Abstract
Background
The corticotropin-releasing factor type 2 receptor (CRFR2) is suggested to play an important role in aiding recovery from acute stress, but any chronic effects of CRFR2 activation are unknown. CRFR2 in the midbrain raphé nuclei modulate serotonergic activity of this key source of serotonin (5-HT) forebrain innervation.
Methods
Transgenic mice overexpressing the highly specific CRFR2 ligand urocortin 3 (UCN3OE) were analyzed for stress-related behaviors and hypothalamic-pituitary-adrenal axis responses. Responses to 5-HT receptor agonist challenge were assessed by local cerebral glucose utilization, while 5-HT and 5-hydroxyindoleacetic acid content were quantified in limbic brain regions.
Results
Mice overexpressing urocortin 3 exhibited increased stress-related behaviors under basal conditions and impaired retention of spatial memory compared with control mice. Following acute stress, unlike control mice, they exhibited no further increase in these stress-related behaviors and showed an attenuated adrenocorticotropic hormone response. 5-HT and 5-hydroxyindoleacetic acid content of limbic nuclei were differentially regulated by stress in UCN3OE mice as compared with control mice. Responses to 5-HT type 1A receptor challenge were significantly and specifically reduced in UCN3OE mice. The distribution pattern of local cerebral glucose utilization and 5-HT type 1A receptor messenger RNA expression levels suggested this effect was mediated in the raphé nuclei.
Conclusions
Chronic activation of CRFR2 promotes an anxiety-like state, yet with attenuated behavioral and hypothalamic-pituitary-adrenal axis responses to stress. This is reminiscent of stress-related atypical psychiatric syndromes such as posttraumatic stress disorder, chronic fatigue, and chronic pain states. This new understanding indicates CRFR2 antagonism as a potential novel therapeutic target for such disorders.
Key Words: Anxiety, corticotropin-releasing factor type 2 receptor, dorsal raphé nucleus, 5-HT type 1A receptor, serotonin, stress
Corticotropin-releasing factor (CRF) plays a fundamental role in regulating the behavioral and neuroendocrine responses to stressors (1). These effects, mediated via stimulation of the CRF type 1 receptor (CRFR1) include hypothalamic-pituitary-adrenal (HPA) axis activation and promotion of anxiety (2). In contrast, urocortins (Ucns) are the endogenous ligands for the CRF type 2 receptor (CRFR2) (3–7), which is suggested to modulate these stress responses.
Affective disorders are associated with CRF hyperactivity (8). CRF and Ucns alter the neurotransmitter systems targeted by antidepressants, in particular the serotonergic system (9,10). CRFR2 is abundant in the midbrain raphé nuclei (11–13), the main source of serotonin (5-HT) innervation to the forebrain. CRFR2 activity increases 5-HT neuronal firing rates and 5-HT release in efferent stress-related nuclei (14–18). This interaction may provide the major link between Ucns/CRFR2 and their effects on stress responses.
While acute pharmacologic stimulation of CRFR2 activates the HPA axis (19,20), both anxiogenic and anxiolytic effects on behavior are reported in rodents (21). Differing experimental paradigms or nonspecificity of the pharmacologic tools employed may be responsible (6,21). Urocortin 3 (Ucn3), however, is a highly specific agonist for CRFR2 (3,6,7). Close anatomical association between major Ucn3 terminal fields and CRFR2 in the limbic system and hypothalamus indicate this peptide is well placed to be an endogenous modulator of CRFR2 activity (22). Ucn3 appears to be anxiolytic when administered in acute experiments (21), but elevated Ucn3 in the perifornical hypothalamus is anxiogenic over a period of days (23). Although long-term CRFR2 stimulation is pertinent to human affective disorders, the effects of chronic CRFR2 activation on stress-related behaviors, the HPA axis, and 5-HT function are largely unknown. We have investigated this key issue by exploiting transgenic mice overexpressing Ucn3 (UCN3OE) widely throughout the brain.
Methods and Materials
Animals
Mice overexpressing Ucn3 were generated by pronuclear DNA microinjection of Ucn3 complementary DNA under the control of the ROSA26 promoter into C57BL/6 × BALB/c first generation oocytes, as previously described (24). Mice were housed in temperature and lighting controlled rooms (lights on 12 hours) with free access to laboratory chow and water. All experimental mice were the offspring of a sire heterozygous for the transgene and a transgene-negative dam from the closed colony. Thus, UCN3OE mice were heterozygous for the transgene, and control mice were transgene-negative littermates. Experiments were carried out on male mice between 10 and 14 weeks old. Principles of Laboratory Animal Care (National Institutes of Health Publication No. 85–23, 1985) were followed. All procedures were approved by The Salk Institute Animal Use and Care Committee, The Weizmann Institute Animal Use and Care Committee, or the United Kingdom Animals (Scientific Procedures) Act, 1986.
Behavioral Testing
All tests were carried out in group-housed mice during the dark phase of the light cycle. Mice were habituated in the home cage in a dark room for 2 hours before testing with constant background white noise (52 dB). For acute restraint stress, mice were subjected to 30 minutes in a ventilated 50 mL plastic centrifuge tube and returned to the home cage for 30 minutes before testing. Separate cohorts of animals were used for 1) rotarod and behavioral testing under basal conditions, n = 14 to 16; 2) behavioral testing following restraint stress, n = 11 to 15; and 3) Barnes maze analysis, n = 13 to 14. Order of testing was rotarod test (cohort 1 only), open-field test, elevated plus-maze (EPM), light-dark transfer test (LDT), and tail suspension test (TST), with 48 to 72 hours between tests. These animals were not further used for other experiments described in this study.
Rotarod Test
Mice were placed on a standard rotarod apparatus with the speed increasing linearly from 5 to 70 rpm over a 5-minute period. Latency to fall from the rotating drum was recorded. For evaluation of motor skill learning, mice were tested three times in quick succession, and this was repeated three times over a period of 4 hours. To test motor memory, another three-run cycle was performed the following day.
Open-Field Test
The apparatus and experimental conditions were as previously described (25). Each mouse was placed in the center of the apparatus to initiate a 10-minute test session. Time spent in the inner squares of the arena and the total number of squares crossed were quantified.
Elevated Plus-Maze
The apparatus and experimental conditions were as previously described (26). Number of open-arm and closed-arm entries and time spent on the open or closed arms were scored. Arm entries were defined as entry of all four paws into the arm. Total arm entries were taken as an index of locomotor activity.
Light/Dark Transfer Test
The apparatus and experimental conditions were as previously described (25). During a 5-minute test session, the latency to enter the light compartment and the number of entries and time spent in the light compartment were measured.
Tail Suspension Test
Mice were suspended from a metal horizontal rod by taping by the base of the tail for 6 minutes. The duration of immobility was scored. Any mouse climbing onto the rod was excluded from analysis.
Barnes Maze
The apparatus consists of a 90 cm diameter Plexiglas circular platform, with 20 holes (5 cm diameter) equidistant around the periphery and surrounded by visual cues. One hole leads to an escape chamber, always located underneath the same randomly determined hole for each mouse. The initial training session was performed by placing the mouse in the escape box for 1 minute and the first trial started 1 minute later. Mice were placed in the middle of the platform under a cup, which was removed to initiate each trial. These were carried out with 80 dB white noise and 400 lux lighting as aversive stimuli. The trial ended upon entry to the escape chamber or after 5 minutes had elapsed. Sound and light were turned off immediately upon successful termination of the trial and the mouse was allowed to remain in the dark for 1 minute. Each mouse was subject to 16 trials over a period of 10 days. First error was scored as the number of holes away from the correct hole first approached by the mouse. An error was defined as searching any hole that did not have the escape chamber beneath it. Time to complete the trial and the search strategy were also recorded: random, unsystematic hole searches with crossings through the maze center; serial, systematic hole searches (every hole or every other hole) in a clockwise or counterclockwise direction; and spatial, moving directly to the target hole or to an adjacent hole before visiting the target. A probe test in which the escape chamber was closed off was run on the final day. For scoring, the maze was divided into four quadrants, with the target hole in the center of the periphery of one quadrant. Latency to first approach the target hole and time spent, crossings into, and the distance travelled in the correct quadrant were scored.
Hypothalamic-Pituitary-Adrenal Axis Activity
Blood samples were collected from individually housed mice by retro-orbital eye bleed from unanesthetized animals within 15 seconds of disturbance of the home cage. For basal HPA axis activity, samples were collected at 7:00 am and 5:00 pm (lights on 6:00 am). For HPA axis response to stress, samples were collected after 2 minutes and 10 minutes of restraint stress at 7:00 am. Individual animals were sampled only once (n = 13–15). Adrenocorticotropic hormone (ACTH) and corticosterone levels were measured in duplicate in unextracted plasma samples using commercially available radioimmunoassay kits as previously described (19). Adrenal glands were weighed following dissection from a separate cohort of animals.
Ucn3 Radioimmunoassay
Ucn3 was measured in whole brain (n = 3) by in-house radioimmunoassay as previously described (27).
Messenger RNA Analysis by In Situ Hybridization Histochemistry and Quantitative Real-Time Polymerase Chain Reaction
Antisense and sense (control) RNA probes were generated using mouse Ucn3 complementary DNA and labeled with DIG-11-UTP using a labeling kit from Roche Molecular Biochemicals (Burgess Hill, United Kingdom). In situ hybridization for Ucn3 messenger RNA (mRNA) (n = 3) and quantitative polymerase chain reaction (qPCR) for CRFR1, CRFR2, and CRF mRNA expression (n = 4−7) were carried out as previously reported (28,29). Primers for serotonin 1A receptor (5-HT1AR) (Htr1a) qPCR: 5'-GTGCACCATCAGCAAGGACC-3' and 5'-GCGCCGAAAGTGGAGTAGAT-3' corresponded to nucleotides 1648-1667 and 1698−1717, respectively. Primers for serotonin reuptake transporter (Slc6a4) qPCR: 5'-GGGTTTGGATAGTACGTTCGCA-3' and 5'-CATACGCCCCTCCTGATGTC-3' corresponded to nucleotides 1490-1511 and 1650-1669, respectively (n = 3−7).
Local Cerebral Glucose Utilization
Local cerebral glucose utilization (LCMRglc) was determined using a 2-deoxyglucose autoradiographic imaging protocol modified from the original technique (30) as described by us previously (31). Equal numbers (n = 7) of animals from each genotype were randomly allocated to a drug treatment group and injected (intraperitoneal) with either 10 mg.kg−1 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT), 25 mg.kg−1 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), or vehicle (.1 mL .9% sodium chloride). Ten minutes after injection of 8-OH-DPAT (or vehicle) or 20 minutes after injection of DOI (or vehicle) measurement of LCMRglc was initiated by injection (intraperitoneal) of 5 μCi [14C]-2-deoxyglucose in .4 mL .9% sodium chloride. After 45 minutes, mice were decapitated and the brains processed for quantitative autoradiographic imaging. Analysis of autoradiograms was performed as described previously (32,33).
High-Performance Liquid Chromatography Analysis of Tissue Concentrations of 5-HT and 5-Hydroxyindoleacetic Acid
Mice (n = 8) were killed by decapitation under basal conditions or 24 hours following restraint stress. Brains were stored at −80°C until analysis. Areas selected for microdissection (Table S1 in Supplement 1) were identified by comparisons with a standard mouse brain stereotaxic atlas (34) and included the raphé nuclei, an anxiety-related raphé-amygdala-subiculum circuit, and septal areas implicated in stress recovery. High-performance liquid chromatography analysis of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were performed as previously described (35).
Statistical Analyses
Statistical analyses employed two-way analysis of variance with post hoc analysis using Fisher's protected least significant difference test or the two-tailed Student t test, as appropriate. Data are presented as mean ± SEM. Differences were considered statistically significant at p < .05.
Results
Brain Overexpression of Ucn3 in UCN3OE Mice
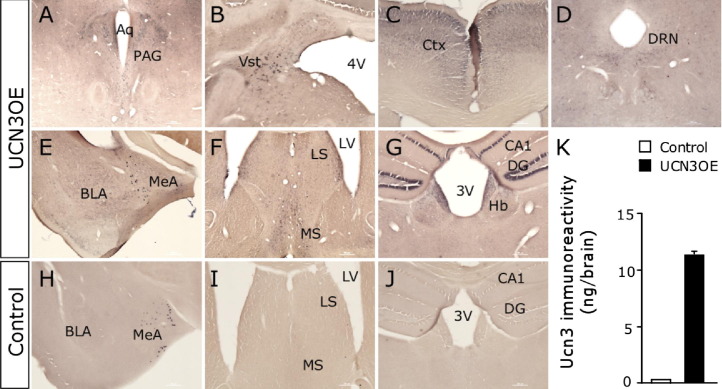
Control brains showed endogenous Ucn3 mRNA expression in the medial amygdala, perifornical area, and the bed nucleus of the stria terminalis as previously reported (6,22). In situ hybridization demonstrated widespread overexpression of Ucn3 mRNA in UCN3OE brains (Figure 1A–G), while increased peptide levels were confirmed by radioimmunoassay (Figure 1K). In addition to expressing Ucn3 mRNA in these endogenous sites, UCN3OE mice have ectopic expression in numerous brain nuclei, including the basolateral amygdala (BLA); cornu ammonis (CA)1, CA3, subiculum, and dentate gyrus of the hippocampus; lateral septum (LS) and medial septum; lateral and medial habenula; caudate putamen; piriform cortex; arcuate nucleus and ventromedial hypothalamus; vestibular nucleus; dorsal raphé nucleus (DRN); periaqueductal gray; and cortex. Several of these brain regions, including the LS, ventromedial hypothalamus, and DRN, express high levels of CRFR2 (12). CRF, CRFR1, and CRFR2 mRNA levels were quantified in their principle sites of expression within anxiety-related circuits (Table S2 in Supplement 1). Ucn3 overexpression did not significantly alter their expression in any area.
Figure 1.
Mice overexpressing urocortin 3 (UCN3OE) express Ucn3 in normal and ectopic brain nuclei. In situ hybridization for Ucn3 messenger RNA in UCN3OE (A–G) and control (H–J) mice showed an endogenous pattern of expression in both control and UCN3OE mice in the MeA (E). Ectopic Ucn3 messenger RNA expression was present in the PAG (A), Vst (B), Ctx (C), DRN (D), BLA (E), LS and MS nuclei (F), and the hippocampal CA1, DG, and Hb (G) of UCN3OE mice. (J) Ucn3 peptide levels were increased in UCN3OE brains (K). n = 3. 3V, third ventricle; 4V, fourth ventricle; Aq, cerebral aqueduct; BLA, basolateral amygdala; CA, cornu ammonis; Ctx, cortex; DG, dentate gyrus; DRN, dorsal raphé nucleus; Hb, habenula; LS, lateral septal; LV, lateral ventricle; MeA, medial amygdala; MS, medial septal; PAG, periaqueductal gray; Ucn3, urocortin 3; Vst, vestibular nucleus.
UCN3OE Mice Show Increased Stress-Related Behaviors
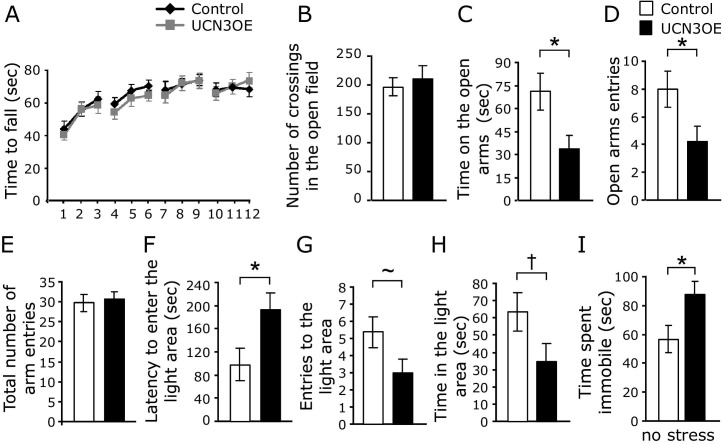
Motor learning and memory were tested using a 2-day, 12-trial rotarod test. Mice overexpressing Ucn3 showed no motor deficits (Figure 2A). Similarly, in the open-field test, UCN3OE mice and littermate control mice showed no differences in total number of crossings, indicating similar locomotor behavior (Figure 2B).
Figure 2.
Mice overexpressing Ucn3 (UCN3OE) show higher anxiety-like behavior under basal conditions. (A) Motor performance in the rotarod test and (B) locomotor activity in the open field test. UCN3OE mice (C) spent less time on and (D) made fewer entries into the open arms of the elevated plus-maze but did not differ in (E) the total number of arm entries made compared with control mice. In the light/dark transfer test, UCN3OE mice showed a (F) longer latency to enter the light compartment and a strong tendency to (G) less entries into the light and (H) less time in the light. (I) UCN3OE mice spent more time immobile in the tail suspension test. *p < .05, ∼p = .06, †p = .07. n = 14 to 16. Ucn3, urocortin 3.
Assessment of stress-related behaviors was initially carried out under basal conditions, without exposing mice to any stress other than that caused by the test itself. Mice overexpressing Ucn3 exhibited higher anxiety-like behavior, as evidenced by less time spent on the open arms (Figure 2C) and less entries made to the open arms (Figure 2D) of the EPM. Again, this was not due to differences in locomotion since the total number of entries was similar between groups (Figure 2E). In the LDT, UCN3OE mice showed increased anxiety-like behavior with a longer latency to enter the light compartment (Figure 2F) and a tendency toward less entries into the light (Figure 2G, p = .06) and less time spent in the light (Figure 2H, p = .07). Mice overexpressing Ucn3 also spent more time immobile than control mice in the TST (Figure 2I) (36).
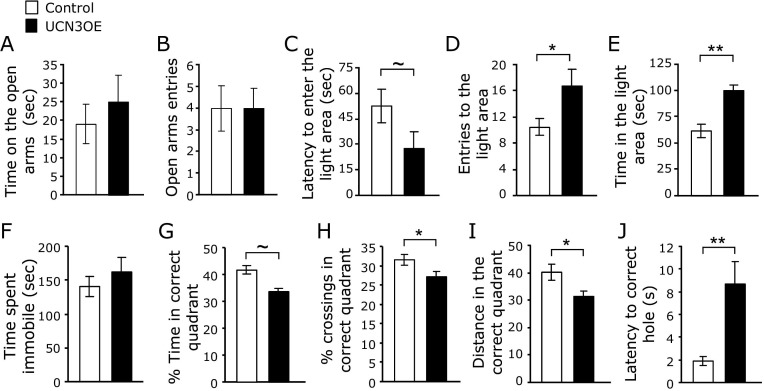
It has been previously shown that activation of CRFR2 affects anxiety-like behavior (23,37,38) under stressed conditions. Following exposure to acute restraint stress (separate cohort), there were no longer differences between behavior of UCN3OE mice and control mice in the EPM (Figure 3A, B; Figure S1 in Supplement 1). This appears due to a stress-related increase in anxiety-like behavior in control but not UCN3OE mice. In the LDT, UCN3OE mice entered the light compartment more often (Figure 3D) and spent significantly more time in the light (Figure 3E) than control mice. This appeared to be due to a decrease in anxiety-like behavior in UCN3OE mice. Stress increased immobility in the TST in both genotypes, but there was no difference between genotypes in immobility following stress (Figure 3F). Overall stress removed the affective behavioral differences between UCN3OE and control mice seen under basal conditions.
Figure 3.
Mice overexpressing urocortin 3 (UCN3OE) show attenuated behavioral responses to acute restraint stress and spatial memory deficits. UCN3OE mice (A) spent similar time on and (B) made a similar number of entries into the open arms of the elevated plus-maze as control mice. UCN3OE mice showed a tendency to (C) a shorter latency to enter and (D) made significantly more entries into and (E) spent more time in the light compartment in the light/dark transfer test. (F) Immobility in the tail suspension test following stress. n = 11 to 15. (G) Time spent, (H) crossings in, and (I) distance travelled in the correct quadrant and (J) the latency to find the escape hole during the probe test in the Barnes maze, n = 13 to 14. *p < .05, **p < .01, ∼p = .09.
Spatial Learning and Memory in the Barnes Maze
Both control and UCN3OE mice showed similar learning in the Barnes maze (39), as shown by measures of reduction in distance of the first hole checked from the escape hole (first error), reduction in number of errors made in each trial set, shorter latency to escape over 16 trials, and the strategy used to locate the escape hole (Figure S1 in Supplement 1). Retention of learning was tested using a single trial probe test. Mice overexpressing Ucn3 explored the correct quadrant significantly less than control mice, as shown by the number of crossings (Figure 3H) and distance travelled (Figure 3I) in the correct quadrant. In addition, UCN3OE mice showed a longer latency to approach the escape hole (Figure 3J). Hence, UCN3OE mice show impaired spatial memory retention.
HPA Axis Responsiveness
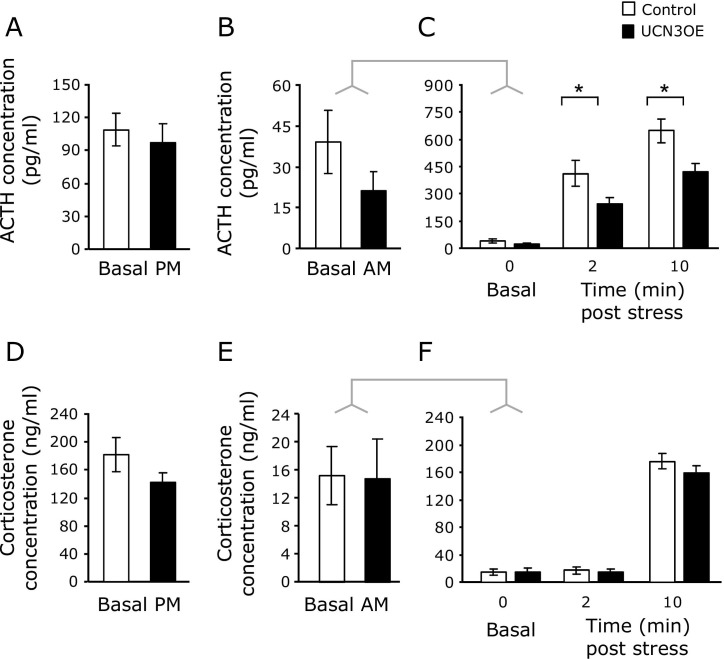
ACTH and corticosterone levels measured under stress-free basal conditions were not different between genotypes at the diurnal peak (Figure 4A, D) and trough (Figure 4B, E) of the circadian rhythm. Following restraint stress, UCN3OE mice showed a significantly attenuated rise in ACTH levels over 10 minutes (Figure 4C), although the rise in corticosterone levels over this time was similar to control mice (Figure 4F). Total adrenal weight did not differ from control mice (control mice 3.62 ± .27 mg, UCN3OE 3.69 ± .33 mg, n = 6–8).
Figure 4.
Mice overexpressing Ucn3 (UCN3OE) and control mice show similar basal hypothalamic-pituitary-adrenal axis activity but an attenuated adrenocorticotropic hormone (ACTH) response upon acute stress. Plasma ACTH levels were measured in the (A) evening and (B) morning under basal conditions. (C) On acute stress, UCN3OE mice show attenuated ACTH levels. No differences were found in plasma corticosterone levels in the (D) evening and (E) morning or (F) following stress. n = 13 to 15. *p < .05.
Serotonergic Function Is Altered in UCN3OE Mice
Following challenge with the 5-HT1AR-specific agonist 8-OH-DPAT, analysis of variance revealed a main effect of treatment in all brain areas examined and of genotype in the hippocampal CA3 [F(1,24) = 15.082, p < .01] and molecular layer [F(1,24) = 5.921, p < .05], prefrontal cortex [F(1,24) = 7.407, p < .05], dorsal subiculum [F(1,24) = 4.645, p < .05], and the medial septum [F(1,24) = 5.538, p < .05] (Table 1 and Table S3 in Supplement 1). There was a significant interaction of treatment × genotype in the hippocampal CA3 [F(1,24) = 11.081, p < .01], prefrontal cortex [F(1,24) = 7.407, p < .05], dorsal subiculum [F(1,24) = 8.258, p < .01], dentate gyrus [F(1,24) = 4.902, p < .05], and lateral striatum [F(1,24) = 4.558, p < .05]. In contrast, when challenged with the serotonin 2C receptor-specific agonist DOI, despite a main effect of treatment in many brain areas, ranging from hippocampal field CA3 [F(1,24) = 33.923] to parietal cortex [F(1,24) = 11.461], there was no main effect of genotype or any significant interaction of genotype with treatment on LCMRglc (Table 2 and Table S4 in Supplement 1), demonstrating a differential response to 8-OH-DPAT only between genotypes.
Table 1.
LCMRglc in Brain Regions of Control and UCN3OE Mice in Response to 5-HT1AR Agonist 8-OH-DPAT
| Control |
UCN3OE |
|||||
|---|---|---|---|---|---|---|
| Saline | 8-OH-DPAT | % | Saline | 8-OH-DPAT | % | |
| Dorsal Raphé Nucleus | 35 ± 3 | 25 ± 3a | −29 | 33 ± 3 | 18 ± 5a | −46 |
| Median Raphé Nucleus | 46 ± 2 | 36 ± 3a | −22 | 45 ± 3 | 24 ± 7a | −47 |
| Neocortex | ||||||
| Orbitofrontal | 62 ± 4 | 39 ± 4a | −37 | 62 ± 5 | 52 ± 4 | −16 |
| Frontal | 46 ± 3 | 33 ± 3a | −28 | 48 ± 4 | 42 ± 3 | −12 |
| Anterior cingulate | 48 ± 4 | 31 ± 4a | −36 | 45 ± 5 | 39 ± 4 | −13 |
| Prefrontalb,c | 47 ± 5 | 22 ± 3a | −53 | 47 ± 4 | 42 ± 2 | −11 |
| Somatosensory | 54 ± 4 | 38 ± 5a | −29 | 52 ± 5 | 45 ± 3 | −13 |
| Parietal | 54 ± 4 | 34 ± 4a | −35 | 48 ± 5 | 43 ± 3 | −10 |
| Posterior cingulate | 51 ± 4 | 32 ± 3a | −37 | 49 ± 5 | 42 ± 2 | −15 |
| Piriform | 39 ± 3 | 24 ± 2a | −38 | 41 ± 3 | 35 ± 5 | −14 |
| Entorhinal | 35 ± 3 | 24 ± 3a | −32 | 33 ± 2 | 29 ± 2 | −12 |
| Hippocampus | ||||||
| Molecular layerb | 41 ± 4 | 26 ± 3a | −38 | 44 ± 3 | 38 ± 2 | −14 |
| Dorsal subiculumb,c | 38 ± 3 | 24 ± 3a | −39 | 36 ± 3 | 32 ± 2 | −11 |
| Dentate gyrusc | 25 ± 1 | 15 ± 1a | −38 | 23 ± 2 | 20 ± 1 | −13 |
| Dorsal CA1 | 35 ± 3 | 23 ± 4a | −36 | 33 ± 2 | 28 ± 3 | −15 |
| CA2 | 33 ± 3 | 21 ± 2a | −36 | 34 ± 3 | 30 ± 4 | −12 |
| Ventral CA1 | 33 ± 2 | 20 ± 2a | −39 | 31 ± 3 | 27 ± 2 | −13 |
| Ventral subiculum | 29 ± 2 | 20 ± 2a | −31 | 28 ± 3 | 25 ± 3 | −11 |
| CA3 | 33 ± 2 | 16 ± 2a | −52 | 34 ± 2 | 29 ± 1 | −15 |
| Extrapyramidal Areas | ||||||
| Medial striatum | 43 ± 3 | 33 ± 3a | −24 | 44 ± 3 | 39 ± 2 | −11 |
| Lateral striatumc | 48 ± 4 | 29 ± 3a | −39 | 46 ± 3 | 41 ± 3 | −11 |
| Globus pallidus | 32 ± 3 | 22 ± 1a | −31 | 33 ± 2 | 29 ± 2 | −12 |
| Substantia nigra, reticulata | 31 ± 2 | 22 ± 2a | −29 | 33 ± 2 | 28 ± 3 | −15 |
| Substantia nigra, compacta | 37 ± 3 | 27 ± 1a | −28 | 39 ± 2 | 34 ± 3 | −13 |
| Limbic Areas | ||||||
| Medial septal nucleusb | 42 ± 2 | 29 ± 3a | −31 | 45 ± 3 | 38 ± 2 | −16 |
| Lateral septal nucleus | 34 ± 3 | 22 ± 3a | −35 | 36 ± 4 | 26 ± 2a | −27 |
| Bed nucleus of the stria terminalis | 28 ± 3 | 17 ± 2a | −41 | 28 ± 2 | 20 ± 3a | −29 |
| Basolateral amygdala | 36 ± 3 | 22 ± 3a | −39 | 34 ± 3 | 30 ± 3 | −12 |
| Central amygdala | 23 ± 3 | 17 ± 2 | −26 | 25 ± 2 | 22 ± 3 | −12 |
LCMRglc shown as mean ± SEM and % change in LCMRglu in DOI compared with saline-treated mice. n = 7. There was a main effect of 8-OH-DPAT treatment for all brain areas.
CA, cornu ammonis; 5-HT1AR, serotonin 1A receptor; LCMRglc, local cerebral glucose utilization; UCN3OE, mice overexpressing urocortin 3.
p < .05 versus saline.
Main effect of genotype.
Main effect of genotype × 8-OH-DPAT treatment.
Table 2.
LCMRglc in Brain Regions of Control and UCN3OE Mice in Response to 5-HT2CR Agonist DOI
| Control |
UCN3OE |
|||||
|---|---|---|---|---|---|---|
| Saline | DOI | % | Saline | DOI | % | |
| Dorsal Raphé Nucleus | 36 ± 3 | 37 ± 2 | 3 | 33 ± 5 | 30 ± 3 | −9 |
| Median Raphé Nucleus | 45 ± 5 | 46 ± 3 | 2 | 45 ± 3 | 44 ± 3 | −2 |
| Neocortex | ||||||
| Orbitofrontala | 58 ± 3 | 47 ± 2b | −19 | 63 ± 5 | 50 ± 3b | −21 |
| Frontala | 43 ± 2 | 36 ± 1b | −16 | 45 ± 3 | 34 ± 3b | −24 |
| Anterior cingulatea | 46 ± 2 | 39 ± 2b | −16 | 48 ± 3 | 38 ± 2b | −21 |
| Prefrontala | 44 ± 3 | 33 ± 3b | −20 | 47 ± 4 | 36 ± 2b | −23 |
| Somatosensory | 51 ± 4 | 47 ± 4 | −8 | 55 ± 6 | 53 ± 3 | −4 |
| Parietala | 54 ± 3 | 42 ± 4b | −22 | 54 ± 5 | 40 ± 3b | −26 |
| Posterior cingulate | 49 ± 2 | 48 ± 2 | −2 | 52 ± 4 | 52 ± 2 | 0 |
| Piriform | 40 ± 3 | 33 ± 2 | −14 | 43 ± 3 | 39 ± 5 | −10 |
| Entorhinal | 37 ± 3 | 32 ± 1 | −13 | 35 ± 2 | 32 ± 3 | −9 |
| Hippocampus | ||||||
| Molecular layera | 42 ± 3 | 34 ± 2b | −19 | 46 ± 3 | 35 ± 2b | −24 |
| Dorsal subiculum | 40 ± 3 | 44 ± 2 | 10 | 37 ± 3 | 36 ± 3 | −3 |
| Dentate gyrusa | 26 ± 2 | 16 ± 2b | −38 | 24 ± 2 | 15 ± 1b | −38 |
| Dorsal CA1a | 37 ± 3 | 25 ± 3b | −32 | 38 ± 3 | 28 ± 2b | −26 |
| CA2 | 35 ± 3 | 36 ± 4 | 3 | 32 ± 3 | 30 ± 3 | −6 |
| Ventral CA1 | 34 ± 2 | 30 ± 2 | −13 | 31 ± 3 | 29 ± 2 | −6 |
| Ventral subiculum | 30 ± 2 | 30 ± 4 | 0 | 33 ± 2 | 35 ± 3 | 6 |
| CA3a | 34 ± 2 | 24 ± 2b | −29 | 36 ± 2 | 25 ± 1b | −31 |
| Extrapyramidal Areas | ||||||
| Medial striatuma | 41 ± 3 | 31 ± 2b | −24 | 42 ± 3 | 30 ± 2b | −22 |
| Lateral striatuma | 46 ± 3 | 29 ± 3b | −36 | 44 ± 3 | 31 ± 3b | −30 |
| Globus pallidus | 30 ± 2 | 32 ± 2 | 7 | 33 ± 2 | 32 ± 3 | −3 |
| Substantia nigra, reticulata | 29 ± 2 | 30 ± 2 | 3 | 28 ± 2 | 28 ± 4 | 0 |
| Substantia nigra, compacta | 36 ± 2 | 37 ± 3 | 2 | 34 ± 3 | 34 ± 3 | 0 |
| Limbic Areas | ||||||
| Medial septal nucleus | 40 ± 2 | 38 ± 3 | −5 | 38 ± 3 | 38 ± 2 | 0 |
| Lateral septal nucleus | 34 ± 3 | 33 ± 3 | −3 | 37 ± 4 | 36 ± 2 | −2 |
| Bed nucleus of the stria terminalis | 27 ± 3 | 27 ± 2 | 0 | 25 ± 6 | 27 ± 3 | 8 |
| Basolateral amygdala | 33 ± 2 | 28 ± 4 | −15 | 34 ± 5 | 30 ± 3 | −12 |
| Central amygdala | 22 ± 2 | 18 ± 2 | −18 | 25 ± 2 | 22 ± 3 | −12 |
LCMRglc shown as mean ± SEM and % change in LCMRglu in DOI compared with saline-treated mice. n = 7. There was no main effect of genotype or interaction of genotype × DOI treatment for any brain area.
CA, cornu ammonis; 5-HT2CR, serotonin 2C receptor; LCMRglc, local cerebral glucose utilization; UCN3OE, mice overexpressing urocortin 3.
Main effect of DOI treatment.
p < .05 versus saline.
Further post hoc analysis (Tables 1 and 2) revealed no significant differences in LCMRglc between vehicle-treated control and UCN3OE mice, indicating no effect of genotype on constitutive cerebral glucose utilization, likely reflecting adaptation to lifelong altered function. Following 8-OH-DPAT, the decrease in LCMRglc was significantly less than in control mice, and, in fact, LCMRglc was not different from vehicle-treated animals in the majority of brain regions studied, indicating an attenuated response to 5-HT1AR stimulation. In contrast, when challenged with DOI, there was no significant difference in LCMRglc between the genotypes (Table 2). Messenger RNA expression of the 5-HT1AR was significantly decreased in the DRN and amygdala of the UCN3OE mice (Figure 5; Figure S2 in Supplement 1). Serotonin reuptake transporter mRNA expression did not differ between genotypes in any brain region examined.
Figure 5.
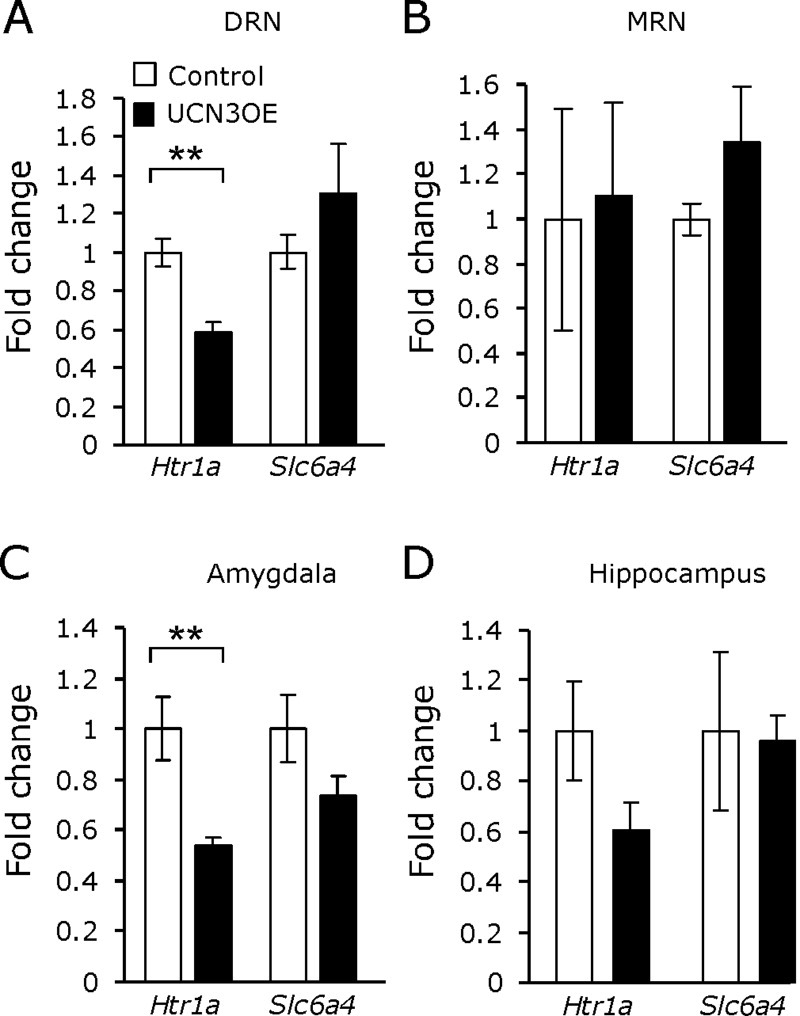
Mice overexpressing Ucn3 (UCN3OE) show decreased basal htr1a messenger RNA expression within the (A) dorsal raphé nucleus (DRN) and (C) amygdala but not (B) median raphé nucleus (MRN) or (D) hippocampus. Basal htr1a and slc6a4 messenger RNA expression within stress-related brain regions in mice overexpressing urocortin 3 and control mice. Panels on the right depict the location of the tissue dissection. n = 4 to 7, except for MRN n = 3 to 4. **p < .01.
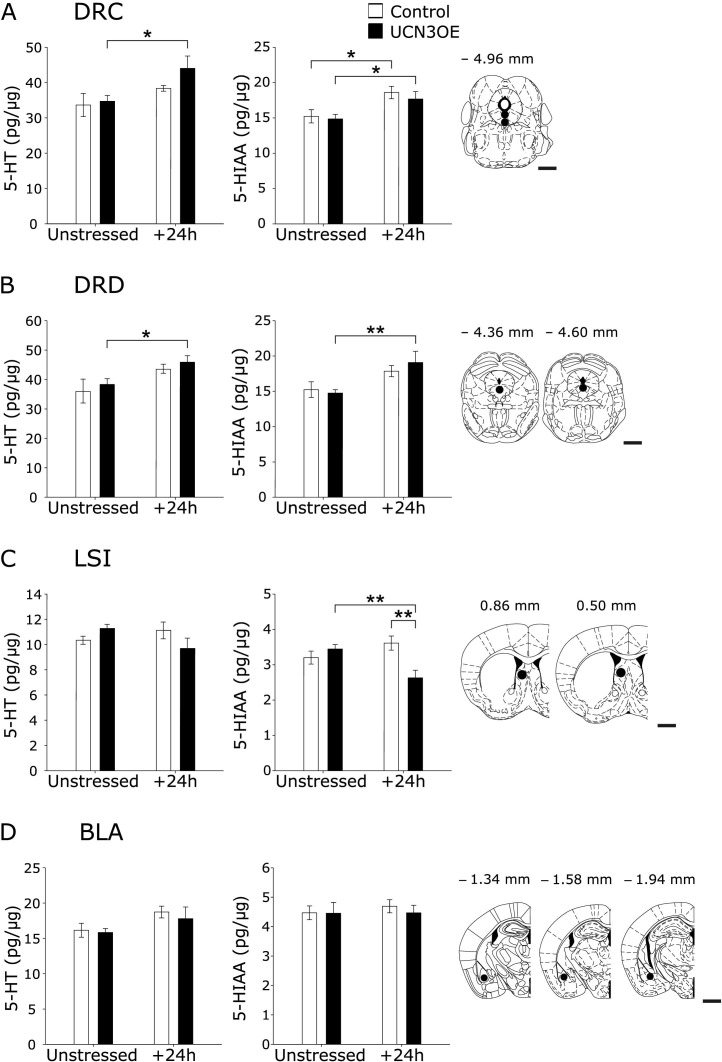
Consistent with the observation of unchanged constitutive brain function in UCN3OE mice, there was no difference in 5-HT and 5-HIAA content of selected brain nuclei when compared with control mice under basal conditions (Figure 6; Table S5 in Supplement 1). There were significant main effects of stress on 5-HT content in the caudal DRN [F(1,28) = 7.566, p < .05], dorsal DRN [F(1,28) = 8.477, p < .01], and the BLA [F(1,28) = 4.337, p < .05]. For 5-HIAA, there was a significant main effect of stress in the caudal DRN [F(1,28) = 13.278, p < .01] and dorsal DRN [F(1,28) = 10.495, p < .01]. There was a significant interaction (genotype × stress) on both 5-HT [F(1,28) = 4.225, p < .05] and 5-HIAA [F(1,28) = 5.371, p < .05] concentrations in the intermediate LS.
Figure 6.
Mice overexpressing Ucn3 (UCN3OE) show stress-induced alterations in tissue concentration of serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) levels in stress-related brain regions. 5-HT (left panel) and 5-HIAA (middle panel) concentrations under unstressed and 24 hours poststress (+24h) conditions in the (A) dorsal raphé nucleus, caudal part (DRC), (B) dorsal raphé nucleus, dorsal part (DRD), (C) lateral septum, intermediate part (LSI), and (D) basolateral amygdala (BLA). Panels on the right depict the location of the microdissection and approximate distance from bregma (reprinted from Paxinos and Franklin [34] with permission from Elsevier, copyright 2001). n = 8. Scale bar = 1 mm. *p < .05; **p < .01. Ucn3, urocortin 3.
Post hoc analysis revealed that 24 hours following a stressor, there was an increase in 5-HIAA content of the caudal DRN (Figure 6A) of control mice. In contrast, UCN3OE mice showed a more robust serotonergic response with significant increases in 5-HT and 5-HIAA in both the caudal and dorsal DRN poststress (Figure 6A, B). They also showed decreased 5-HIAA, in the intermediate LS poststress (Figure 6C). There were no effects of stress or genotype in the central amygdala, medial septum, or subiculum (data not shown).
Discussion
We show that specific CRFR2 activation by chronic Ucn3 overexpression increases baseline stress-related behaviors and yet attenuates the affective impacts of stress and aspects of HPA axis function. The underlying mechanism for this is plausibly reduced 5-HT1AR signaling in the raphé nuclei.
Ucn3 overexpression should result in continuous and chronic stimulation and relative hyperactivation of CRFR2. Ucn3 overexpression has potential to stimulate endogenous CRFR2 at the site of expression, e.g., in the LS, BLA, hippocampal CA1, and the DRN (12,13), or to exert effects via axonal transport of Ucn3 to CRFR2 fields, e.g., the habenula has no CRFR2 but provides projections to the DRN (40–42). Importantly, CRFR2 levels in functionally significant sites, including the paraventricular nucleus, LS, median raphé nucleus (MRN), and DRN, are not affected by the continuous overexpression of ligand, a finding in keeping with unaltered peripheral expression of CRFR2 in these mice (24).
Mice overexpressing Ucn3 display an increase in stress-related behaviors under basal circumstances, suggesting chronic CRFR2 activation promotes an anxiety-like state. This extends and accords with the previously observed increase in anxiety seen following viral overexpression of Ucn3 in hypothalamus (23). Anxiety impairs spatial memory (43–48), plausibly accounting for the reduced retention of the spatial memory seen in UCN3OE mice. Conversely, following a stressor, indices of anxiety-like behavior and immobility in the TST in UCN3OE mice were similar to or lower than in control mice. This could be due to a ceiling effect of the enhanced anxiety levels in the UCN3OE mice. However, UCN3OE mice even showed some anxiolysis under stressful conditions. Overall, CRFR2 hyperactivation in this model displays a dual and contrasting effect under basal and stress conditions, in agreement with the suggested roles of CRFR2 in contributing to the recovery phases of the stress response (49–51). These effects may be, at least in part, due to direct effects of CRFR2 stimulation by Ucn3 on behavior. However, current understanding of CRFR2 function in relation to this aspect has moved mechanistic theories away from the originally proposed direct opposition of CRFR1 action and toward more complex regulation of other neurotransmitter systems by CRFR2 activity, in particular 5-HT function (15,16,29,52). Consistent with this, 5-HT function is significantly altered in UCN3OE mice, with evidence of attenuated 5-HT1AR responsiveness.
The response to a 5-HT1AR agonist was altered in extrapyramidal brain areas lacking in 5-HT1AR (53,54) but which receive 5-HT projections from the DRN (55,56) in UCN3OE mice, inferring the effect is mediated in the DRN rather than in the forebrain structures themselves. Serotonin innervation of the forebrain is supplied by the MRN and DRN (57), both of which express CRFR2 (12), and it is likely the MRN may be similarly affected. However, 5-HT1AR mRNA expression and 5-HT and 5-HIAA content following stress exposure were altered only in the DRN of UCN3OE mice, suggesting this may be the main site where effects on 5-HT function by CRFR2 are mediated. In keeping with this, 5-HT1AR was downregulated in the UCN3OE amygdala, an area where 5-HT projections are supplied by the DRN (57).
Mice overexpressing Ucn3 show enhanced stress-induced 5-HT and 5-HIAA content in the DRN, consistent with their attenuated response to 5-HT1AR agonist and reduced 5-HT1AR expression here, as less autoinhibition by 5-HT1AR correlates with increased activation and firing rates of 5-HT neurons. While the dorsal DRN is the classic anxiety-related subregion of the DRN activated by anxiogenic drugs, social defeat, fear-potentiated startle, and urocortin 2 (15,58–60), the caudal DRN may mediate stress-induced alterations of 5-HT in forebrain regions including the LS (61). A differential genotype effect in the LS was also observed, with decreased 5-HT and 5-HIAA levels in UCN3OE mice poststress. This area not only receives both DRN and MRN projections but is also implicated in promoting stress-related behaviors mediated by direct activation of the CRFR2 expressed here (62,63).
Corticotropin-releasing factor receptor stimulation in the raphé nuclei regulates efferent 5-HT release in a site-specific manner with increases in cortex and hippocampus but decreases in other regions including the LS and striatum (10). This is presumed due to differential CRFR1 versus CRFR2 activation (9). Our results implicate CRFR2 in modulating these effects following a stressor. In contrast, no difference in the 5-HT responses of UCN3OE mice in amygdala or subiculum compared with control mice indicates this anxiety-related circuit may not be key to their stress-related phenotype or, given the well-documented effects of stress here, that 24 hours is not the crucial time point to observe any differential effects.
Dysregulated 5-HT functioning is recognized to underlie the pathophysiology of stress-related psychopathologies, including anxiety disorders and depression, with decreased 5-HT1AR activity associated with these disorders (64–66). Learned helplessness, a behavioral model for anxiety and affective disorders, is dependent on 5-HT activity in the DRN and is proposed to result from hyperactivation of 5-HT neurons in the DRN during exposure to uncontrollable stress (67), leading to internalization of inhibitory serotonin 1A autoreceptors, thus sensitizing other DRN 5-HT neurons to subsequent stress (68,69). In addition to modulating 5-HT neuronal activity, CRFR2 stimulation mediates the behavioral aspects of this process (70). Thus, we hypothesize the phenotype of the UCN3OE mice is a consequence of the continued activation of CRFR2 resulting in a similar process. We propose a model where in the healthy animal, Ucn3 is rapidly released in response to an acute stressor (19,71) and is an important mediator of the stress recovery process (51). However, in UCN3OE mice, ongoing Ucn3 stimulation of CRFR2 models a chronically stressed animal with increased anxiety-like behaviors and decreased responsiveness of 5-HT1AR autoreceptors.
Contrary to what might be anticipated given their behavioral phenotype, UCN3OE mice showed no differences in ACTH or corticosterone levels under basal conditions but exhibited attenuated HPA axis activation (ACTH) in response to stress. Adrenal weight and corticosterone response to stress were not different between genotypes, suggesting possibly greater adrenocortical responses to ACTH in UCN3OE mice, but more detailed analyses over a longer time course are required to draw any definite conclusions.
Blunting of ACTH responses is observed in a subgroup of affective disorders, including atypical depression, posttraumatic stress disorder, and in adults following childhood abuse (72–74), and HPA axis hyporeactivity has been reported in rodents exposed to maternal separation (75–77). Proposed mechanisms include desensitization of CRF receptors and hence the HPA axis by chronically elevated ligand (78) or hypoactivity of CRF neurons based on findings of low CRF in cerebrospinal fluid in patients in certain disorders including chronic fatigue and atypical depression (79–82). In either case, this would appear to be a maladaptation to chronic stress exposure.
Therefore, while attenuated HPA axis and behavioral responses to stress may well reflect the proposed contrasting role of CRFR2 under basal and stressed conditions (49,50), they may also represent maladaptation of the stress response, akin to that observed in illnesses such as atypical depression and posttraumatic stress disorder. Atypical depression has been reported to have a higher comorbidity of anxiety disorders than other subtypes of depression (83,84). Furthermore, gender differences in clinical presentation, with women reported to show more atypical and anxiety symptoms than men (85–87), have led to suggestions that similar serotonergic dysfunctioning underpins both atypical depression and anxiety disorders and that selective serotonin reuptake inhibitors may be more effective than other antidepressant medications in women. Our findings in UCN3OE mice provide further evidence for this link between anxiety, hyporesponsiveness of the HPA axis, and associated dysregulation of serotonergic function, specifically attenuated 5-HT1AR responsiveness. Thus, the role of CRFR2 and urocortins in relation to these psychiatric conditions appears worthy of further study. There is much to be elucidated regarding the complex regulation of serotonergic function by CRFR2, and UCN3OE mice may provide a useful model in this respect.
Acknowledgments
The article is dedicated to our dear friend and mentor Wylie W. Vale, Ph.D., who passed away unexpectedly on January 3, 2012, while this article was under revision.
This work was supported by Wellcome Trust Grant 083192/Z/07/Z and, in part, by award DK026741-32 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Award MH086539 from the National Institute of Mental Health, the Adler Foundation, the Clayton Medical Research Foundation Inc., the Kleberg Foundation, the European Research Council (FP7 Grant 260463), the Israel Science Foundation, and the Legacy Heritage Biomedical Science Partnership. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Mental Health, or National Institutes of Health.
We thank Jordana Wingate for excellent technical assistance.
W. W. Vale was a cofounder, member of the Board of Directors, and shareholder of Neurocrine Biosciences. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online.
Supplementary data
References
- 1.Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41 residue ovine hypothalamic peptide that stimulates the secretion of corticotropin and b-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Muller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 3.Lovenberg T.W., Liaw C.W., Grigoriadis D.E., Clevenger W., Chalmers D.T., De Souza E.B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan J.M., Donaldson C., Bittencourt J., Perrin M.H., Lewis K., Sutton S. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 5.Reyes T.M., Lewis K., Perrin M.H., Kunitake K.S., Vaughan J., Arias C.A. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis K., Li C., Perrin M.H., Blount A., Kunitake K., Donaldson C. Identification of Urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu S.Y., Hsueh A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd R.B., Nemeroff C.B. The role of corticotropin-releasing hormone in the pathophysiology of depression: Therapeutic implications. Curr Top Med Chem. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- 9.Valentino R.J., Lucki I., Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linthorst A.C.E. Stress, corticotropin-releasing factor and serotonergic neurotransmission. In: Steckler T., Kalin N.H., Reul J.M.H.M., editors. Handbook of Stress and the Brain. 1st ed. Elsevier; Amsterdam: 2005. pp. 503–524. [Google Scholar]
- 11.Lowry C.A. Functional subsets of serotonergic neurones: Implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Pett K., Viau V., Bittencourt J.C., Chan R.K.W., Li H.-Y., Arias C. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers D.T., Lovenberg T.W., De Souza E.B. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pernar L., Curtis A.L., Vale W.W., Rivier J.E., Valentino R.J. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staub D.R., Evans A.K., Lowry C.A. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 16.Amat J., Tamblyn J.P., Paul E.D., Bland S.T., Amat P., Foster A.C. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 17.Forster G.L., Pringle R.B., Mouw N.J., Vuong S.M., Watt M.J., Burke A.R. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukkes J.L., Forster G.L., Renner K.J., Summers C.H. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson P.M., Li C., Kukura C., Vaughan J., Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama H., Makino S., Noguchi T., Nishioka T., Hashimoto K. Central type 2 corticotropin-releasing hormone receptor mediates hypothalamic-pituitary-adrenocortical axis activation in the rat. Neuroendocrinology. 2007;86:1–16. doi: 10.1159/000103556. [DOI] [PubMed] [Google Scholar]
- 21.Fekete E.M., Zorrilla E.P. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Vaughan J., Sawchenko P., Vale W. Urocortin III-immunoreactive projections in rat brain: Partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuperman Y., Issler O., Regev L., Musseri I., Navon I., Neufeld-Cohen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci U S A. 2010;107:8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamieson P.M., Cleasby M.E., Kuperman Y., Morton N.M., Kelly P.A., Brownstein D.G. Urocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high-fat diet. Diabetologia. 2011;54:2392–2403. doi: 10.1007/s00125-011-2205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bale T.L., Contarino A., Smith G.W., Chan R., Gold L.H., Sawchenko P.E. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Gen. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 26.Contarino A., Dellu F., Koob G.F., Smith G.W., Lee K.-F., Vale W., Gold L.H. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- 27.Li C., Chen P., Vaughan J., Blount A., Chen A., Jamieson P.M. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 28.Korosi A., Veening J.G., Kozicz T., Henckens M., Dederen J., Groenink L. Distribution and expression of CRF receptor 1 and 2 mRNAs in the CRF over-expressing mouse brain. Brain Res. 2006;1072:46–54. doi: 10.1016/j.brainres.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Neufeld-Cohen A., Evans A.K., Getselter D., Spyroglou A., Hill A., Gil S. Urocortin-1 and −2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 2010;15:426–441. doi: 10.1038/mp.2009.115. 339. [DOI] [PubMed] [Google Scholar]
- 30.Sokoloff L., Reivich M., Kennedy C., Des Rosiers M.H., Patlak C.S., Pettigrew K.D. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 31.Dawson N., Ferrington L., Olverman H.J., Kelly P.A. Novel analysis for improved validity in semi-quantitative 2-deoxyglucose autoradiographic imaging. J Neurosci Methods. 2008;175:25–35. doi: 10.1016/j.jneumeth.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Dawson N., Ferrington L., Lesch K.P., Kelly P.A. Cerebral metabolic responses to 5-HT2A/C receptor activation in mice with genetically modified serotonin transporter (SERT) expression. Eur Neuropsychopharmacol. 2011;21:117–128. doi: 10.1016/j.euroneuro.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Dawson N., Ferrington L., Olverman H.J., Harmar A.J., Kelly P.A. Sex influences the effect of a lifelong increase in serotonin transporter function on cerebral metabolism. J Neurosci Res. 2009;87:2375–2385. doi: 10.1002/jnr.22062. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G., Franklin K.B.J. 2nd ed. Academic Press; San Diego: 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- 35.Evans A.K., Reinders N., Ashford K.A., Christie I.N., Wakerley J.B., Lowry C.A. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Todorovic C., Radulovic J., Jahn O., Radulovic M., Sherrin T., Hippel C. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur J Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- 38.Bakshi V.P., Smith-Roe S., Newman S.M., Grigoriadis D.E., Kalin N.H. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S., Rakoczy S., Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amat J., Sparks P.D., Matus-Amat P., Griggs J., Watkins L.R., Maier S.F. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 41.Aghajanian G.K., Wang R.Y. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- 42.Varga V., Kocsis B., Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- 43.Santin L.J., Bilbao A., Pedraza C., Matas-Rico E., Lopez-Barroso D., Castilla-Ortega E. Behavioral phenotype of maLPA1-null mice: Increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav. 2009;8:772–784. doi: 10.1111/j.1601-183X.2009.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q., Tang M., Mamiya T., Im H.I., Xiong X., Joseph A., Tang Y.P. Bi-directional effect of cholecystokinin receptor-2 overexpression on stress-triggered fear memory and anxiety in the mouse. PLoS One. 2010;5:e15999. doi: 10.1371/journal.pone.0015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espana J., Gimenez-Llort L., Valero J., Minano A., Rabano A., Rodriguez-Alvarez J. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Ryu J., Futai K., Feliu M., Weinberg R., Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression, and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung A.Y., Futai K., Sala C., Valtschanoff J.G., Ryu J., Woodworth M.A. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moseley A.E., Williams M.T., Schaefer T.L., Bohanan C.S., Neumann J.C., Behbehani M.M. Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Kloet E.R., Joels M., Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 50.Joels M., Baram T.Z. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufeld-Cohen A., Tsoory M.M., Evans A.K., Getselter D., Gil S., Lowry C.A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci U S A. 2010;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanahashi S., Yamamura S., Nakagawa M., Motomura E., Okada M. Effect of lamotrigine and carbamazepine on corticotropin-releasing factor-associated serotonergic transmission in rat dorsal raphe nucleus. Psychopharmacology (Berl) 2012;220:599–610. doi: 10.1007/s00213-011-2506-y. [DOI] [PubMed] [Google Scholar]
- 53.Laporte A.M., Lima L., Gozlan H., Hamon M. Selective in vivo labelling of brain 5-HT1A receptors by [3H]WAY 100635 in the mouse. Eur J Pharmacol. 1994;271:505–514. doi: 10.1016/0014-2999(94)90812-5. [DOI] [PubMed] [Google Scholar]
- 54.Verge D., Daval G., Marcinkiewicz M., Patey A., el Mestikawy S., Gozlan H., Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McQuade R., Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- 56.Vertes R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 57.Vertes R.P., Fortin W.J., Crane A.M. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 58.Spannuth B.M., Hale M.W., Evans A.K., Lukkes J.L., Campeau S., Lowry C.A. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience. 2011;179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams J.K., Johnson P.L., Hay-Schmidt A., Mikkelsen J.D., Shekhar A., Lowry C.A. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Gardner K.L., Thrivikraman K.V., Lightman S.L., Plotsky P.M., Lowry C.A. Early life experience alters behavior during social defeat: Focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 61.Waselus M., Galvez J.P., Valentino R.J., Van Bockstaele E.J. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Bakshi V.P., Newman S.M., Smith-Roe S., Jochman K.A., Kalin N.H. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: Functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henry B., Vale W., Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nemeroff C.B., Owens M.J. The role of serotonin in the pathophysiology of depression: As important as ever. Clin Chem. 2009;55:1578–1579. doi: 10.1373/clinchem.2009.123752. [DOI] [PubMed] [Google Scholar]
- 66.Drevets W.C., Thase M.E., Moses-Kolko E.L., Price J., Frank E., Kupfer D.J. Serotonin-1A receptor imaging in recurrent depression: Replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maier S.F., Watkins L.R. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 68.Greenwood B.N., Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 69.Rozeske R.R., Evans A.K., Frank M.G., Watkins L.R., Lowry C.A., Maier S.F. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammack S.E., Schmid M.J., LoPresti M.L., Der-Avakian A., Pellymounter M.A., Foster A.C. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venihaki M., Sakihara S., Subramanian S., Dikkes P., Weninger S.C., Liapakis G. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: Modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- 72.Strohle A., Scheel M., Modell S., Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res. 2008;42:1185–1188. doi: 10.1016/j.jpsychires.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Demitrack M.A., Dale J.K., Straus S.E., Laue L., Listwak S.J., Kruesi M.J. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 74.Carpenter L.L., Carvalho J.P., Tyrka A.R., Wier L.M., Mello A.F., Mello M.F. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daniels W.M., Pietersen C.Y., Carstens M.E., Stein D.J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt M.V., Oitzl M.S., Levine S., de Kloet E.R. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- 77.Ladd C.O., Thrivikraman K.V., Huot R.L., Plotsky P.M. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Newport D.J., Heim C., Owens M.J., Ritchie J.C., Ramsey C.H., Bonsall R. Cerebrospinal fluid corticotropin-releasing factor (CRF) and vasopressin concentrations predict pituitary response in the CRF stimulation test: A multiple regression analysis. Neuropsychopharmacology. 2003;28:569–576. doi: 10.1038/sj.npp.1300071. [DOI] [PubMed] [Google Scholar]
- 79.Demitrack M.A., Crofford L.J. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci. 1998;840:684–697. doi: 10.1111/j.1749-6632.1998.tb09607.x. [DOI] [PubMed] [Google Scholar]
- 80.Gold P.W., Licinio J., Wong M.L., Chrousos G.P. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci. 1995;771:716–729. doi: 10.1111/j.1749-6632.1995.tb44723.x. [DOI] [PubMed] [Google Scholar]
- 81.Gold P.W., Chrousos G.P. The endocrinology of melancholic and atypical depression: Relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 82.Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 83.Posternak M.A., Zimmerman M. The prevalence of atypical features across mood, anxiety, and personality disorders. Compr Psychiatry. 2002;43:253–262. doi: 10.1053/comp.2002.33498. [DOI] [PubMed] [Google Scholar]
- 84.Blanco C., Vesga-Lopez O., Stewart J.W., Liu S.M., Grant B.F., Hasin D.S. Epidemiology of major depression with atypical features: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) J Clin Psychiatry. 2012;73:224–232. doi: 10.4088/JCP.10m06227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorman J.M. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- 86.Keers R., Aitchison K.J. Gender differences in antidepressant drug response. Int Rev Psychiatry. 2010;22:485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- 87.Halbreich U., Kahn L.S. Atypical depression, somatic depression and anxious depression in women: Are they gender-preferred phenotypes? J Affect Disord. 2007;102:245–258. doi: 10.1016/j.jad.2006.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.