Abstract
Objectives
We sought to establish a novel method to generate nano-sized carbon black particles (nano-CBPs) with an average size smaller than 100 nm for examining the inhalation exposure risks of experimental rats. We also tested the effect of nano-CBPs on the pulmonary and circulatory systems.
Methods
We used chemical vapor deposition (CVD) without the addition of any additives to generate nano-CBPs with a particle size (electrical mobility diameter) of less than 100nm to examine the effects of inhalation exposure. Nano-CBPs were applied to a nose-only inhalation chamber system for studying the inhalation toxicity in rats. The effect on the lungs and circulatory system was determined according to the degree of inflammation as quantified by bronchoalveolar lavage fluid (BALF). The functional alteration of the hemostatic and vasomotor activities was measured by plasma coagulation, platelet activity, contraction and relaxation of blood vessels.
Results
Nano-CBPs were generated in the range of 83.3-87.9 nm. Rats were exposed for 4 hour/day, 5 days/week for 4 weeks to 4.2 × 106, 6.2 × 105, and 1.3 × 105 particles/cm3. Exposure of nano-CBPs by inhalation resulted in minimal pulmonary inflammation and did not appear to damage the lung tissue. In addition, there was no significant effect on blood functions, such as plasma coagulation and platelet aggregation, or on vasomotor function.
Conclusion
We successfully generated nano-CBPs in the range of 83.3-87.9 nm at a maximum concentration of 4.2 × 106 particles/cm3 in a nose-only inhalation chamber system. This reliable method can be useful to investigate the biological and toxicological effects of inhalation exposure to nano-CBPs on experimental rats.
Keywords: Nano-sized particle, Carbon black, Nose-only inhalation chamber, Plasma coagulation, Platelet
Introduction
Carbon black (CB; CAS No. 1333-86-4) is virtually pure elemental carbon in the form of colloidal particles that are produced by incomplete combustion or thermal decomposition of gaseous or liquid hydrocarbons under controlled conditions. Current worldwide production is about 8.1 million metric tons per year, which ranks as one of the top 50 industrial chemicals manufactured worldwide. Approximately 90% of CB is used in rubber applications, 9% as a pigment, and the remaining 1% as an essential ingredient in hundreds of diverse applications [1].
Ten workplaces in Korea with 168 workers produce approximately 282,000 tons per year of CB (the Korea Occupational Safety and Health Agency). Additionally, more than 2,100 workers in 379 workplaces are involved in various manufacturing industries that consume approximately 1,823,000 tons of CB as the raw material [2]. Therefore, occupational exposure of CB may be a health problem to Korean workers.
As an ultrafine particle (UFP), CB can disperse in ambient conditions and be readily exposed by inhalation in workplaces. Therefore, the respiratory system serves as the primary target of CB exposure. The inhalation of particulate matters (PM) primarily leads to inflammation in the pulmonary system and, subsequently, spreads to systemic inflammation, which is the general mechanism for PM-induced toxicity [3]. UFPs, generally smaller than 100 nm in size, can even enter into the circulatory system directly, leading to diverse systemic problems such as cardiovascular toxicities and neurological disorders [4]. Recently, UFPs were demonstrated to exhibit general properties in biological systems to some extent, regardless of their chemical composition, e.g., the induction of inflammation, penetration of biological membranes, and generation of free radicals/oxidative stress [5]. These suggest that the biological effects of CB may not be different from those of other UFPs.
In this study, we examined the first line effect of nano-sized CB particles (nano-CBPs) in experimental animal models. To test the biological effect of nano-CBPs, we established the methods to generate CBPs smaller than 100 nm by chemical vapor deposition (CVD). We exposed Sprague-Dawley rats to the resulting particles via a nose-only inhalation chamber. Pathological analysis was performed with lung tissue. Inflammatory cytokines were analyzed with bronchoalveolar lavage fluid (BALF) to test whether nano-CBPs induce inflammation. In addition, plasma coagulability, platelet aggregation and vasomotor function were examined to evaluate the cardiovascular consequence of nano-CBP exposure.
Materials and Methods
Chemicals and reagents
Hematologic reagents for prothrombin time (PT) and activated partial thromboplastin time (aPTT) measurement were from Fisher Diagnostics (Middletown, VA, USA). ELISA kits for interleukin-6 (IL-6), IL-1b, IL-4, IL-10, tumor necrosis factor-α (TNF-α), and interferon-gamma (IFN-γ) were supplied by BD Biosciences (San Diego, CA, USA). Thrombin, phenylephrine and serotonin were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of the highest purity available and purchased from standard suppliers.
Animals
Specific pathogen-free 5-week-old Sprague-Dawley rats were purchased from Central Lab Animal Inc. (Seoul, Korea) and underwent 4-week quarantine and acclimatization before use. The rats were housed in a room maintained at a temperature of 22 ± 3℃ and a relative humidity of 50 ± 20% with artificial lighting from 8 AM to 8 PM and with 12-15 air changes per hour. The rats were housed in polycarbonate cages and allowed sterilized tap water and commercial rodent chow (LabDiet 5053, PMI Nutrition, St. Louis, MO, USA) ad libitum. The rat studies were approved by an animal ethics committee to ensure appropriate animal care before the rats were obtained for research.
Dispersion and characterization of CB with airborne CB generation system
The ethylene used as a precursor was of commercial grade (99.999%, RIGAS, Daejeon, Korea). Argon (99.999%, Deokyang Energen Co., Seosan, Korea) was used as the carrier gas. A picture of the CVD equipment is shown in Fig. 1. The reactor consisted of a vertical quartz tube (D 22 × 1,000 mm) heated by an electric resistance furnace. The temperature was controlled and monitored by a thermocouple. The reactant gases (C2H4 and Ar) were fed into the reactor through electronic mass flow controllers. The effluent gases were tripped by a water cooling system, and effluent gases and CB were diluted in the mixing chamber with air. The flow rates of ethylene and argon were 10 mL/min and 1,300 mL/min, respectively. The furnace temperature was adjusted to 900℃.
Fig. 1.

Picture of the chemical vapor deposition equipment.
Particle size analysis by scanning nano-particle spectrometer and measurement of the particles' weight concentration
Measurements were performed with a scanning nano-particle spectrometer (SNPS; HCT, Icheon, Korea). The SNPS system comprised a condensation particle counter (HCT, Icheon, Korea), which measured the particle count concentration. The instrument measured particles in the size range from 7-300 nm. A Gilian air sampler (Sensidyne Inc., Clearwater, FL, USA) with mixed cellulose ester membrane filter (37 mm, 0.2 µm) was used to measure the weight concentration of CBP. Air was sampled at a rate of 2.0 liter/min in the highest nose-only inhalation chamber.
Nose-only inhalation chamber for CB
Total flow rates (100 L/min) were supplied to 4 nose-only inhalation chambers. The flow rate was considered sufficient for the maximum number of rodents (1.0 L/min/rat) and to monitor the particle concentration. The nose-only inhalation chamber for CB is shown in Fig. 2. The Sprague-Dawley rats were exposed to CB for 4 hour/day, 5 days/week for 4 weeks, after which they were sacrificed on the next day.
Fig. 2.
Nose-only inhalation chamber (A), Scanning Nano-particle Spectrometer and Condensation Particle Counter (B).
Analysis of BALF
The rats were euthanized with isoflurane (Ilsung Pharm, Seoul, Korea). A tracheal cannula was inserted and BAL was performed 10 times through the cannula using 3 mL calcium- and magnesium-free, phosphate buffer solution, pH 7.4. The induction of inflammation was assessed by analyzing the inflammatory cytokines in BALF.
Plasma coagulation test
Plasma coagulability was evaluated by PT and aPTT, which were measured with a Coagulator2 coagulation analyzer (Behnk Elektronik, Norderstedt, Germany) using thromboplastin-D or CaCl2 and APTT-XL reagents (Fisher Diagnostics), respectively, according to the manufacturer's instructions.
Platelet function analysis
Ex-vivo platelet aggregation study was performed with a 4-channel aggregometer (Chrono-log, Havertown, PA, USA). Platelet-rich plasma (PRP) was isolated from CB-exposed rats, as described previously [6]. PRP was preincubated for 5 min at 37℃, and then 5-8 unit/mL thrombin or 20 g/mL collagen was added to induce aggregation.
Assessment of vasomotor function
The rats were sacrificed by decapitation and then exsanguinated. We carefully isolated the thoracic aorta and cut it into ring segments. The prepared aortic rings were mounted in four-channel organ baths filled with Krebs-Ringer solution (115.5 mM NaCl, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 25.0 mM NaHCO3, and 11.1 mM glucose, pH 7.4). The organ bath was continuously gassed with 95% O2/5% CO2 and maintained at 37℃. The rings were stretched gradually to an optimal resting tension of 2 g and equilibrated for 30 min. The change in tension was measured isometrically with Grass FT03 force transducers (Grass Instrument Co., Quincy, MA, USA) and recorded using the PowerLab 8/30 data acquisition system. Data were analyzed with the LabChart pro computer program (AD Instruments, Australia).
Statistical analysis
The means and standard deviation of the means were calculated for all experiment groups. The data were subjected to one way analysis of variance, followed by Dunnett's test to determine significant difference from the control. Statistical analyses were performed using SigmaStat software (Version 3.5, Systat Software, San Jose, CA, USA). In all cases, p-value of < 0.05 was used to determine significance.
Results
Generation and characterization of nano-sized carbon black particles (nano-CBPs)
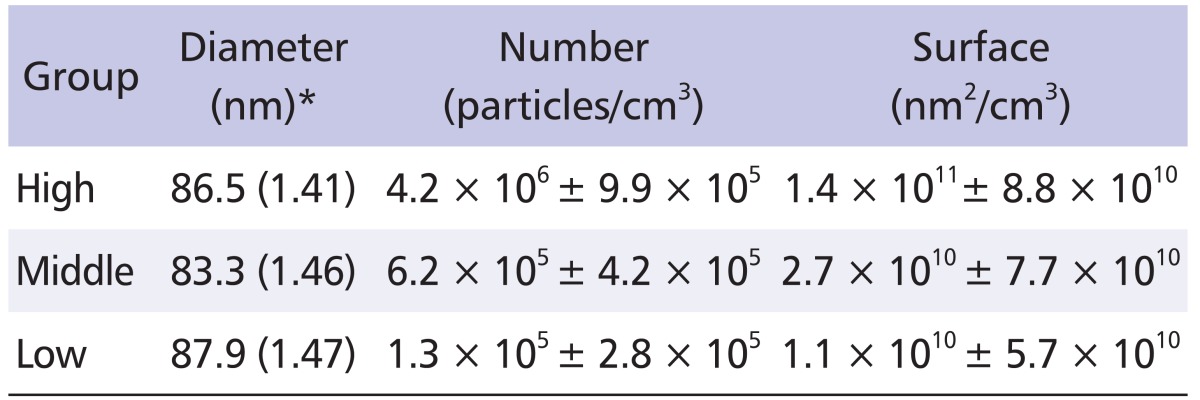
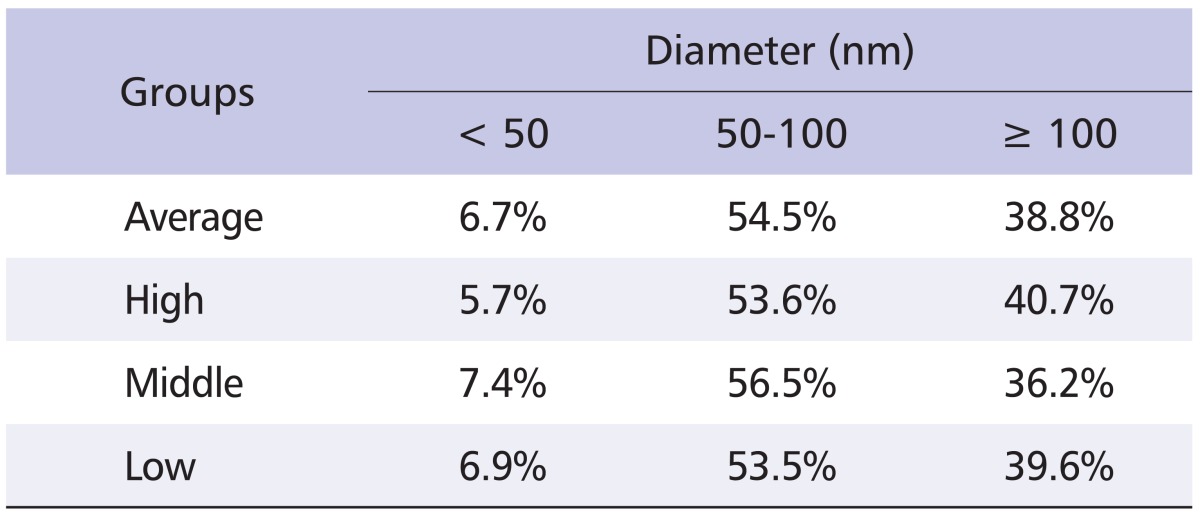
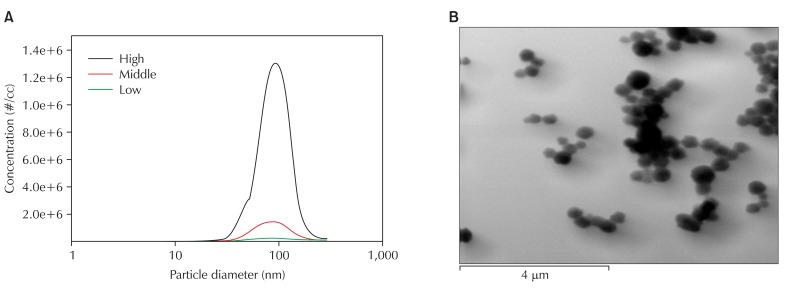
Ultrafine CB was generated by gas phase CVD and the characteristics of the dispersed particles were investigated. We established aerosol generator equipped with quart tube, and dispersed nano-CBPs at concentrations of 104, 105 and 106 particle/cm3 for low, middle, and high levels of exposure, respectively. We thus obtained nano-CBPs with electrical mobility diameters of 86.5 ± 1.41, 83.3 ± 1.46 and 87.9 ± 1.47 nm, which were quite comparable in size, for each level of exposure, respectively. The number concentration was 4.2 × 106, 6.2 × 105 and 1.3 × 105 particle/cm3 for high, middle or low levels of exposure, and the specific surface area was 1.4 × 1011, 2.7 × 1010 and 1.1 × 1010 nm2/cm3, respectively (Table 1). Approximately 61% of particles were smaller than 100 nm (Table 2). The particle morphology was examined with transmission electron microscopy image analysis, which exhibited a mixed presence of individual particles and an aciniform of small aggregates, which is the typical shape mixture of CB (Fig. 3). These results suggest that nano-CBPs were obtained successfully as intended with the gas phase CVD method.
Table 1.
Distribution of carbon black particles (mean ± standard deviation)
*GM (GSD).
Table 2.
Size distribution of carbon black particles
Fig. 3.
Distribution of CB (A) and TEM analysis of CB with TEM (B). CB: carbon black, TEM: transmission electron microscopy.
Effect of nano-CBP exposure on pulmonary inflammation
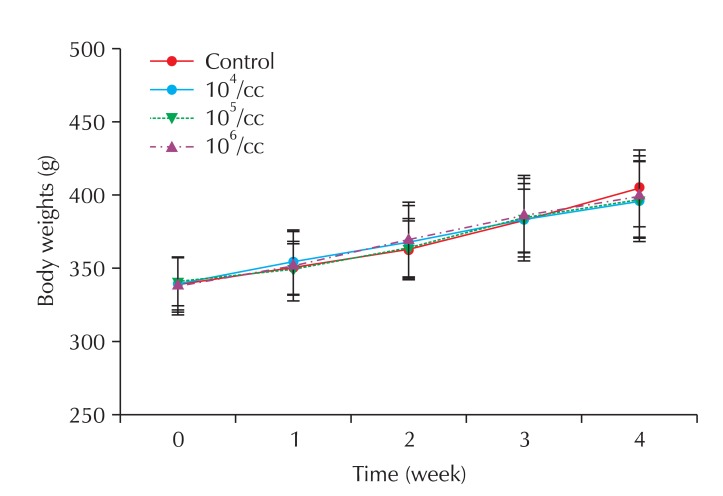
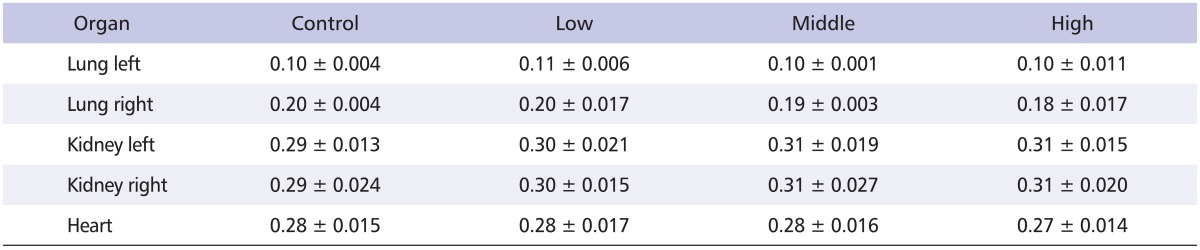
Rats were exposed to nano-CBPs for 4 weeks and the effects on the lungs were examined. All the rats gained weight steadily during the experiment, and no significant difference was observed among the groups (Fig. 4). At the end of the experiment, the weight of the major organs (lung, kidney and heart) did not differ significantly among the treated groups (Table 3).
Fig. 4.
Body weight changes of rats exposed to carbon black.
Table 3.
Relative organ weight in rats of the exposure groups
All values are expressed as mean ± standard deviation (%).
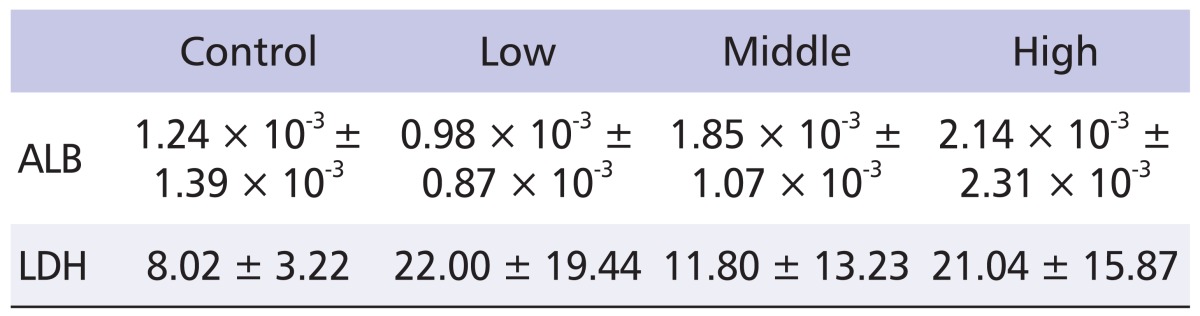
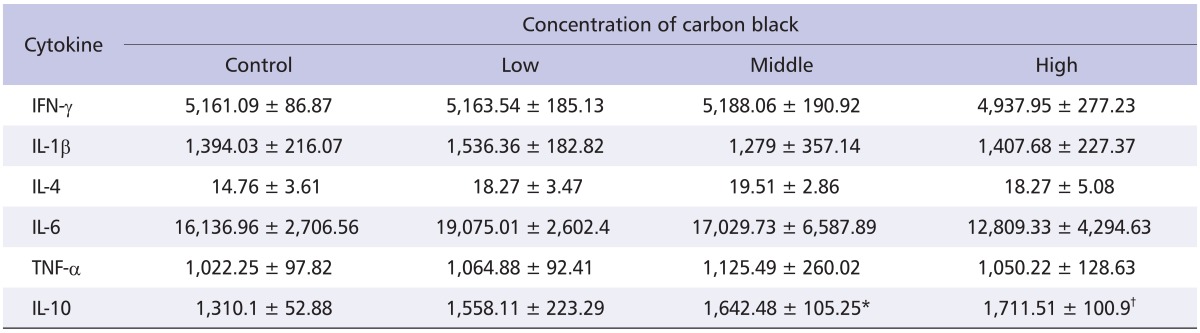
To test if inhalation exposure to nano-CBPs damages the lung tissue, BALF was prepared, and albumin and lactate dehydrogenase (LDH) leakage were measured. However, no detectable increase was observed in either albumin or LDH level (Table 4). Consistent with these results, no pathological symptom was observed in the histological analysis of the lungs. Pulmonary inflammation was examined by analysis of inflammatory cytokines in BALF. As shown in Table 5, most of the samples, including IL-1β, IL-4, IL-6, TNF-α and INF-γ, exhibited little change in levels. However, IL-10 level was significantly elevated in BALF from nano-CBP-exposed rats, and this elevation was CB concentration-dependent (Table 5). These results suggest that exposure of nano-CBPs by inhalation does not damage the lung tissue and only induces minimal inflammation in the respiratory system.
Table 4.
Bronchoalveolar lavage fluid biochemical values in rats of the exposure groups
All values are expressed as mean ± standard deviation.
ALB: albumin (mg/dl), LDH: lactate dehydrogenase (IU/l), n = 5.
Table 5.
Cytokine assay result in bronchoalveolar lavage fluid of standard deviation male rats exposed to carbon black (pg/mL)
*p < 0.05, †p < 0.01, n = 5.
Effect of nano-CBP exposure on blood and vasomotor function
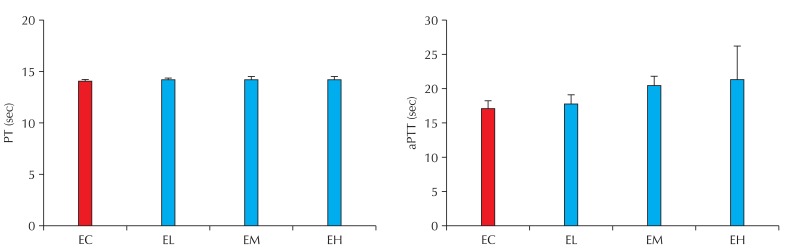
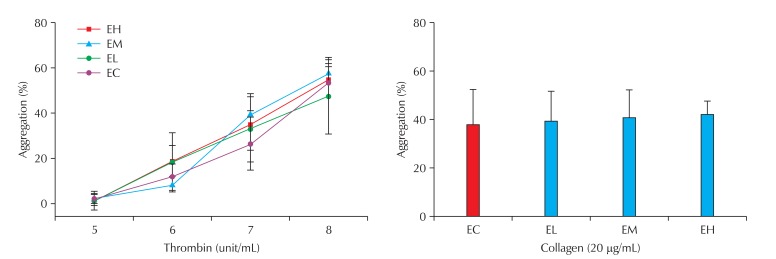
Based on the reports that nano-CBPs are capable of entering the circulatory system and causing circulatory disorders, we evaluated the plasma coagulability and platelet aggregability of blood from rats exposed to nano-CBPs. Plasma coagulability was assessed with PT and aPTT measurements. Four-week exposure to nano-CBPs did not affect PT. However, aPTT tended to increase in the nano-CBP-exposed groups and this increase was dependent on the carbon concentration but was not statistically significant (Fig. 5).
Fig. 5.
Effect of carbon black on plasma coagulation in rats of the exposure groups. PT: prothrombin time, aPTT: activated partial thromboplastin time, EC: control, EL: low, EM: middle, EH: high. n = 5.
The effect of nano-CBP exposure on platelet activity was examined by aggregation study. PRP was isolated from the blood and the aggregation was induced with 20 µg/mL collagen or 5-8 U/mL thrombin. However, the platelet reactivity of the rats exposed to these agonists was not different from that of the control group (Fig. 6). The blood cell numbers, including red blood cells, white blood cells and platelets, showed no significant difference among the treated groups (Table 6). These results suggest that the respiratory exposure of nano-CBPs does not affect the blood cell count and has only a minimal effect on blood functions such as plasma coagulation and platelet aggregation.
Fig. 6.
Effect of carbon black (CB) on platelet activity in rats of the exposure groups. EC: control, EL: low, EM: middle, EH: high. n = 5.
Table 6.
Serum biological values of rats
All values are expressed as mean ± standard deviation.
WBC: white blood cell count (103/mm3), RBC: red blood cell count (106/mm3), PLT: platelet (103/µ3).
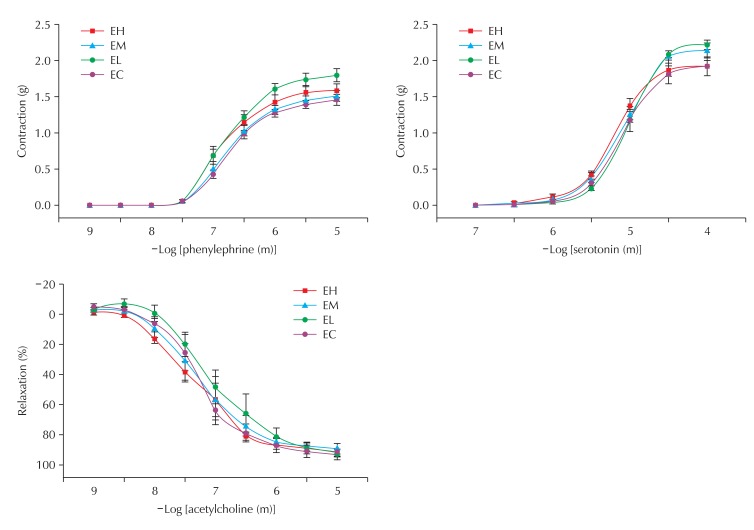
To examine the effect of CB on vascular function, we tested the response of the aorta to vasoconstrictors and vasorelaxant. The aortic rings were isolated from the thoracic aorta and the vasomotor function was tested with a physiometer. The aortas from all experimental groups exhibited normal constriction to serotonin and phenylephrine and relaxation to acetylcholine and there was no significant difference among groups, suggesting that nano-CBPs did not affect vasomotor function (Fig. 7).
Fig. 7.
Effect of carbon black on contraction and relaxation of blood vessels in rats of the exposure groups. EC: control, EL: low, EM: middle, EH: high. n = 5.
Discussion
The aim of this study was to establish a novel method to generate nano-CBPs with an average size smaller than 100 nm for examining the inhalation exposure risks of experimental rats. We tested the effect of nano-CBPs generated by this method on the lungs and circulatory system. We generated 83.3-87.9 nm nano-CBPs up to a concentration of 4.2 × 106 particles/cm3 and successfully exposed experimental rats to these particles in a nose-only inhalation chamber. Inhalation-exposure of nano-CBPs resulted in only minimal pulmonary inflammation, did not damage the lung tissue, and did not significantly affect the blood functions such as plasma coagulation, platelet aggregation and vasomotor function. These study results suggest that subacute inhalation-exposure of nano-CBPs up to about 106 particles/cm3 does not critically affect the tested pulmonary and circulatory systems.
We intended to expose the rats to a higher concentration than 106 particle/cm3 to test the intoxication of nano-CBPs, but generating a higher concentration enlarged the particle size in our experimental system (data not shown), for unknown reasons. Therefore, 106 particles/cm3 was set as the highest exposure concentration. Several studies have reported the generation of CBPs for inhalation exposure. Ma-Hock et al. employed a dust feeder to generate CBPs with a median particle size of 150 nm in scanning mobility particle sizer and 400 nm in optical particle counter [7]. Renwick et al. reported an average particle size of 260.2 nm [8], and 0.6-0.9 µm CBPs were generated by Elder et al. [9]. Recently, Niwa et al. succeeded in generating a relatively smaller particle size ranging from 188 to 122 nm at a high CB concentration of 1.57 ± 0.4 × 104 particles/cm3 [10]. Hence, our work is the first study to successfully generate ultrafine CB with a mean particle size of 83.3-87.9 nm at a high number concentration of up to 4.2 × 106 particles/cm3. This allowed us to investigate the biological effect of nano-CBPs.
The potential for the respiratory exposure of CB to induce pulmonary inflammation has remained controversial. CB is generally regarded as less toxic than other types of carbon-based PM such as single walled carbon nanotube and residual oil fly ash which were reported to elicit toxicity and inflammation in the lungs [11]. Indeed, Li et al. failed to detect the increment of LDH in BALF from the animals treated with CBPs intratracheally [12]. On the other hand, the intratracheal instillation of CBPs at relatively larger amounts of 125 and 500 µg/kg significantly elevated LDH in BALF in experimental animals [8]. In our experiment, the inhalation of CBPs with a diameter of 83.3-87.9 nm neither induced toxicity of the lungs nor elicited pulmonary inflammation up to the concentration of 4.2 × 106 particles/cm3. Intriguingly, the IL-10 level was elevated in BALF with nano-CBPs in a concentration-dependent manner whereas most of the tested cytokines did not change (Table 5). We could not elucidate why only the IL-10 level was increased; however, histological analysis suggested that nano-CBPs do not induce inflammatory responses in the respiratory system despite the increase in IL-10. Further study will be needed to elucidate the consequence of IL-10 elevation.
Most of the studies investigating ultrafine or nano-sized materials revealed that inhaled particles can enter into the circulation directly and may exhibit biological activity, as indeed was the case with CB [13]. Therefore, it is reasonable to assume that CB may translocate into the blood and thereby affect the functions of the circulation system. To test this hypothesis, we examined platelet aggregation, plasma coagulability and vasomotor function, but none was altered by CBP exposure. These results suggest that inhaled nano-CBPs have minimal effect on hemostatic and vascular functions. At present, we could not clarify whether this is because nano-sized CB does not move into circulation or because nano-CB is inert and cannot therefore induce any functional changes. It is also possible that quantity of nano-CBPs reaching circulation is not enough to affect physiological function of circulatory system. In any case, it seems clear that the inhalation of nano-CBPs, up to a substantial exposure level of 106 particles/cm3, does not critically damage the circulatory functions tested in our study.
To summarize, we successfully generated nano-CBPs, and the exposure of these particles to rats did not induce any toxicity or inflammatory response in the lungs or make any functional alteration of the hemostatic and vasomotor activities. The exposure protocol established in this study can be a useful and reliable method to investigate the biological or toxicological effect of inhaled nano-CBPs.
Acknowledgements
This article contains the data from the work at the college of pharmacy, Dong-Guk University, and was supported by grants from OSHRI (Grant No. 2010-119-968 to Lee MY).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.What is Carbon Black? [Internet] Boston (MA): International Carbon Black Association; 2005. [cited 2011 Feb 1]. Available from http://www.carbon-black.org/index.html. [Google Scholar]
- 2.Surveys on Work Environment in Korea, 2009. Incheon (Korea): Korea Occupational Safety and Health Agency, Ministry of Employment and Labor (KR); 2009. [Google Scholar]
- 3.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 4.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 5.Stone V, Donaldson K. Nanotoxicology: signs of stress. Nat Nanotechnol. 2006;1:23–24. doi: 10.1038/nnano.2006.69. [DOI] [PubMed] [Google Scholar]
- 6.Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol Appl Pharmacol. 2002;179:83–88. doi: 10.1006/taap.2001.9356. [DOI] [PubMed] [Google Scholar]
- 7.Ma-Hock L, Gamer AO, Landsiedel R, Leibold E, Frechen T, Sens B, Linsenbuehler M, van Ravenzwaay B. Generation and characterization of test atmospheres with nanomaterials. Inhal Toxicol. 2007;19:833–848. doi: 10.1080/08958370701479190. [DOI] [PubMed] [Google Scholar]
- 8.Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, Oberdörster G. Effects of subchronically inhaled carbon black in three species I Retention kinetics, lung inflammation, and histopathology. Toxicol Sci. 2005;88:614–629. doi: 10.1093/toxsci/kfi327. [DOI] [PubMed] [Google Scholar]
- 10.Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72:144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- 11.Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]