
Having demonstrated previously that patent filarial infection suppresses the production of malaria-specific IL-12p70, IFN-γ and CXCL-10 in an IL-10-dependent manner [8], we sought to examine those factors associated with the regulation of IL-12p70 in the context of malaria/filaria co-infection. To this end, expression of il12rb1, il12rb2 and irf-1 was assessed in filaria-infected (Fil+) and filaria-uninfected (Fil−) patients (Fig. 1). Although there were no differences in the baseline expression levels of il12rb1, il12rb2 and irf-1 (Fig. 1A), when cells were stimulated with malaria antigen (MalAg), cells from Fil+ had significantly lower expression levels of irf-1 (GM (range) 3.03 (1.34–5.34) versus 7.71 (3.4–52.79), p=0.008) compared with those from Fil− (Fig. 1B). The expression of il12rb1 and il12rb2 were lower in the Fil+ group but the difference did not reach statistical significance. Moreover, neutralizing anti-IL-10 antibody was clearly capable of reversing this malaria-specific suppression of irf-1 expression in response to malaria antigen stimulation (Fig. 1C).
Figure 1.
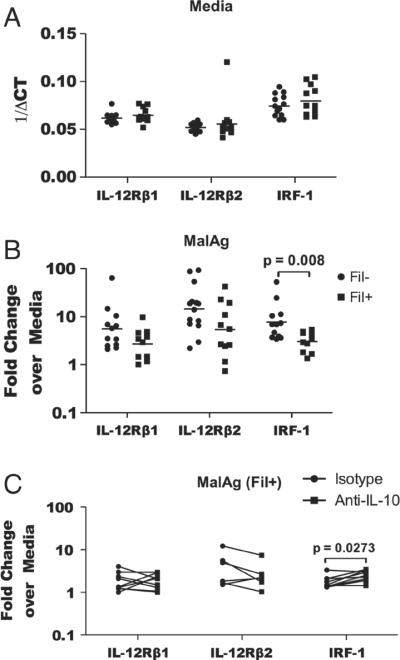
Patent filarial infection is associated with lower expression of IRF-1 in response to malaria antigen stimulation that can be reversed with anti-IL10 antibodies. Whole blood cells from filaria-infected and-uninfected subjects were stimulated with MalAg or left unstimulated for 24 h, and RNA expression was assessed by reverse transcriptase real-time PCR (RT-PCR). Expression levels of il12rb1, il12rb2 and irf-1 (A) at baseline, (B) in response to MalAg alone, or (C) in response to MalAg in the presence of neutralizing antibody to IL-10 are shown. The graph represents all the date obtained from the study subjects (n = 38). (A and B) Each symbol represents a subject and the bar represents the geometric mean. (C) Six Fil+subjects were assessed in the presence of neutralizing antibodies to IL-10 (square) or of the isotype control antibody (circle). Statistical significance was determined using Mann-Whitney (B) or Wilcoxon signed-rank test (C).
Frequency of IL-12p70/40-producing DCs in response to MalAg stimulation
The transcription factor IRF-1 regulates, among other genes, the expression of IL-12 in DCs and the IL-12Rs in CD4+ T cells [13, 17]. Because there were no differences between the patient groups in the expression of the IL-12Rs, we sought to determine the frequency of IL-12-producing DCs in Fil+ and Fil− cells in response to MalAg (Fig. 2; see Supporting Information Fig. 1 for gating strategy) assuming that these cells reflected best the IRF regulated IL-12 production. As can be seen in Fig. 2, the frequency of IL-12p70/40-producing mDCs was significantly lower in Fil+ compared with that in Fil− subjects (GM (range) 2.9 (0.7–22.2) versus 17.7 (2.6–38.4), p=0.0037) (Fig. 2A) as was the integrated geometric mean fluorescence (iGMFI) of IL-12p70/40-producing mDCs (Fig. 2B) (GM (range) 11 765 (1325–64 365) versus 21 367 (1772–155 587), p=0.0049). However, there were no differences in either the frequency or the iGMFI of Type 1 interferon-producing pDCs between the two groups (Fig. 2C and D).
Figure 2.
Patent filarial infection is associated not only with lower frequency of IL-12p70/40-producing mDCs but also with the per-cell cytokine production following malaria antigen stimulation. Whole blood cells were cultured as in Fig. 1, with Brefeldin A added in the last 12 h of incubation. The cells were then stained with antibodies to mDC and pDC surface markers (Alexa fluor 700-mouse anti-human CD3, CD19, CD19, PE-Cy7-mouse anti-human CD123 and PE-Cy5-mouse anti-human CD11c), and antibodies specific to IL-12p70/40, IFN-β and IL-10. The (A, C) net frequency and (B, D) iGMFI of cytokine-producing (A, B) mDCs and (C, D) pDCs were determined. The graph represents all the date obtained from the study subjects (n = 38). Each symbol represents the frequency or the iGMFI of mDCs or pDCs from one subject and the bars represent the geometric mean. Statistical significance was determined using the Mann–Whitney test.
Live mf alter mDC production of Th1-associated proinflammatory cytokines in response to MalAg
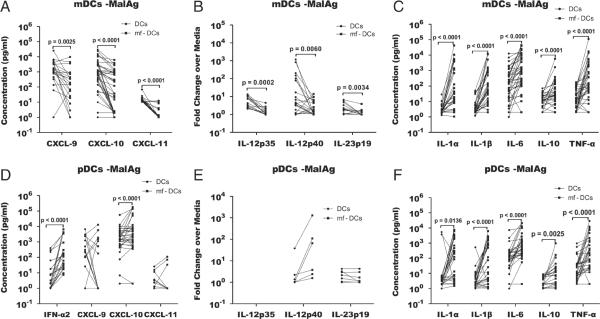
To address more specifically how parasitic helminths can affect DC responses to MalAg, in vitro-derived mDCs (see Supporting Information Fig. 2 for the in vitro-derived DC phenotypes and cytokine response to TLR ligands) pre-exposed to mf were examined after stimulation with MalAg. As can be seen in Fig. 3, mf-pre-exposed mDCs had a significantly lower production of CXCL-9 (GM (range) 99.7 (1–9761) versus 997.8 (1–25 205), p=0.0025), CXCL-10 (GM (range) 41.1 (1–6186) versus 553.5 (1–29 741), p<0.0001), and CXCL-11 (GM (range) 1.2 (1–11.4) versus 13.4 (9.3–68.55), p<0.0001) in response to MalAg (Fig. 3A) when compared with mf-unexposed DCs. In accord with the protein data, the transcription levels of IL-12p35 (p=0.0002), IL-12p40 (p=0.0060) and IL-23p19 (p=0.0034) were significantly lower in mf pre-exposed mDCs compared with that in non-exposed mDCs (Fig. 3C). Interestingly, the production of other inflammatory cytokines by mDCs in response to malaria antigen IL-1α (GM (range) 66.87 (0.05–42 497) versus 1.62 (0.29–27.27), p<0.0001), IL-1β (GM (range) 89.55 (1–12 304) versus 2.06 (0.19–50.18) p<0.0001), IL-6 (GM (range) 887.3 (1–44 510) versus 1208 (1–12 837), p<0.0001) and TNF-α (GM (range) 224.1 (1–9761) versus 11.91 (1–178.4), p<0.0001) and the regulatory cytokine IL-10 (GM (range) 22.1 (1–5790) versus 6.91 (1–69.59), p<0.0001) was significantly increased by pre-exposure to mf (Fig. 3B). Unlike mDCs, pre-exposure of pDCs to live mf significantly boosted the production of cytokines in response to MalAg stimulation (Fig. 3D and F). However, there was no significant difference in the expression levels of IL-12p19 and IL-12p40 between mf pre-exposed and unexposed pDCs (Fig. 3E).
Figure 3.
Pre-exposure of monocyte-derived mDCs and pDCs to Brugia malayi mf suppresses the production of Th1-associated proinflammatory cytokines in response to malaria antigen stimulation in mDCs but not pDCs. mDCs and pDCs were differentiated in vitro from monocytes for 7 days using a cocktail of GM-CSF/IL-4 and IFN-β/IL-3 respectively, exposed to mf for 48 h, then harvested, counted and stimulated with MalAg for 24 h. The supernatants were screened for the indicated cytokines and the cells were used for RNA purification and RT-PCR. (A, C, D and F) represent the net concentration of cytokines measured in culture supernatants, and (B and E) represent the fold change of mRNA expression of the listed cytokines in MalAg-stimulated over the unstimulated DCs. The figure represents data from 32 individual experiments; each symbol represents a monocyte donor, and the bars join the data point from an individual sample exposed or unexposed to mf. Statistical significance was determined comparing cytokine values from the DC-exposed or -unexposed to mf using the Wilcoxon signed-rank test. The p-values were corrected for multiple comparisons using Holm's correction.
Mf inhibit the transcription of IRFs by mDCs in response to MalAg
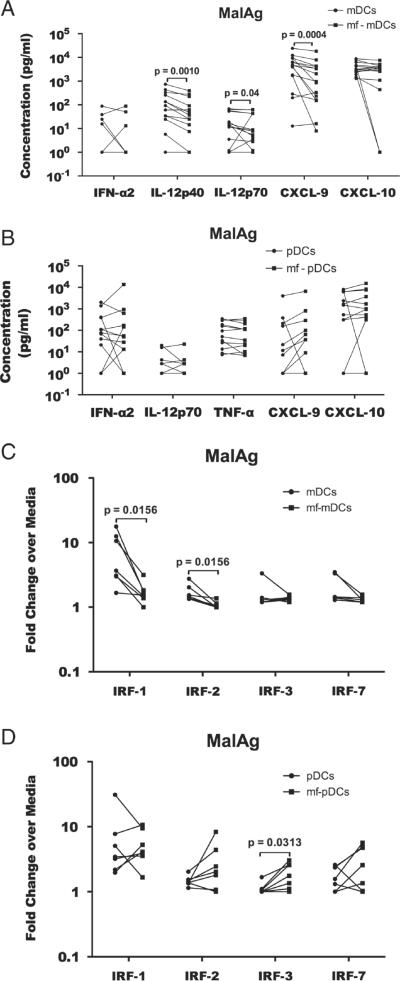
To assess the mechanisms underlying the downregulation of Th1-associated pro-inflammatory cytokines in mf pre-exposed mDCs, we assessed the expression of the interferon regulatory factors (IRF) – IRF-1, IRF-2, IRF-3 and IRF-7 – each implicated in the regulation of IL-12, type I interferons and/or the CXCR3 ligands CXCL-9 and -10. As shown in Fig. 4, in response to malaria antigen stimulation, mf pre-exposed mDCs had significantly lower RNA expression levels of IRF-1 (GM (range) 1.87. (1–7.95) versus 4.2 (1.2–37.02), p=0.0031), IRF-2 (GM (range) 1.12 (1–1.85) versus 1.89 (1–5.54), p=0.0001) and IRF-3 (GM (range) 1.11 (0.79–2.43) versus 1.47 (1–3.07), p=0.0039) compared with that found in non-exposed mDCs (Fig. 4A). However, there were no differences in the expression levels of these transcription factors between mf pre-exposed and non-exposed pDCs (Fig. 4B).
Figure 4.
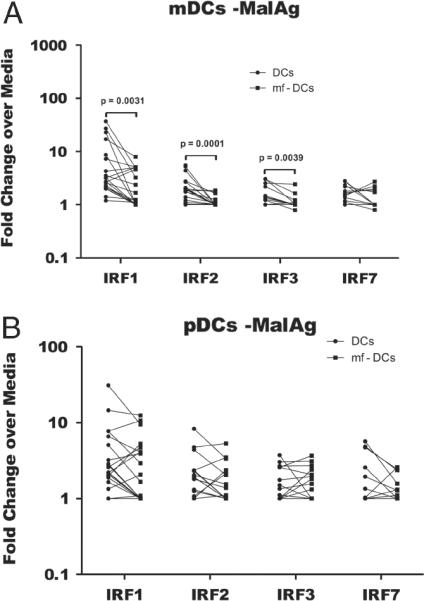
Pre-exposure of monocyte-derived DCs induces a down-regulation of interferon regulatory factors 1, 2 and 3 in mDCs but not pDCs. In vitro generated (A) mDCs and (B) pDCs were exposed to mf for 48 h, stimulated with MalAg for 24 h and the cells were used for RNA purification and RT-PCR. The fold change of RNA expression over unstimulated DCs or mf-pre-exposed DCs from 22 experiments is shown. Each symbol represents a value from one monocyte donor, and the lines join the values from mDCs and mf pre-exposed DCs from the same volunteer. Statistical significance was determined using Wilcoxon signed-rank test.
Mf induce IL-12p70, IL-12p40, IRF-1 and IRF-2 down-regulation in mDCs co-cultured with MalAg
With the recent data showing that monocyte-derived in vitro generated DCs differ from blood DCs in terms of their antigen processing machinery [18], we sought to extend the results obtained in vitro with blood derived mDCs and pDCs. Thus, we isolated DCs from PBMCs (Supporting Information Fig. 3) and assessed the effect of live mf on the innate response to MalAg (Fig. 5). As seen, mf pre-exposed-mDCs stimulated with MalAg had significantly lower production of IL-12p40 (p=0.0010) and IL-12p70 (p=0.04) compared with un-exposed mDCs (Fig. 5A). Mf had no effect on pDC production of cytokines in response to MalAg (Fig. 5B). In fact there were no difference in the amount of cytokines produced between unexposed pDCs and mf-exposed pDCs. To ascertain whether mf mediated the suppression of IL-12 production in blood-derived mDCs, through the same process seen in in vitro derived mDCs, we assessed the expression of IRF-1, -2, -3 and -7 in MalAg stimulated mf pre-exposed mDCs purified from peripheral blood. As seen in Fig. 5C, the expression of irf-1 and irf-2 were significantly down regulated in mf pre-exposed-mDCs, though there were no differences in the expression of irf-3 and irf-7. There were no differences in the expression of these genes in pDCs also purified from peripheral blood. In fact, irf-3 was up regulated in the mf pre-exposed-pDCs in response to MalAg stimulation (Fig. 5D).
Figure 5.
Pre-exposure of blood derived mDCs and pDCs with B. malayi mf induce a downregulation of Th1-associated proinflammatory cytokines and suppression of IRF-1 and 2 in mDCs but not pDCs. mDCs and pDCs were purified from blood using mDCs and BDCA-4/Neuropilin-1 MicroBead Kit, pre-exposed to mf and stimulated with MalAg for 24 h. (A, B) The supernatants were collected and screened for cytokines and (C, D) the cells were used for RNA purification and RT-PCR. The figure represents data from 15 individual experiments/donors. Each symbol represents cells from one volunteer and the line joins the cytokine values from the same donor. Comparisons were done using the Wilcoxon signed-rank tests and the p-values were corrected for multiple comparisons using Holm's correction.
Inhibition of irf-1 in mDCs suppresses the expression of IL-12p35 and the production of IL-12p70
After demonstrating that mf downregulated the transcription of il12-p35 and several interferon regulatory factors including irf-1, as well as the production of IL-12p70 protein, we sought to determine whether the diminished production of IL-12p70 was directly related to irf-1 expression. Thus, we inhibited the expression of irf1 in mDCs using siRNA and examined the effect on the production of IL-12p70 in response to a strong TLR-mediated agonist Poly (I:C) and MalAg (Fig. 6). As shown in Fig. 6A, the silencing of irf-1 (p=0.0003 compared with control siRNA) markedly inhibited the expression of il12p35 (p=0.002) but did not affect the expression of il12p40 in response to Poly (I:C) stimulation. Furthermore, the production of IL-12p70 protein (p=0.006) was significantly downregulated in irf-1-silenced mDCs compared with mDC-treated with control siRNA (Fig. 6B) whereas irf-1 silencing failed to alter the production of IL-12p40, CXCL-10, CXCL-9, and CXCL-11 in mDCs stimulated with Poly (I:C) (Fig. 6B). In response to MalAg (Fig. 6C), silencing of irf-1 significantly suppressed the expression of il12p35 and il12p40 (p=0.0001 and p=0.01, respectively) but did not affect the expression of cxcl10 when compared with mDCs exposed to control siRNA (Fig. 6C).
Figure 6.
Inhibition of IRF-1 down-regulate the production of IL-12p70 in mDCs. Monocyte-derived mDCs were transfected with either IRF-1 siRNA or control siRNA and stimulated with (A, B) Poly (I:C) or (C) MalAg for 24 h. (A, C) The cells were used for RNA extraction and RT-PCR and (B) the supernatants were collected and screened for cytokine production. The figure represents data from 18 experiments/donors; each symbol represents data from one volunteer with the lines joining data points from the same cells treated with either control siRNA or IRF-1 siRNA constructs. The bars represent the geometric mean and the error bars the 95% confidence interval. Statistical significance was estimated using the Wilcoxon signed-rank test.
Discussion
Having previously demonstrated that malaria-specific Th1 responses (IFN-γ) are attenuated in the context of concomitant filarial infections – an attenuation that reflects diminished IL-12p70 and CXCL-10 production – we examined those factors that regulate IL-12 production and responsiveness. A Th1 immune response typically is induced by IL-12p70 produced by DCs that act on receptors expressed on CD4+ T cells [19–21]. The expression of both IL-12p70 and its receptors is regulated, in part, by IRF-1 and IRF-2 [12, 13, 20–22]. Our data showed that upon stimulation with malaria antigen, cells from filarial-infected subjects had not only significant lower frequency of IL-12-producing mDCs but also diminished expression of IRF-1, a transcription factor known to regulate the expression of IL-12p70 in mDCs and its receptors on CD4+ T cells. Although the expression of irf-1 was not determined in DCs and T cells specifically in Fil+ subject, IRF-1 is known to modulate the differentiation of Th1 cells despite its expression and discreet role in other cell types [23]. Although, we did not address the mechanisms involved in IL-12 signaling or how IL-12R function on CD4+ T cells, we did not find any significant differences in the expression of either IL-12Rβ1 or IL-12Rβ2, suggesting the diminished malaria-specific Th1 response is likely regulated at the level of IL-12 production, through IRF-1 silencing [24].
DCs play critical role in inducing Th1 effector responses, hence their importance in immune responses to malaria [25–27]. Although, their role in inducing Th1-cell responses is unequivocal, data from in vitro studies assessing their function in malaria are varied [26, 28–33]. Our data clearly show that monocyte-derived DCs stimulated with malaria antigen produced high levels of not only pro-inflammatory cytokines (IL-1α, IL-1β, TNF-α and IL-6) but also high levels of Th1-associated cytokines (CXCL-9, CXCL-10, CXCL-11 and IL-12 (p35 and p40) the production of which was profoundly attenuated by live mf (see Fig. 3).
In contrast to human malaria, a recent study of filarial-infected subjects showed that this helminth infection was associated with an increased number of circulating mDCs (the major producer of IL-12) while the number of circulating pDCs was not different when compared with filaria-uninfected subjects [34]. Despite this increase in the number of circulating mDCs during filarial infection, those capable of making IL-12p70 were significantly limited (see Fig. 2A and B) in Fil+ individuals compared with Fil− individuals. Interestingly, the effect of live mf on DCs was very specific to the induction Th1-associated cytokines (e.g. IL-12p70, CXL-9, CXCL-10 and CXCL-11) as mf-pre-exposed monocyte-derived or blood-derived mDCs produced higher levels of IL-1β, IL-6 and TNF-α compared with un-exposed mDCs. These Th1-associated cytokines are regulated among many other factors by the interferon regulatory factors (IRF-1, IRF-3 and IRF-7) and the NF-κb pathway. The differential effect of mf on the mDC production of Th1-associated cytokines (IL-12 and CXCR3 ligands) and the other pro-inflammatory cytokines suggest that mf may alter specifically the IRF pathway in mDCs. To this end we found that mf significantly inhibited the expression of IRF-1, IRF-2 and IRF-3, transcription factors known to regulate the production of IL-12p35, IL-12p40 and CXCL-10 [35–37].
Because helminths have been shown to directly or indirectly condition DCs to induce Th2 responses [38–40], the impairment of the IRFs (major regulators of IL-12 and CXCR3 ligands) in DCs may be one mechanism used by filarial parasites to promote Th2 responses by dampening the Th1 responses. The concept is supported by our data using purified DCs (both mDCs and pDCs) from whole blood in which IL-12p40, IL-12p70, CXCL-9 and CXCL-10 (see Fig. 5) production was diminished by the presence of mf. These results corroborate the data from in vitro generated mDCs and suggest that mf alter the function of those DCs that produce IL-12 required to drive the Th1-cell immune responses.
To evaluate the importance of IRF-1 in regulating IL-12p70 production, we showed that IRF-1 siRNA constructs significantly inhibited the expression of IL-12p35 and the production of IL-12p70. IL-12p40 and IL-12p35, the two subunits of IL-p70 are differentially regulated, IL-12p35 by IRF-1 and IL-12p40 by IRF-1, IRF-2 and NF-κB [13, 41, 42]. IRF-1 has also been shown to be responsible for the impaired production of IL-12 by DCs and macrophages that in turn impair Th1-cell differentiation [19, 20]. The down regulation of IRF-1, 2 and 3 in mDCs in mf-exposed DCs was seen specifically in response to malaria antigen (and to poly (I:C)) stimulation and did not reflect an mf-imprinted mDC phenotype. Moreover, analyzing the transcriptional profile of mf-pre-exposed mDCs failed to find mf-induced alteration of IRF expression [15]. Although the mechanism by which filaria parasites mediate the down regulation of IRFs still remains to be elucidated, filarial parasites are known to suppress the expression and function of TLRs [43–45]. Because signaling through some TLRs activates IRFs [23, 37, 46, 47], modulating TLRs expression and function could be one mechanism by which filarial parasites suppress IRF expression and function.
Our data demonstrate that in the context of malaria/filaria co-infection, filarial parasites directly modulate the ability of mDCs to produce Th1-associated cytokines in response to malaria antigen stimulation. This modulation (of IL-12p70, CXCR3 ligands) appears to reflect altered control of IRFs (IRF-1, IRF-2 and IRF-3) in mDCs that has broad implications for the development of appropriate Th1-cell-dominated responses following malaria infection.
Materials and methods
Patients and study site
The patient population used in this study (NCT00471666) has been described previously [48] and studied under protocols approved by the NIAID IRB and the Ethical Committee of the University of Mali. Individual informed consent was obtained from all participants and/or their legal guardians in French or Bambara.
Whole blood culture
Whole blood cultures were set up as described previously [8, 48]. Malaria parasite extract (MalAg) was prepared using standard methods described previously [8] and used at 104 infected erythrocyte/mL of culture. For RNA assays, the cells were stored at −70°C in buffer RLT (Qiagen, Valencia, CA, USA) until used for RNA purification. For flow cytometry, Brefeldin A (Sigma-Aldrich, St. Louis, MO, USA) was added to the cultures after 12 h of incubation.
Gene expression
RNA was purified from frozen samples using the RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions.
cDNA synthesis
Totally, 200 ng of RNA was used to generate cDNA using TaqMan reverse transcription reagents (ABI, Carlsbad, CA, USA), according to the manufacturer's protocol.
Real-time RT-PCR
Real-time quantitative RT-PCR was performed in an ABI 7900HT sequence detection system (ABI, Carlsbad, CA, USA) using TaqMan Assays for IRF-1 (Hs00971960_m1), IRF-2 (Hs00180006_m1), IRF-3 (Hs01547282_m1) and IRF-7 (Hs00185375_m1). An endogenous 18s ribosomal RNA was used for RNA control. Relative transcripts were determined by the formula:
ΔCT=CTsample−CT18S control, the relative expression was calculated; ΔΔCT = AverageΔCTstimulated−ΔCTunstimulated, as was the fold change by the formula 2−ΔΔCT, in which CT is the threshold cycle during the exponential phase of amplification.
Flow cytometry
The flow cytometry assay and analysis were performed as described earlier [48]. The cells were stained with anti-human CD3-Alexa Fluor 700 (clone UCTH), CD123-PE-Cy7 (clone 6H6) (eBioscience, San Diego, CA, USA), anti-human CD19-Alexa Fluor 700 (Clone SJ25C1), CD56-Alexa Fluor 700 (clone NCAM16.2), CD11c (clone B-ly6), CD14-APC-Cy7 (clone MφP9) (BD Bioscience, San Jose, CA, USA), PE-conjugated BDCA-1 and BDCA-3 (Miltenyi, Auburn, CA, USA). Samples were acquired on a BD LSRII (BD PharMingen) and analyzed using FlowJo (TreeStar, Ashland, OR, USA).
Generation of monocyte-derived myeloid and plasma-cytoid DCs
Peripheral blood monocytes were obtained by centrifugal elutriation from normal donors under a protocol approved by the IRB of the Department of Transfusion Medicine, NIH (IRB 99-CC-0168). Monocytes were plated at 8.4×106 cells/well in a 6-well plate (3506 costar) (Corning, NY USA) in serum-free RPMI 1640 media (incomplete media) supplemented with 1% glutamine 200 mM, 1% HEPES 1 M, 1% penicillin/streptomycin 10 000 units/10 000 μg (Invitrogen, Carlsbad, CA, USA) and incubated for two hours at 37°C in a CO2 incubator. Non-adherent cells were aspirated and the media was replaced with 2% human serum containing-media. The cells were incubated for 7 days with GM-CSF and IL-4 (1000/100 units) (Peprotech, Rocky Hill, NJ, USA) for mDCs or IL-3 (Peprotech, Rocky Hill, NJ, USA) and IFN-β (Avonex, Research Triangle Park, NC, USA) (1500/150 units) for pDCs, added on days 0, 3 and 5. On day 7, the cells were harvested and counted. In vitro generated mDCs and pDCs expressed markers and produced cytokines known to be expressed and produced by blood derived DCs (Supporting Information Fig. 2).
Purification of mDCs and pDCs from peripheral blood
mDCs and pDCs were isolated from peripheral blood mono-nuclear cells (PBMCs) of normal donors using the Myeloid DC Isolation Kit and the CD304 (BDCA-4/Neuropilin-1) MicroBead Kit respectively according to the manufacturer's protocol (Miltenyi). The purity of the DCs based on flow cytometry using anti-BDCA-1, anti-BDCA3 and anti-BDCA-2 antibodies was always>95% (Supporting Information Fig. S3).
DC stimulation
mDCs or pDCs were exposed to live B. malayi mf at a final concentration of 50 000 mf/1×106 cells/well as described previously [49] or were left unexposed for 48 h. The cells were then harvested, counted, plated at 1×106 cells/mL and either cultured in media alone, or with MalAg or with 2.5 μg/mL of Poly (I:C) (Invitrogen) for 24 h. The supernatants and the cells were collected and stored at −80°C for cytokine assay and real-time quantitative PCR respectively.
Inhibition of Irf-1 in mDCs
IRF-1 siRNAs were purchased from Invitrogen. The sequences of IRF-1 siRNA used were, sense 5′-AAU UAA UCU GCA UCU CUA GCC AGG G-3′ and antisense 5′-CCC UGG CUA GAG AUG CAG AUU AAU U; sense 5′-AAA CAG GCA UCC UUG UUG AUG UCC C and antisense 3′-GGG ACA UCA ACA AGG AUG CCU GUU U-5′. A control stealth RNAi with low GC content was also purchased from Invitrogen. IRF-1 siRNA and control RNA transfections of in vitro generated mDCs were done by electroporation using the human DC Nucleofector® kit and the Amaxa Nucleofector® II Device (Lonza Walkersville, MD, USA) following the manufacturer's protocol. After transfection, cells were left unstimulated or stimulated with Poly (I:C) or MalAg extract for 24 h. The supernatants were collected and stored for cytokine screening, and the cells were used for RNA extraction and real-time RT-PCR.
Cytokine analysis
Cytokine analysis in supernatants was performed as described in [8]. The assay was done using Milliplex kits for human cytokines (Millipore, Billerica, MA USA) according to the manufacturer's protocol.
Statistical analyses
The Mann–Whitney and Wilcoxon signed-rank tests were used for paired and unpaired analyses, respectively; and p-values were corrected for multiple comparisons using Holm's correction. All analyses were performed using Prism version 5.0 (GraphPad, San Diego, CA, USA).
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- Fil+
filaria-infected individuals
- Fil−
uninfected individuals
- Fo
frequency
- GM
geometric mean
- iGMFI
integrated geometric mean fluorescence intensity
- IRF
interferon regulatory factor
- MalAg
malaria antigen
- mDC
myeloid dendritic cell
- mf
microfilariae
- pDCs
plasmacytoid dendritic cells
Footnotes
Conflict of Interest: Because S. Metenou, A. D. Klion, and T. B. Nutman are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
References
- 1.Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology. 2008;135:783–794. doi: 10.1017/S0031182008000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Malaria Report 2009. Geneve: 2010. http://www.who.int/malariaworld_malaria_report_2009/en.index.html. [Google Scholar]
- 4.Le Hesran JY, Akiana J, Ndiaye el HM, Dia M, Senghor P, Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2004;98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, Guindo A, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am. J. Trop. Med. Hyg. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 6.Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Vannaphan S, Traore B, Gay F, et al. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am. J. Trop. Med. Hyg. 2001;65:834–836. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 7.Hartgers FC, Obeng BB, Kruize YC, Dijkhuis A, McCall M, Sauerwein RW, Luty AJ, et al. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 2009;199:1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 8.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, et al. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J. Immunol. 2009;183:916–924. doi: 10.4049/jimmunol.0900257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King CL, Kumaraswami V, Poindexter RW, Kumari S, Jayaraman K, Alling DW, Ottesen EA, et al. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J. Clin. Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahanty S, Ravichandran M, Raman U, Jayaraman K, Kumaraswami V, Nutman TB. Regulation of parasite antigen-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect. Immun. 1997;65:1742–1747. doi: 10.1128/iai.65.5.1742-1747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Unutmaz D, Vilcek J. IRF1: a deus ex machina in TH1 differentiation. Nat. Immunol. 2008;9:9–10. doi: 10.1038/ni0108-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonios D, Rousseau P, Larange A, Kerdine-Romer S, Pallardy M. Mechanisms of IL-12 synthesis by human dendritic cells treated with the chemical sensitizer NiSO4. J. Immunol. 2010;185:89–98. doi: 10.4049/jimmunol.0901992. [DOI] [PubMed] [Google Scholar]
- 15.Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, Gilden JK, Nutman TB. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J. Immunol. 2003;171:1950–1960. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 16.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly YI, Diallo AA, Soumaoro L, et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J. Immunol. 2011;186:4725–4733. doi: 10.4049/jimmunol.1003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohoff M, Duncan GS, Ferrick D, Mittrucker HW, Bischof S, Prechtl S, Rollinghoff M, et al. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J. Exp. Med. 2000;192:325–336. doi: 10.1084/jem.192.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCurley N, Mellman I. Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PLoS One. 2010;5:e11949. doi: 10.1371/journal.pone.0011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohoff M, Ferrick D, Mittrucker HW, Duncan GS, Bischof S, Rollinghoff M, Mak TW. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 20.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suzuki G, et al. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GX, Yu S, Gran B, Li J, Siglienti I, Chen X, Calida D, et al. Role of IL-12 receptor beta 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J. Immunol. 2003;171:4485–4492. doi: 10.4049/jimmunol.171.9.4485. [DOI] [PubMed] [Google Scholar]
- 22.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J. Immunol. 1999;163:1529–1536. [PubMed] [Google Scholar]
- 23.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 24.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, Zhou W, et al. Inhibition of IFN regulatory factor-1 down-regulate Th1 cell function in patients with acute coronary syndrome. J. Clin. Immunol. 2010;30:241–252. doi: 10.1007/s10875-010-9367-8. [DOI] [PubMed] [Google Scholar]
- 25.Bruna-Romero O, Rodriguez A. Dendritic cells can initiate protective immune responses against malaria. Infect. Immun. 2001;69:5173–5176. doi: 10.1128/IAI.69.8.5173-5176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ing R, Stevenson MM. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect. Immun. 2009;77:770–782. doi: 10.1128/IAI.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhorne J, Albano FR, Hensmann M, Sanni L, Cadman E, Voisine C, Sponaas AM. Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection. Immunol. Rev. 2004;201:35–47. doi: 10.1111/j.0105-2896.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 28.Urban BC, Mwangi T, Ross A, Kinyanjui S, Mosobo M, Kai O, Lowe B, et al. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood. 2001;98:2859–2861. doi: 10.1182/blood.v98.9.2859. [DOI] [PubMed] [Google Scholar]
- 29.Voisine C, Mastelic B, Sponaas AM, Langhorne J. Classical CD11c+dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int. J. Parasitol. 2010;40:711–719. doi: 10.1016/j.ijpara.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Sponaas AM, Cadman ET, Voisine C, Harrison V, Boonstra A, O'Garra A, Langhorne J. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettiol E, Carapau D, Galan-Rodriguez C, Ocana-Morgner C, Rodriguez A. Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation. Malar. J. 2010;9:64. doi: 10.1186/1475-2875-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott SR, Spurck TP, Dodin JM, Maier AG, Voss TS, Yosaatmadja F, Payne PD, et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 34.Semnani RT, Mahapatra L, Dembele B, Konate S, Metenou S, Dolo H, Coulibaly ME, et al. Expanded numbers of circulating myeloid dendritic cells in patent human filarial infection reflect lower CCR1 expression. J. Immunol. 2010;185:6364–6372. doi: 10.4049/jimmunol.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol. Immunother. 2010;59:489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tailor P, Tamura T, Ozato K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 2006;16:134–140. doi: 10.1038/sj.cr.7310018. [DOI] [PubMed] [Google Scholar]
- 37.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 38.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl. Acad. Sci. USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J. Exp. Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa K, Funaba M, Tsujimoto M. Suppression of NF-kappaB and IRF-1-induced transcription of the murine IL-12 p40 by transforming growth factor-beta Smad pathway in macrophages. Mol. Cell. Biochem. 2008;308:9–15. doi: 10.1007/s11010-007-9605-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, Beg AA. Distinct roles of different NF-kappa B subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J. Immunol. 2007;178:6777–6788. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- 43.Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, et al. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl. Trop. Dis. 2009;3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J. Immunol. 2005;175:1170–1176. doi: 10.4049/jimmunol.175.2.1170. [DOI] [PubMed] [Google Scholar]
- 45.Semnani RT, Venugopal PG, Leifer CA, Mostbock S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood. 2008;112:1290–1298. doi: 10.1182/blood-2008-04-149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur. J. Immunol. 2007;37:306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 47.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur. J. Immunol. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 48.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semnani RT, Venugopal PG, Mahapatra L, Skinner JA, Meylan F, Chien D, Dorward DW, et al. Induction of TRAIL- and TNF-alpha-dependent apoptosis in human monocyte-derived dendritic cells by microfilariae of Brugia malayi. J. Immunol. 2008;181:7081–7089. doi: 10.4049/jimmunol.181.10.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.