Abstract
Impaired sleep, particularly in the context of sleep-disordered breathing (SDB), is associated with a vast array of comorbidities, including obesity. It is well known that the etiology of obesity is both complex and multifactorial. Recent trends have shown that obesity rates have risen at an alarming rate in children, and this has likely contributed to an increased prevalence of SDB in children. Like the ‘chicken and the egg’ hypothesis, the temporal relationship of obesity and SDB is unclear but it is speculated that these two conditions converge to promote a fundamental disruption to normal lipid homeostasis. In this review, the effect of sleep disruption and SDB on lipid homeostasis in both murine and human models will be critically examined, with the intent of demonstrating that disrupted sleep in children is itself a precursor to obesity via disordered lipid homeostasis.
Keywords: atherosclerosis, children, intermittent hypoxia, lipids, metabolism, obesity, obstructive sleep apnea, sleep, sleep-disordered breathing, sleep fragmentation
Over the past century, obesity and obesity-related complications have emerged as a leading contributor to mortality and morbidity. Of equally paramount concern, the prevalence of obesity continues to increase at an alarming rate across all age groups, including childhood. Obesity during childhood increases the risk for end-organ injury, particularly the hepatic, metabolic and cardiovascular systems, in common with obesity during adulthood. Central to the pathophysiology of obesity-related morbidity is disordered lipid homeostasis, which predisposes to fatty deposition, impacting tissue and organ function. As an example, excess adipose tissue in the neck, abdominal wall and thorax mass increases the mechanical loads to the pulmonary system, elevating the work of breathing, and thereby increasing the risk of sleep-disordered breathing (SDB).
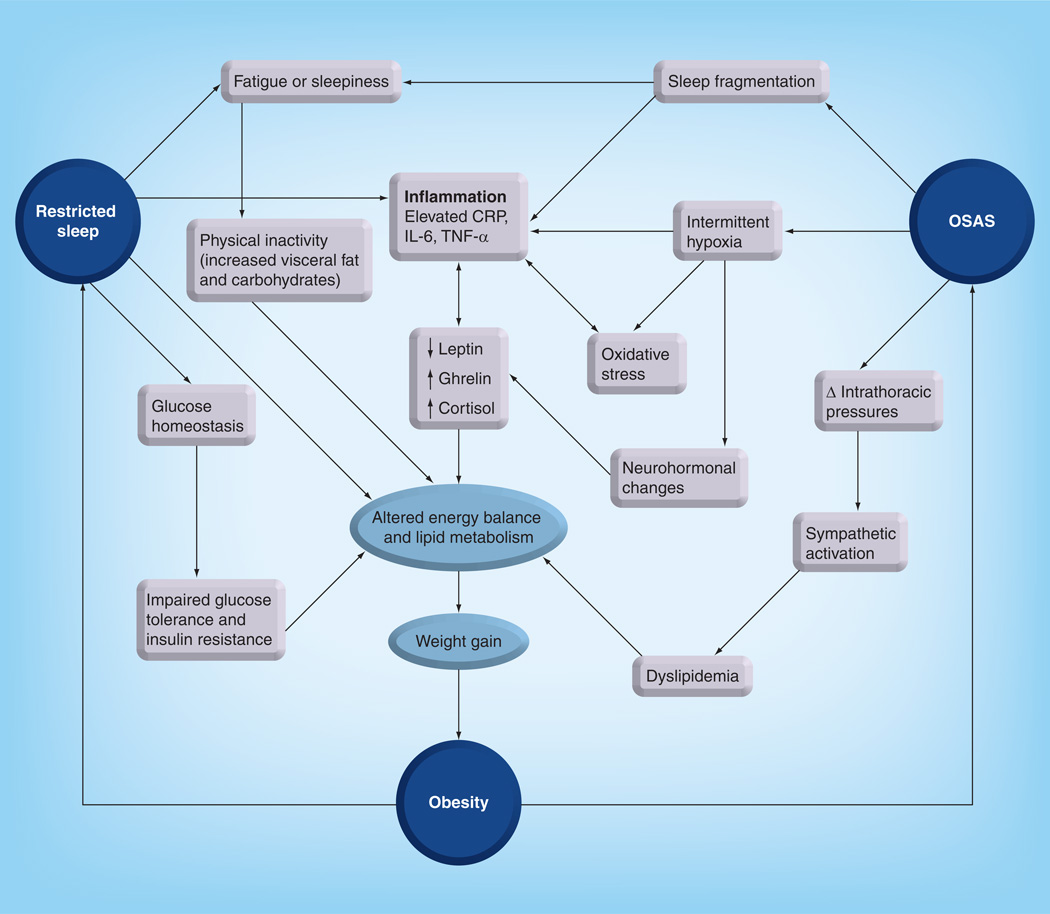
As obesity elevates the risk of SDB, similarly there is emerging evidence suggesting that SDB, by a mechanism involving associated alterations in respiratory gas exchange and sleep fragmentation, elevates the risk of disordered lipid homeostasis, leading to obesogenic behaviors and proinflammatory adipose tissue responses, and thus promoting insulin resistance. These interactions between sleep and lipid-related biological pathways suggest the presence of a circular risk model of obesity, disrupted sleep and SDB, which all converge to disordered lipid metabolism (Figure 1). This review presents the cumulative evidence on the existence of this circular association in both children and mice, and then presents the plausible underlying putative mechanisms that link disordered sleep with disordered lipid homeostasis.
Figure 1. The circular model of sleep restriction, obstructive sleep apnea syndrome and obesity, emphasizing the central role of disrupted lipid metabolism.
CRP: C-reactive protein; OSAS: Obstructive sleep apnea syndrome.
Sleep restriction, obesity & lipid homeostasis: evidence from children
The impact of sleep duration and/or disrupted sleep has been shown to have profound influences on neurocognitive function and quality of life in children [1–3]. The influence of sleep restriction on the risk of childhood obesity has only been described recently. Using a telephone-based questionnaire of 422 children aged 5–10 years, Chaput et al. [4] revealed the odds ratio of developing obesity was 1.42 for children who reported 10.5–11.5 h of sleep, and 3.45 for children who reported 8–10 h sleep compared with children who reported 12–13 h of sleep following adjustment for age, sex and other risk factors. From the FLAME study, using a cohort of 244 children aged 3–7 years, Carter et al. [5] report that for each additional hour of sleep at ages 3–5 years there was an associated reduction in BMI of 0.48 and a reduced risk of being overweight (BMI ≥85th centile) of 0.39 at age 7 years. Using bioelectrical impedance, the differences in BMI were explained by differences in fat mass index more than by differences in fat-free mass index [5]. Using longitudinal follow-up data from adolescent girls, short sleep duration during adolescence was found to have lasting effects of obesity during young adulthood [6]. In a meta-analysis of 30 studies, 12 examining children, Cappucio and colleagues established an association of short sleep duration with obesity [7]. Of the 12 pediatric studies, the pooled odds ratio for short sleep duration and obesity was 1.89, which was statistically highly significant (p < 0.0001). A meta-analysis by Chen et al. [8] found that children with shorter sleep duration had a 58% elevated risk for overweight or obesity, and children with the shortest sleep duration had an even higher risk (92%) when compared with children having longer sleep duration. Furthermore, for each hour increase in sleep, the risk of overweight or obesity was reduced on average by 9% [8]. Recent evidence has also suggested that children who have weekend or holiday ‘catch-up’ sleep have a reduced risk of being overweight [9]. Thus, although a causative link has not been definitively established between sleep and obesity, the plausibility of such an association has incrementally gained credibility in the last decade.
In a study of children aged 4–10 years using actigraphy, a measurement tool providing objective measurements of total sleep duration over a 7-day period, Spruyt et al. demonstrated that, while total sleep duration over a week of measurements did not vary significantly between obese children and nonobese children, it was evident that obese children had significantly increased night-to-night variability in their sleep duration as well as decreased amounts of weekend catch-up sleep, compared with nonobese children [10]. In a subset of 156 children from whom blood samples were drawn, these investigators uncovered significant associations between sleep duration and cardiovascular and metabolic biomarkers. Indeed, restricted sleep duration was associated with elevated fasting serum C-reactive protein and insulin levels, as well as with increased LDL levels. Although further studies are needed to critically examine the relationship between sleep restriction and lipid homeostasis in children, this study does imply that reduced sleep duration may adversely impact lipid homeostasis in children and lead to increased LDL-C concentrations. Putative mechanisms that could be involved in sleep restriction-associated lipid changes may include changes in the levels of specific neuro-peptides that regulate satiety or appetite, such as increased levels of ghrelin and reduced levels of leptin, which in turn lead to increased food intake (with preferences for high-fat-containing food items) and promotion of obesity risk [11]. Of note, a recent study from Hong Kong has corroborated the presence of an association between restricted sleep duration and the risk for increased lipid levels in secondary school children; however, this association was not apparent in primary school children [12].
SDB & obesity: evidence from children
The spectrum of SDB in children ranges from primary snoring during the night to overt obstructive sleep apnea syndrome (OSAS). The incidence of OSAS in children is estimated to be 2–3% of all children, with a peak prevalence found in children aged between 2 and 8 years [13–20].
The pathophysiology of OSAS is characterized by repetitive short or prolonged periods of increased upper airway resistance that leads to either partial or complete obstruction of the upper airway during sleep, leading to loud intermittent snoring, frequent arousals from sleep and secondary sleep fragmentation. Episodic upper airway collapse in children can subsequently disturb respiratory gas exchange, for example, repetitive decreases in oxygen saturation followed by rapid reoxygenation as well as episodic hypercapnia. Finally, occlusion of the upper airway leads to large intrathoracic pressure fluxes, which, coupled with the gas exchange disturbances and frequent brain arousals, induce sustained activation of sympathetic nervous system activity, even during daytime.
The interaction between the various patho-physiological disturbances associated with OSAS is linked to a plethora of serious end-organ morbidities in children; including neurocognitive and behavioral disturbances [21–27], somatic growth failure [28], enuresis [29,30], systemic inflammation [31–33], cardiovascular morbidity [34–36] and finally, of special interest in this review, impaired lipid homeostasis.
Before examining the interactions between OSAS, obesity and lipid homeostasis in children, it is essential to review the etiology of OSAS in childhood. The principal abnormality associated with the increased likelihood of OSAS in children is hypertrophy of adenotonsillar tissues. Enlargement of these tissues in the upper airway reduces the anatomical patency of the airway and thus leads to exponential increases in pharyngeal resistance, which will ultimately result in episodic airway collapse during sleep, characteristic of OSAS [37,38]. While the presence of enlarged tonsils and adenoids does not reliably predict the likelihood of OSAS in children [39], the concurrent presence of habitual snoring and adenotonsillar enlargement should prompt timely diagnostic efforts to confirm or rule out the presence of OSAS.
Notwithstanding the major contribution of adenotonsillar hypertrophy, the presence of obesity in children significantly increases the risk of developing OSAS [40–43]. With prevalence rates as high as 7–22% of children in various western countries [44,45], studies examining the prevalence of OSAS in children have shown substantial increases with obesity [46], such that for each increase of 1 kg/m2 of BMI above the mean in children, the risk of OSAS appears to increase by 12% [47]. Furthermore, when the standard initial treatment of OSAS is implemented (i.e., adeno-tonsillectomy [AT]), the risk for residual OSAS is markedly greater in obese children, as recently shown in a large multicenter retrospective study [48]. Moreover, follow-up data from the large longitudinal cohort TuCASA study reveals that children with residual OSAS are at an elevated risk of developing obesity after 5 years [49]. Evidence has suggested that, at any level of OSAS severity, the degree of adenotonsillar hypertrophy required to develop OSAS of such magnitude is reduced in obese children [50]. Recent evidence in children has also suggested that OSAS is related to specific areas of fat deposition; more specifically, OSAS severity was shown to be strongly predicted by visceral fat deposition as defined by MRI [51]. Finally, the phenotype of obesity-induced childhood OSAS is markedly different from the OSAS phenotype that is exclusively induced by adenotonsillar hypertrophy [52]. In contrast to OSAS induced by adenotonsillar hypertrophy, which is typically associated with symptoms of poor attention and/or hyperactivity, OSAS induced by obesity in children strikingly resembles that of adult patients with OSAS, the vast majority of whom are obese, presenting with symptoms of excessive daytime sleepiness as well as metabolic and cardiovascular disease [53], suggesting that similar mechanisms in adults and obese children may lead to the morbid consequences of OSAS in these patients.
SDB & lipid homeostasis: evidence from children
Childhood obesity is a well-defined risk factor for insulin resistance and dyslipidemia [54–59]. As OSAS has been identified as a concomitant comorbidity of obesity, it is not surprising that OSAS has also been identified as a risk factor for dyslipidemia and insulin resistance in many adult-based studies [60–64]. However, when obesity and OSAS coexist, it is difficult to ascertain whether the effect of OSAS on lipid metabolism is indeed independent of the dyslipidemia induced by obesity in adults.
Studies linking OSAS in children to metabolic disturbances are relatively sparse and divergent. In a study of 135 children, Tauman et al. did not reveal any association between most major polysomnographic indices used in quantifying OSAS severity and fasting serum insulin, glucose, homeostatic model assessment or serum lipid levels [54]. This association study did not, however, examine whether OSAS alone or in combination with obesity contributes to lipid abnormalities. Similarly, in a study of 110 children who did not satisfy obesity criteria, Kaditis et al. found no correlations between the severity of OSAS and fasting insulin or homeostatic model assessment levels, with serum lipids not being reported [65].
In contrast to such studies, Verhulst et al. performed a cross-sectional study of 104 relatively older obese children (42% postpubertal), and showed significant associations between the degree of oxyhemoglobin desaturation and serum lipid and cholesterol levels even after controlling for gender, puberty and BMI [66]. Of note, however, this study did not show a significant association between these metabolic markers and the respiratory disturbance index, an index that reflects the severity of OSAS. In a retrospective study of obese and nonobese children undergoing polysomnography, Alexopoulos et al. reported that the risk of low HDL-C was threefold higher in nonobese children with moderate- to-severe OSAS compared with children with mild OSAS or primary snoring, following adjustment for age and gender; however, the same association was not found in obese children [67]. In a prospective cohort study of adolescents recruited from the general community, Redline et al. reported an odds ratio of 6.49 for development of the metabolic syndrome in subjects with OSAS when compared with subjects without OSAS [68]. The metabolic syndrome was defined by abnormalities in acceptable threshold levels in three of the following five measures: waist circumference, blood pressure, triglyceride level, HDL-C level and glucose level. Furthermore, the study did report a significant association between the presence of OSAS in adolescents and serum LDL concentrations. However, similar to the previously cited studies, the apnea–hypopnea index was not significantly correlated with serum triglyceride or cholesterol concentrations.
In a study of 62 consecutive prepubertal children (40% of whom were nonobese), the authors’ group examined the metabolic parameters of all children undergoing AT as the primary treatment of OSAS [33]. AT led to improvement of OSAS in all children; however, effective resolution of OSAS was more likely in the nonobese group, with a substantial proportion of obese children manifesting residual OSAS after surgery. In nonobese children, AT resulted in significant improvements in LDL, HDL and LDL:HDL levels (Table 1). In obese children, improvements emerged in total cholesterol, total triglyceride, LDL, HDL and LDL:HDL levels as well as in fasting insulin and insulin:glucose ratios following AT. Of note, AT did not result in any significant change in BMI in obese children, suggesting the improvements in lipid homeostasis following AT occurred independently from any changes in BMI or adiposity. Moreover, the severity of OSAS (apnea–hypopnea index) and the degree of oxyhemoglobin desaturation correlated significantly with LDL, HDL and LDL:HDL levels following adjustment for BMI and age. ApoB serum levels were improved in both nonobese and obese groups following AT. Finally, C-reactive protein levels were reduced following AT suggesting marked improvements in systemic inflammation [33]. Taken together, these findings strongly support the hypothesis that disrupted sleep and episodic hypoxia in the context of OSAS impose substantial changes in lipid regulatory mechanisms in children.
Table 1.
Metabolic changes in nonobese and onese children with obstructive sleep apnea syndrome before and 6–12 months after under going adenotonsillectomy.
| Metabolic parameter |
Nonobese Obese | Obese | ||||
|---|---|---|---|---|---|---|
| Pre-AT | Post-AT | p-value | Pre-AT | Post-AT | p-value | |
| Triglycerides (mg/dl) |
76.60 ± 7.40 | 76.20 ± 7.10 | NS | 104.40 ± 8.00 | 89.70 ± 8.10 | <0.01 |
| Total cholesterol (mg/dl) |
157.80 ± 5.20 | 154.40 ± 6.10 | NS | 171.30 ± 6.30 | 164.30 ± 7.10 | <0.01 |
| LDL (mg/dl) | 92.00 ± 5.10 | 66.00 ± 2.30 | <0.01 | 117.60 ± 4.90 | 91.30 ± 4.30 | <0.01 |
| HDL (mg/dl) | 44.60 ± 2.80 | 64.20 ± 3.60 | <0.01 | 37.80 ± 1.30 | 51.70 ± 2.70 | <0.01 |
| LDL:HDL | 2.25 ± 0.20 | 1.13 ± 0.10 | <0.01 | 3.26 ± 0.30 | 2.10 ± 0.30 | <0.01 |
| ApoB (mg/dl) | 102.20 ± 5.40 | 56.30 ± 2.90 | <0.01 | 96.10 ± 3.10 | 62.50 ± 3.40 | <0.01 |
| Insulin (µIU/ml) | 8.80 ± 2.10 | 8.70 ± 1.40 | NS | 26.20 ± 1.90 | 20.40 ± 1.10 | <0.01 |
| Insulin:glucose | 0.10 ± 0.02 | 0.10 ± 0.02 | NS | 0.29 ± 0.03 | 0.21 ± 0.03 | <0.01 |
| CRP (µg/ml) | 4.00 ± 0.90 | 1.10 ± 0.20 | <0.01 | 6.10 ± 1.00 | 2.40 ± 0.60 | <0.01 |
AT: Adenotonsillectomy; CRP: C-reactive protein; NS: Not significant.
Reproduced with permission from [33]
However, based on the aforementioned studies examining the effect of OSAS in childhood on lipid homeostasis, it is apparent that the findings somewhat diverge. The precise putative mechanisms linking the pathophysiological manifestations of OSAS with lipid metabolism in children remain to be elucidated and have been more precisely addressed in murine models (see below). It is noteworthy in this context to mention a recent study of 309 consecutive children by Bhushan and colleagues, which attempted to resolve the discrepant findings in pediatric OSAS and dyslipidemia by examining gene variants in FABP4 and assaying FABP4 protein levels [69]. FABP4 is an important protein involved in the interaction of inflammation and metabolism and one of its main functions is to regulate the intracellular processing of lipids in critical tissues such as adipose tissue and macrophages. Children with OSAS were found to have elevated concentrations of FABP4 in plasma, and the expression of the rs1054135 polymorphism in the FABP4 gene was found to result in significantly elevated levels of FABP4 after adjustment for BMI, suggesting that genetic predisposition may serve as a plausible mechanism accounting for the presence of impaired lipid homeostasis in some children with OSAS but not in others.
Lipid metabolism in rodent models of OSAS
As alluded to in previous paragraphs, the manifestations of OSAS reflect the interactions of intermittent hypoxia (IH), intermittent hypercapnia, increased intrathoracic pressure swings and sleep fragmentation, all of which result from repetitive upper airway obstruction during sleep. Although an animal model that collectively mimics OSAS is unavailable, over the last decade or so researchers have tried to elucidate the contribution of each of the OSAS components and more specifically have attempted to identify some of the mechanisms leading to alterations in lipid metabolism in the context of OSAS.
Lipid metabolism associated with IH
The most commonly used model of OSAS in rodents has relied on the imposition of IH as the surrogate paradigm. Extensive data using this model indicate that IH alters lipid metabolism by upregulating lipid biosynthesis in the liver leading to dyslipidemia, and that IH also modulates increases in adipose tissue lipolysis and inhibits lipoprotein clearance [70].
In the past decade, a substantial amount of evidence has emerged in the effort to identify the mechanism(s) of IH-induced hyperlipidemia. Development of hypercholesterolemia during IH may be a consequence of accelerated lipid biosynthesis, which occurs in the liver and is regulated by a family of transcription factors, SREBPs, which include SREBP-1a, SREBP-1c, and SREBP-2. These SREBPs regulate enzymes such as stearoyl-CoA desaturase 1 (SCD-1), which underlie the synthesis of fatty acids and cholesterol. Li and colleagues studied lean mice exposed to 5 days of IH and reported an increase in fasting serum levels of total cholesterol, HDL-C, phospholipids and triglycerides, as well as liver triglyceride content [71]. These changes were not observed in obese mice exposed to IH, despite the fact that they already had hyperlipidemia and fatty liver at baseline. In lean mice IH also increased SREBP-1 levels in the liver; increased mRNA and protein levels of SCD-1 increased monounsaturated fatty acid content in serum, indicating augmented SCD-1 activity [71]. The same research group also studied genetically obese mice exposed to IH and again found 30% increases in liver triglyceride and phospholipid content with a higher gene expression of cholesterol and fatty acid biosynthesis regulatory enzymes [72]. This response to IH is therefore obesity independent, can be specifically attributed to IH during sleep and has been documented in nonobese children [32]. However, it should be noted that the magnitude of metabolic dysregulation seems to be dependent on the level of the hypoxia stimulus and may also be amplified by existing obesity [73]. In fact, exposure to IH in combination with a high-fat dietary intake will lead to a synergistic effect on the dyslipidemic response [74].
The reported upregulation of key hepatic transcription factors of lipid biosynthesis induced by IH [72] is an important pathway by which SCD-1 converts saturated fatty acids into monounsaturated fatty acids. Abundance of monounsaturated fatty acids increases the biosynthesis of cholesterol esters and triglycerides, which are incorporated into secreted VLDL particles [75,76]. Indeed, an important observation from the Polotsky laboratory came to support this hypothesis when they studied knockout mice of SCAP in the liver and compared them with wild-type controls exposed to 5 days of IH [77]. As anticipated, wild-type mice exposed to IH had increased fasting levels of serum total cholesterol and HDL-C, serum triglycerides, serum and liver phospholipids, mRNA levels of SREBP-1 and mtGPAT, and protein levels of SCAP, nSREBP-1, and mtGPAT in the liver. By contrast, IH did not have any effect on serum and liver lipids, or on expression of lipid metabolic genes in L-Scap-null mice [77]. Savransky and coworkers depleted SCD-1 in C57BL/6J mice using antisense oligonucleotide approaches and showed that they could prevent hyperlipidemia in these mice during 10 weeks of IH exposure [78]. In their recent review, Drager et al. proposed that the increase in SCD-1 under IH conditions may be mediated through HIF-1, which is a master regulator of metabolic responses to hypoxia [70]. Mice with reduced functional HIF-1 are partially protected against hypertriglyceridemia and hepatic lipid accumulation during IH and also show attenuated increases in the active nuclear isoforms of SREBP-1c and SCD-1 [79].
Another important mechanism that has been advanced in IH-induced hyperlipidemia is the activation of lipolytic pathways. Indeed, while IH increased hepatic triglycerides and hepatic VLDL secretion, there was no evidence of an increase in de novo fatty acid synthesis [71]. This observation suggests that peripheral lipolysis, especially in adipose tissue, may operate as the supplier of hepatic fatty acids for the synthesis of triglycerides and VLDL. If this lipolysis exceeds normal needs, a large flux of free fatty acid (FFA) to the liver will lead to steatohepatitis. Furthermore, under most physiological conditions, adipose tissue lipolysis is a catabolic process that leads to the breakdown of triglycerides stored in fat cells and the release of fatty acids, followed by further degradation into acetyl units by β-oxidation. This pathway involves regulatory mechanisms recruited in the CNS, endocrine system, liver and pancreas [80]. Jun and colleagues used ApoE-deficient mice as an atherogenesis-prone murine model and reported that 12 weeks of IH exposure led to significant increases in serum FFA levels suggesting increased adipose tissue lipolysis during IH [81]. One of the mechanisms underlying lipolysis may reside in the increased sympathetic nerve activity associated with IH [82], which also functions as an important regulator of lipolysis [83]. However, hypoxic conditions also attenuate β-oxidation of FFAs, which then leads to FFA accumulation and higher influx of FFAs to the liver.
Another putative mechanism that was recently explored by Drager and colleagues involved the hypothesis that IH can lead to hyperlipidemia not only by inducing lipid biosynthesis and lipolysis, but also by inhibiting the clearance of triglyceride-rich lipoproteins (TRLPs) [84], a known leading pathway in the pathogenesis of atherosclerosis [85]. TRLPs include liver-synthesized VLDL and dietary chylomicrons, which are cleared from the circulation via a multistep process beginning with lipoprotein lipase (LpL), a key enzyme in lipoprotein metabolism that is preferentially expressed in the adipose tissue, skeletal muscle and heart [86,87]. Drager and coworkers exposed mice fed on a high-fat diet to 4 weeks of IH and found that, as anticipated, IH induced significant increases in levels of total cholesterol and triglycerides, which occurred in TRLP and LDL fractions. These investigators further documented an inhibition of TRLP clearance during IH and a fivefold decrease in LpL activity, as well as an 80% increase in mRNA and protein levels of ANGPTL4, a potent inhibitor of LpL activity in the epididymal fat. These findings indicate that IH decreases TRLP clearance and inhibits LpL activity in adipose tissues, which may contribute to the increased atherogenesis observed in OSAS [84].
Effects of IH in adipose tissue
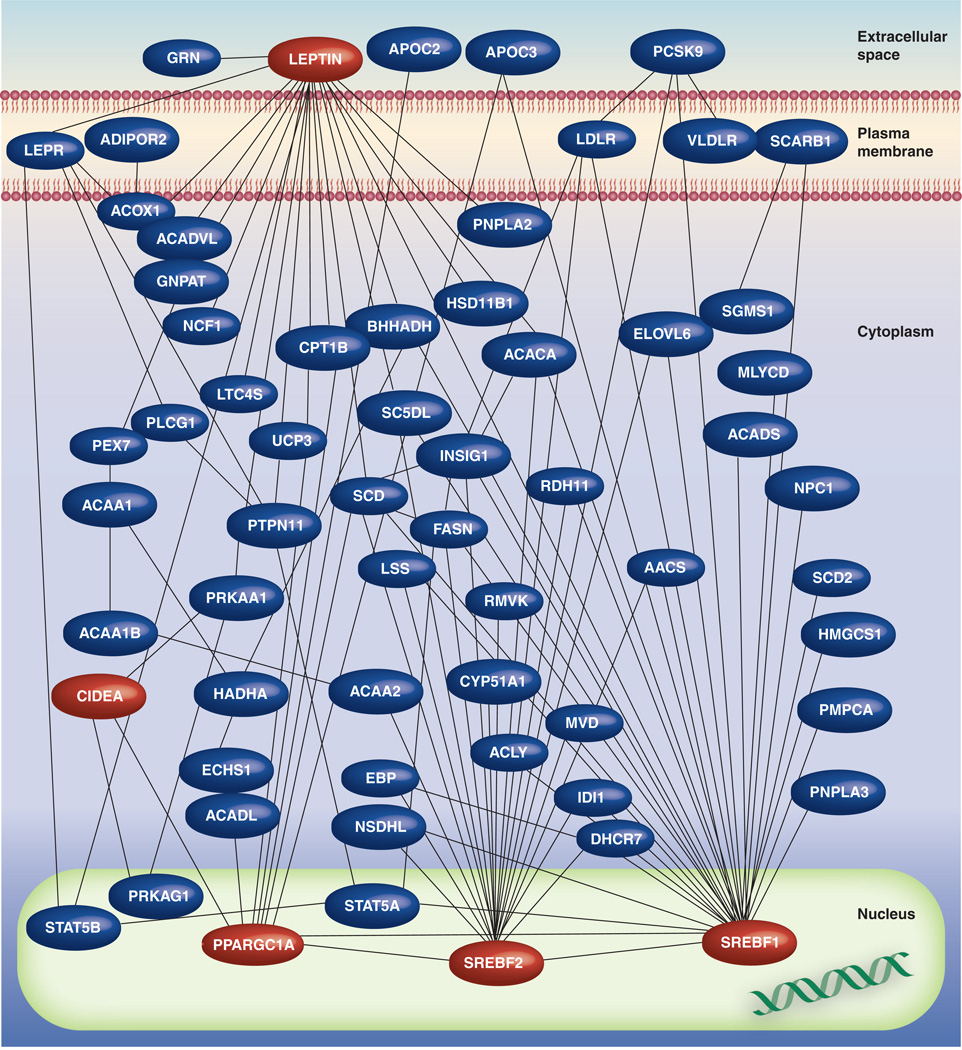
Since adipose tissue is the major production site of critical regulators of metabolism such as leptin and adipokines [88], it is highly likely that the systemic and adipose tissue-specific perturbations of OSAS may be due to the effects of IH on adipocytes. To further investigate this issue, Polotsky et al. examined the effects of chronic IH on obesity and insulin resistance and also performed microarray experiments in subcutaneous adipose tissue samples from mice exposed to 5 days of IH [89]. Significant changes in the expression of a restricted number of metabolic genes that are involved in lipid and glucose metabolism emerged, indicating the potential involvement of fat tissue in IH-induced metabolic perturbations [89]. However, this study was limited to subcutaneous fat. It is now apparent that visceral adipose tissue mass is more strongly associated with abnormal metabolic parameters compared with abdominal subcutaneous fat [90], and that removal of visceral fat by omentectomy results in decreased glucose and insulin levels in humans [91]. By contrast, removal of subcutaneous fat by liposuction does not always result in improvements in glucose metabolism or lipid levels [92]. Therefore, the authors have systematically interrogated the transcriptional programs activated in visceral fat tissue of lean mice in response to 13 days of IH during sleep (Figure 2) [93]. Functional pathway enrichment analytical procedures and network analyses on differentially expressed genes revealed that over 3000 genes exhibit significant alterations in their expression patterns during the time course of IH exposures. Among these genes, the most enriched pathways mapped to metabolic processes, mitochondria and oxidative stress responses. The pathophysiological relevance of these findings was also confirmed by demonstrating that mice exposed to chronic IH develop dyslipidemia and undergo significant lipid and protein oxidation within their visceral adipose depots [90]. Gene–gene interaction network analysis led to the identification of critical controllers of IH-induced transcriptional programs in adipocytes, whereby network hubs represent putative targets that may enable us in the future to alter the effects of IH on adipose tissue. Furthermore, a subnetwork mapping to lipid metabolic pathways was particularly prominent, and was topographically characterized by several densely connected hubs that are well-known master regulators of metabolism, including leptin, and of lipid biosynthesis, including SREBF1 and SREBF2.
Figure 2. Lipid metabolism interactome in visceral adipose tissue of mouse, comprised of differentially expressed genes in response to intermittent hypoxia during sleep, as a model for obstructive sleep apnea syndrome.
Colored in red are selected high-density network nodes, such as leptin, PPARGC1A, SREBF1 and SREBF2, which may represent regulators of adipocyte metabolic responses during intermittent hypoxia.
Reproduced with permission from [93].
Of added interest, another member of the lipid metabolism interactome – CIDEA – was the most differentially upregulated gene in response to IH. Cidea−/− mice have enhanced metabolic rates and lipolysis, and are resistant to diet-induced obesity and diabetes [94]. These findings suggest that activation of CIDEA in visceral adipocytes may represent a mechanism whereby chronic IH induces metabolic dysregulation and dyslipidemia. Interestingly, another important network hub was PPARGC1A (also known as PGC-1α), a transcriptional coactivator of many genes that are involved in metabolism, which exhibited progressive timedependent increases in expression during IH. This study also explored mitochondrial function in adipocytes exposed to IH and confirmed that IH imposed significant oxidative burden on visceral adipocytes by demonstrating a dramatic increase in metabolite levels, as an indicator of lipid peroxidation in the adipose tissues, with genes such as NADH dehydrogenase, ATP synthase, cytochrome reductase and mitochondrial translocase being markedly regulated. These findings demonstrate that chronic exposure to IH activates transcriptional programs encompassing distinct functional units within adipocyte mitochondria [93].
In summary, similar to the human studies, rodent models of OSAS, such as IH during sleep, manifest time-dependent perturbations in adipocyte gene expression and these changes map to distinct functional pathways and networks, most significantly those involved in metabolism, mitochondrial function and oxidative stress.
Conclusion
Taken together, the cumulative evidence in children and in murine models suggests an inter-relationship between disrupted sleep and disrupted lipid homeostasis mediated via either total sleep restriction or sleep fragmentation, SDB and obesity. The translational models presented in this review support a putative paradigm whereby disrupted lipid homeostasis is central to this circular model (Figure 1). Delineation of a cause-and-effect explanation linking sleep with obesity will require future longitudinal studies addressing treatment of OSAS, and interventions aimed at increasing total sleep duration in order to demonstrate reversibility, further providing compelling mechanistic evidence in support of our proposed model. Nonetheless, translational studies of lipid homeostasis aiming to identify tissue-specific mechanisms governing the dyslipidemia of SDB and sleep restriction are not only important, but may provide for development of therapeutic interventions.
Executive summary.
Sleep restriction, obesity & lipid metabolism
-
▪
Short sleep and sleep restriction are risk factors of childhood obesity, and compelling evidence is emerging favoring longer sleep to lower the risk of metabolic consequences and obesity.
-
▪
Recent evidence has suggested that children who have weekends or holiday ‘catch-up’ sleep have a reduced risk of being overweight or of manifesting altered serum lipids. This phenomenon is less evident in obese versus nonobese children.
-
▪
Restricted sleep is associated with elevated fasting C-reactive protein and insulin levels as well with increased LDL levels.
Obstructive sleep apnea syndrome & obesity & lipid homeostasis
-
▪
The presence of obesity in children dramatically increases the risk of developing obstructive sleep apnea syndrome (OSAS).
-
▪
In contrast to OSAS induced by adenotonsillar hypertrophy, which is typically associated with symptoms of poor attention or hyperactivity, OSAS induced by obesity in children resembles the adult phenotype of OSAS, mainly presenting with symptoms of excessive day sleepiness as well as cognitive, metabolic and cardiovascular morbidities.
-
▪
A correlation between the degree of the oxyhemoglobin desaturation and increases in serum cholesterol levels has emerged in children, and these alterations will be abrogated after effective surgical treatment of OSAS.
Lipid metabolism in the rodent model of OSAS
-
▪
Intermittent hypoxia during sleep activates multiple metabolic pathways and is a major contributing mechanism to development of dyslipidemia and insulin resistance.
-
▪
Intermittent hypoxia-induced dyslipidemia appears to be due to excessive adipose tissue lipolysis and increased free fatty acids flux to the liver, as well as by upregulation of hepatic triglyceride biosynthesis and lipoprotein secretion.
-
▪
The effect of intermittent hypoxia on lipid metabolism may also be due to inhibition of the clearance of triglyceride-rich lipoproteins.
Effect of intermittent hypoxia on adipose tissue
-
▪
Adipose tissue is an important regulator of metabolism and is affected by the alterations associated with OSAS, particularly intermittent hypoxia during sleep.
-
▪
Time-dependent perturbations in adipocyte transcriptome occur during intermittent hypoxia during sleep, and map to distinct functional pathways and networks linked to metabolism, especially mitochondrial function and oxidative stress.
Acknowledgments
This study was supported by NIH grants HL-065270 and HL-086662.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Montgomery-Downs HE, Gozal D. Toddler behavior following polysomnography: effects of unintended sleep disturbance. Sleep. 2006;29(10):1282–1287. doi: 10.1093/sleep/29.10.1282. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien LM. The neurocognitive effects of sleep disruption in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2009;18(4):813–823. doi: 10.1016/j.chc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien LM, Gozal D. Neurocognitive dysfunction and sleep in children: from human to rodent. Pediatr. Clin. North. Am. 2004;51(1):187–202. doi: 10.1016/s0031-3955(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 4. Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int. J. Obesity (Lond.) 2006;30(7):1080–1085. doi: 10.1038/sj.ijo.0803291.. ▪ Population-based study linking sleep duration and obesity.
- 5.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangwisch JE, Malaspina D, Babiss LA, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33(7):956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity. 2008;16(2):265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 9.Wing YK, Li SX, Li AM, Zhang J, Kong AP. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009;124(5):e994–e1000. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
- 10. Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127(2):e345–e352. doi: 10.1542/peds.2010-0497.. ▪▪ Perspective on the associations between objectively measured short sleep duration and regularity on risks of obesity, systemic inflammation and metabolic dysfunction.
- 11.Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 2008;23:75–83. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- 12.Kong AP, Wing YK, Choi KC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011;12(7):659–665. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/ hyperactivity disorder. Pediatrics. 2003;111(3):554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 14.Blunden S, Lushington K, Lorenzen B, Wong J, Balendran R, Kennedy D. Symptoms of sleep breathing disorders in children are underreported by parents at general practice visits. Sleep Breath. 2003;7(4):167–176. doi: 10.1007/s11325-003-0167-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaditis AG, Finder J, Alexopoulos EI, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr. Pulmonol. 2004;37(6):499–509. doi: 10.1002/ppul.20002. [DOI] [PubMed] [Google Scholar]
- 16.Lofstrand-Tidestrom B, Hultcrantz E. The development of snoring and sleep related breathing distress from 4 to 6 years in a cohort of Swedish children. Int. J. Pediatr. Otorhinolaryngol. 2007;71(7):1025–1033. doi: 10.1016/j.ijporl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27(1):87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J. Pediatr. 2003;142(4):383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 19.Schlaud M, Urschitz MS, Urschitz-Duprat PM, Poets CF. The German study on sleep-disordered breathing in primary school children: epidemiological approach, representativeness of study sample, and preliminary screening results. Paediatr. Perinat. Epidemiol. 2004;18(6):431–440. doi: 10.1111/j.1365-3016.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 20.Spruyt K, O’Brien LM, Macmillan Coxon AP, Cluydts R, Verleye G, Ferri R. Multidimensional scaling of pediatric sleep breathing problems and bio-behavioral correlates. Sleep Med. 2006;7(3):269–280. doi: 10.1016/j.sleep.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 Pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 22.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am. J. Respir. Crit. Care Med. 2007;176(2):188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4–5 year olds. Arch. Dis. Child. 1993;68(3):360–366. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chervin RD, Archbold KH, Dillon JE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109(3):449–456. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- 25.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20(12):1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 26.Marcotte AC, Thacher PV, Butters M, Bortz J, Acebo C, Carskadon MA. Parental report of sleep problems in children with attentional and learning disorders. J. Dev. Behav. Pediatr. 1998;19(3):178–186. doi: 10.1097/00004703-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Owens J, Spirito A, Marcotte A, McGuinn M, Berkelhammer L. Neuropsychological and behavioral correlates of obstructive sleep apnea syndrome in children: a preliminary study. Sleep Breath. 2000;4(2):67–78. doi: 10.1007/BF03045026. [DOI] [PubMed] [Google Scholar]
- 28.Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2006;70(5):769–778. doi: 10.1016/j.ijporl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Sans Capdevila O, Crabtree VM, Kheirandish-Gozal L, Gozal D. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based study. Pediatrics. 2008;121(5):e1208–e1214. doi: 10.1542/peds.2007-2049. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos EI, Kaditis AG, Kostadima E, Gourgoulianis K. Resolution of nocturnal enuresis in snoring children after treatment with nasal budesonide. Urology. 2005;66(1):194. doi: 10.1016/j.urology.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 31. Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr. Pulmonol. 2011;46(4):313–323. doi: 10.1002/ppul.21370.. ▪ Comprehensive review that critically assesses the evidence linking pediatric sleep apnea to systemic inflammation and their interactions with obesity.
- 32.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am. J. Respir. Crit. Care Med. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC.. ▪▪ Provides initial evidence that obstructive sleep apnea syndrome in children adversely affects several components associated with the metabolic syndrome.
- 34. Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in nonhypertensive children: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–691. doi: 10.1378/chest.11-1777.. ▪ Initial assessment of the interactions between obesity and sleep apnea in children and their effects on the vascular endothelium.
- 35.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog. Cardiovasc. Dis. 2009;51(5):416–433. doi: 10.1016/j.pcad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51(1):84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 37.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J. Appl. Physiol. 1994;77(2):918–924. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 38.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27(5):997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 39.Wang RC, Elkins TP, Keech D, Wauquier A, Hubbard D. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol. Head Neck Surg. 1998;118(1):69–73. doi: 10.1016/S0194-5998(98)70377-8. [DOI] [PubMed] [Google Scholar]
- 40.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12(1):25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 41.Rudnick EF, Walsh JS, Hampton MC, Mitchell RB. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol. Head Neck Surg. 2007;137(6):878–882. doi: 10.1016/j.otohns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr. Respir. Rev. 2006;7(4):247–259. doi: 10.1016/j.prrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch. Dis. Child. 2007;92(3):205–208. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 45.Lobstein T, Jackson-Leach R. Child overweight and obesity in the USA: prevalence rates according to IOTF definitions. Int. J. Pediatr. Obes. 2007;2(1):62–64. doi: 10.1080/17477160601103948. [DOI] [PubMed] [Google Scholar]
- 46.Ievers-Landis CE, Redline S. Pediatric sleep apnea: implications of the epidemic of childhood overweight. Am. J. Respir. Crit. Care Med. 2007;175(5):436–441. doi: 10.1164/rccm.200606-790PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am. J. Respir. Crit. Care Med. 1999;159(5 Pt 1):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 48. Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am. J. Respir. Crit. Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC.. ▪▪ Compelling evidence on the low efficacy of adenotonsillectomy as the current treatment of choice for pediatric sleep apnea.
- 49.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6- to 17-year old children – the Tucson Children’s Assessment of Sleep Apnea Study. J. Pediatr. 2010;157(1):57–61. doi: 10.1016/j.jpeds.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136(1):137–144. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J. Clin. Sleep Med. 2011;7(3):268–273. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med. Clin. 2007;2(3):433–444. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc. Am. Thorac. Soc. 2008;5(2):274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tauman R, O’Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116(1):e66–e73. doi: 10.1542/peds.2004-2527. [DOI] [PubMed] [Google Scholar]
- 55.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002;346(11):802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 56.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51(1):204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 57.Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J. Pediatr. 1995;126(5 Pt 1):690–695. doi: 10.1016/s0022-3476(95)70394-2. [DOI] [PubMed] [Google Scholar]
- 58.Sung RY, Tong PC, Yu CW, et al. High prevalence of insulin resistance and metabolic syndrome in overweight/obese preadolescent Hong Kong Chinese children aged 9– years. Diabetes Care. 2003;26(1):250–251. doi: 10.2337/diacare.26.1.250. [DOI] [PubMed] [Google Scholar]
- 59.Invitti C, Guzzaloni G, Gilardini L, Morabito F, Viberti G. Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care. 2003;26(1):118–124. doi: 10.2337/diacare.26.1.118. [DOI] [PubMed] [Google Scholar]
- 60.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middleaged and overweight men. Am. J. Respir. Crit. Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 61.Strohl KP. Diabetes and sleep apnea. Sleep. 1996;19(10 Suppl):S225–S228. doi: 10.1093/sleep/19.suppl_10.s225. [DOI] [PubMed] [Google Scholar]
- 62.Grunstein RR. Metabolic aspects of sleep apnea. Sleep. 1996;19(10 Suppl):S218–S220. doi: 10.1093/sleep/19.suppl_10.s218. [DOI] [PubMed] [Google Scholar]
- 63.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 64.Davies RJ, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnoea and snoring; their comparison with matched controls and response to treatment. J. Sleep Res. 1994;3(3):180–185. doi: 10.1111/j.1365-2869.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 65.Kaditis AG, Alexopoulos EI, Damani E, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr. Pulmonol. 2005;40(6):515–523. doi: 10.1002/ppul.20306. [DOI] [PubMed] [Google Scholar]
- 66. Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. J. Pediatr. 2007;150(6):608–612. doi: 10.1016/j.jpeds.2007.01.051.. ▪ Important study on the association between the metabolic syndrome and sleep apnea in obese children.
- 67.Alexopoulos EI, Gletsou E, Kostadima E, et al. Effects of obstructive sleep apnea severity on serum lipid levels in Greek children with snoring. Sleep Breath. 2011;15(4):625–631. doi: 10.1007/s11325-010-0410-z. [DOI] [PubMed] [Google Scholar]
- 68. Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am. J. Respir. Crit. Care Med. 2007;176(4):401–408. doi: 10.1164/rccm.200703-375OC.. ▪ Important study on the association between metabolic syndrome and sleep apnea in obese adolescents.
- 69. Bhushan B, Khalyfa A, Spruyt K, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12(7):666–671. doi: 10.1016/j.sleep.2010.12.014.. ▪ First study to examine the role of gene polymorphisms on the metabolic profile responses to sleep apnea and obesity in children.
- 70.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24(5):843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9.. ▪▪ Important paper that critically and systematically addresses the effects of intermittent hypoxia on metabolic functions in a nonobese murine model of sleep apnea.
- 72.Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005;99(5):1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. 2007;102(2):557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 74.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am. J. Respir. Crit. Care Med. 2007;175(12):1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 2000;275(39):30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 76.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004;43(2):91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 77. Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol. Genomics. 2007;31(2):273–280. doi: 10.1152/physiolgenomics.00082.2007.. ▪ Interesting paper that defines the role of hepatic pathways on intermittent hypoxia induced hyperlipidemia and lipid peroxidation.
- 78.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ. Res. 2008;103(10):1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Bosch-Marce M, Nanayakkara A, et al. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol. Genomics. 2006;25(3):450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 80.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006;53(6):482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209(2):381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010;174(1–2):156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. (Lond.) 2010;34(Suppl. 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Drager LF, Li J, Shin MK, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur. Heart J. 2012;33(6):783–790. doi: 10.1093/eurheartj/ehr097.. ▪▪ Exploration of alternative aspect of the effects of intermittent hypoxia on lipid metabolism, with emphasis on triglyceride-rich protein clearance.
- 85.Dallinga-Thie GM, Franssen R, Mooij HL, et al. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211(1):1–8. doi: 10.1016/j.atherosclerosis.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J. Clin. Invest. 2007;117(1):94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009;297(2):E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 88.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 89.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J. Physiol. 2003;552(Pt 1):253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 91.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int. J. Obes. Relat. Metab. Disord. 2002;26(2):193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 92.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 2004;350(25):2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 93. Gharib SA, Khalyfa A, Abdelkarim A, et al. Intermittent hypoxia activates temporally coordinated transcriptional programs in visceral adipose tissue. J. Mol. Med. (Berl.) 2011 doi: 10.1007/s00109-011-0830-7. (Epub ahead of print). ▪▪ Transcriptomic analysis of gene–gene interaction networks to identify critical controllers of intermittent hypoxia-induced alterations in metabolic pathways in adipocytes.
- 94.Puri V, Ranjit S, Konda S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl Acad. Sci. USA. 2008;105(22):7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]