Abstract
Objectives
Studies examining healthcare workers' exposure to antineoplastic drugs have focused on the drug preparation or drug administration areas. However, such an approach has probably underestimated the overall exposure risk as the drugs need to be delivered to the facility, transported internally and then disposed. The objective of this study is to determine whether drug contamination occurs throughout a facility and, simultaneously, to identify those job categories that are potentially exposed.
Methods
This was a multi-site study based in Vancouver, British Columbia. Interviews were conducted to determine the departments where the drugs travel. Subsequent site observations were performed to ascertain those surfaces which frequently came into contact with antineoplastic drugs and to determine the job categories which are likely to contact these surfaces. Wipe samples were collected to quantify surface contamination.
Results
Surface contamination was found in all six stages of the hospital medication system. Job categories consistently found to be at risk of exposure were nurses, pharmacists, pharmacy technicians, and pharmacy receivers. Up to 11 job categories per site may be at risk of exposure at some point during the hospital medication system.
Conclusion
We found drug contamination on select surfaces at every stage of the medication system, which indicates the existence of an exposure potential throughout the facility. Our results suggest that a broader range of workers are potentially exposed than has been previously examined. These results will allow us to develop a more inclusive exposure assessment encompassing all healthcare workers that are at risk throughout the hospital medication system.
Keywords: Antineoplastic drugs, Occupational exposure, Healthcare, Hospital medication system
Introduction
Antineoplastic (cytotoxic) drugs are widely used agents for the treatment of cancer. Some of these drugs act by interfering directly with the deoxyribonucleic acid (or its synthesis) of tumour cells and thereby interrupt their growth. Unfortunately, antineoplastic drugs are generally non-selective and therefore normal (non-tumour) cells may also be damaged which, in turn, results in toxic effects [1]. Given this, there is a risk to healthcare workers who handle, prepare, and/or administer antineoplastic drugs.
Numerous studies have examined antineoplastic drug contamination in healthcare facilities. Studies from several countries have demonstrated surface contamination of biological safety cabinets, countertops, cabinets and floors within the drug preparation area [2-6]. Detectable levels of environmental drug contamination have also been found in patient care areas where antineoplastic drugs are administered [7]. A recently published summary of the contamination levels found in the literature reported that cyclophosphamide (CP) ranged in concentration from not detected to 3,834 nanograms per square centimeter (ng/cm2), which suggests that existing control measures are not effective in reducing contamination levels [8]. In the absence of any current occupational exposure limits for CP (and most other antineoplastic drugs), it is therefore important to minimize contamination.
A literature review revealed that these surface contamination studies have primarily focused on two departments within a healthcare facility - the pharmacy, where the drugs are prepared, and the administration units where the prepared drugs are given to patients. The emphasis on these two departments is warranted since direct handling of the drugs is expected during both preparation and administration. However, given the fact that the drugs need to be initially delivered to the pharmacy, then transported to the wards and eventually disposed of as part of the hospital medication system (process flow of the drugs throughout a facility from cradle-to-grave), it is conceivable that other areas of a healthcare facility may have drug residual and, therefore, the number of healthcare workers at risk of exposure is underestimated.
The potential for other areas to be contaminated with antineoplastic drugs and, in turn, for additional healthcare workers to be at risk of occupational exposure is supported by the mechanisms of drug contamination spread that have been proposed in the literature. Surface contamination may arise as early as the facility receiving stage in the hospital medication system as it has been documented that drug vials are often contaminated on the outside [9-11]. It is also possible that drug residue is spread by the footwear of workers or during cleaning of floors and that external drug contamination on gloves may be transferred to other objects or surfaces [12]. Overall, this suggests a need to examine the healthcare facility as a whole, not just the pharmacy and drug administration units, to determine the extent of antineoplastic drug contamination from the point at which the agents are received at the facility through to disposal of or excretion. To our knowledge, no existing literature has investigated this issue.
The current study aims to identify surfaces throughout the hospital medication system whereby antineoplastic drug contamination may be possible and to ascertain the various healthcare job categories that may be at risk of dermal exposure to antineoplastic drugs via contact with the contaminated surfaces - not just drug administration nurses and pharmacy personnel.
Materials and Methods
Selection of participating sites
Participating sites were selected from healthcare facilities situated within the Metro Vancouver area of British Columbia, Canada that prepare and administer CP, the marker drug investigated in this study. The sites finalized for inclusion were determined by asking a pharmacy member from each participating health administrative authority which of their facilities is the largest users of CP on an annual basis (based on overall frequency of compounding). In total, 5 major acute care hospitals and 1 cancer treatment centre participated in the study.
Informant interviews
Interviews with key informants were conducted in order to understand the site-specific hospital medication system and to predict how and where a worker may be exposed to antineoplastic drugs. Key informants included supervisors/managers, clinical nurse leaders, and team leaders such as the senior pharmacy technician. At all sites, the initial interview was conducted in the pharmacy department as their personnel would be familiar with how the drugs arrive at their department and where the prepared drugs are transported for eventual administration. Subsequently, additional interviews were scheduled with those departments identified by the pharmacy informant as being part of the hospital medication system. All departments at each site involved in the hospital medication system were interviewed with the exception of housekeeping which is operated by the same external contractor at each site; the company declined to participate in the study.
The duration of each interview was at least twenty minutes. All interviewees were asked a series of standard questions related to the shift when antineoplastic drugs are primarily handled, prepared or administered, circumstances under which workers may be exposed to antineoplastic drugs, and the likely job categories which may be at risk of exposure. In addition, pharmacy personnel were interviewed about their understanding of the hospital medication system at their site.
Site observations
Passive site observations at each site were conducted by members of the research team to visually establish the hospital medication system and to identify which surfaces/objects may be contaminated with antineoplastic drugs as well as those job categories potentially at risk of dermal exposure via contact with the contaminated surfaces [13]. Employees were considered "at-risk" if they physically handled the drugs, contacted a potentially drug-contaminated surface/object, or used an object previously touched by another worker suspected of having drug-contaminated hands/gloves. A standard observation checklist was developed and used to record: a) the surfaces/objects which came into contact with the drug products, b) the job category of the worker that contacted the drugs and/or the contaminated surfaces and c) the associated frequency of contact of surfaces/objects for each worker.
At all sites, the observations began in the pharmacy department where antineoplastic drug preparation took place. We then followed the drugs as they were transported to the unit where they would be administered. Where prepared drugs were delivered to more than one unit, we randomly selected one unit to follow the drug. We conducted observations of the other unit(s) on separate occasions. There was considerable variation with respect to delivery times of the antineoplastic drugs to the healthcare facility. To accommodate this, we scheduled site visits to specifically observe the receiving process but did not necessarily review other areas of the hospital medication system.
Overall, in order to understand the entire hospital medication system, each site was observed on at least 5 separate occasions over a course of twelve months starting in June 2009. For each site visit, at least 1 task cycle was observed in each department. By performing repeated observations the sequence of each site's hospital medication system was determined and the job categories with potential risk of exposure to antineoplastic drugs were identified.
Surface sampling and analyses
The contact frequency of a surface/object was averaged over the number of observation periods at each site and then ranked by order of frequency for each stage of the hospital medication system. At each participating site, the top 5 most frequently contacted surfaces from the drug preparation and drug administration stage were selected for sampling and the top 3 most frequently contacted surfaces were sampled from all other hospital medication system stages. The rationale for sampling a varying number of surfaces is that drug preparation and drug administration are more complex tasks with greater variability than other stages and therefore more surfaces/objects are contacted overall. Surfaces were sampled and analyzed according to a previously described procedure [14]. Wipes were analyzed for the amount of CP present using high-performance liquid chromatography-tandem mass spectrometry and results reported in nanograms per wipe (ng/wipe).
Data analysis
A histogram was developed for the type and frequency of surfaces contacted at each stage. Summary statistics are presented for the drug contamination levels found at each stage of the medication circuit. Data were analyzed and figures generated using SPlus 8.0 for Windows (Insightful Corp., Seattle, WA, USA).
Theory
A thorough understanding of the medication system within a hospital is necessary to determine those surfaces/objects throughout the facility which may be contaminated with antineoplastic drugs. In turn, it is then possible to ascertain the various categories of healthcare workers that may contact the aforementioned surfaces/objects as dermal exposure is the primary route of occupational exposure to antineoplastic drugs [15-17]. Subsequently, this information can be used to provide guidance in developing an appropriate sampling strategy to assess occupational exposure to antineoplastic drugs of all at-risk healthcare job categories as part of a future study.
Results
General hospital medication system
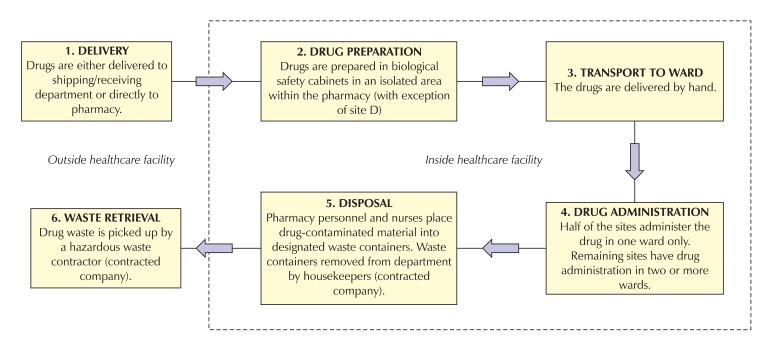
As shown in Fig. 1, the general sequence of the hospital medication system for each of the participating facilities has a minimum of the following 6 stages: 1) delivery of the antineoplastic drugs to the facility, 2) drug preparation, 3) transport to ward, 4) drug administration, 5) disposal and, 6) waste retrieval. As disposal of the waste containers and waste retrieval is performed by contracted companies that declined to participate in the study, more specific details for these 2 steps were unavailable.
Fig. 1.
Overview of the hospital medication system of antineoplastic drugs at the participating facilities.
Description of participating facilities
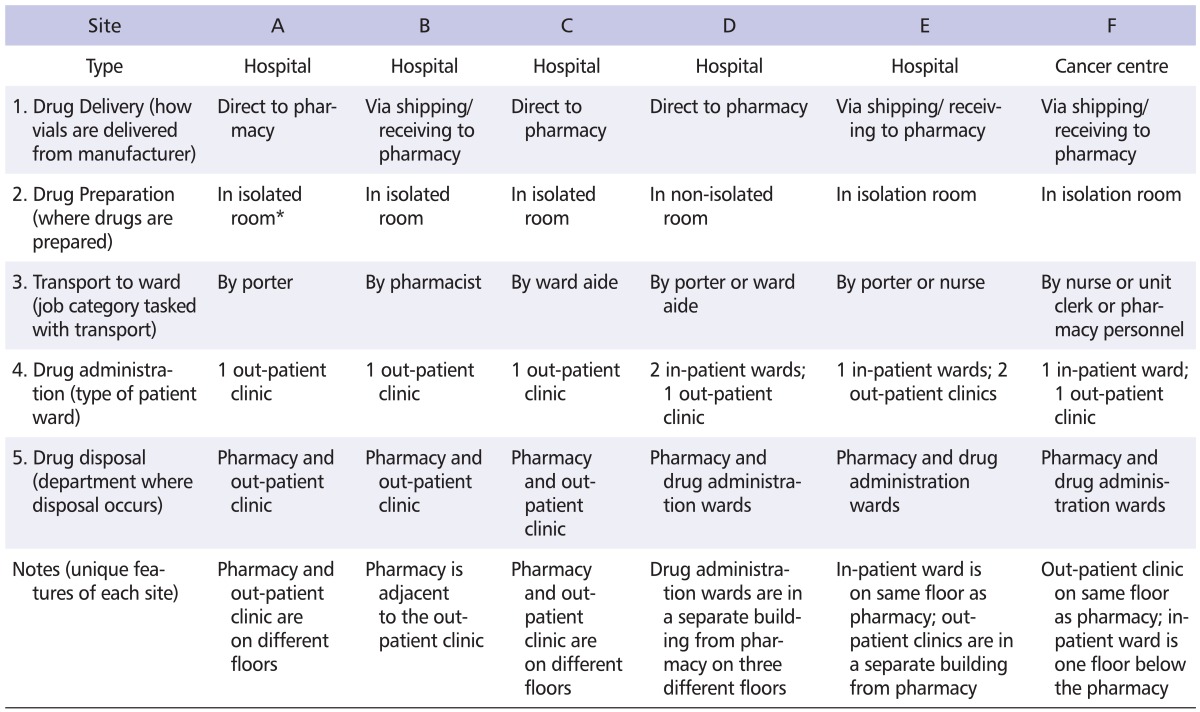
Table 1 provides a descriptive summary of each of the participating facilities. All facilities are acute care hospitals except Site F which is a cancer centre. Sites B, E and F have the drugs initially delivered to the shipping/receiving department whereas the remaining sites have the drugs delivered directly to the pharmacy department. The antineoplastic drugs are prepared in dedicated isolation rooms situated in the pharmacy department, except at Site D where they are prepared adjacent to an area for drugs that are not used for chemotherapy.
Table 1.
Description of participating facilities
*Isolated: room is designated strictly for antineoplastic drug preparation, non-isolated: room is open-concept with biological safety cabinets for preparing antineoplastic drugs and other hoods for preparing non-cytotoxic drugs.
The prepared drugs are delivered by various job categories to the administration wards, with one site (Site F) having up to 3 different job categories performing this task. Drugs are either administered in an out-patient clinic or within in-patient wards. The antineoplastic drugs are administered in 3 wards at Sites D and E. In all instances, the antineoplastic drugs are disposed of within the pharmacy (the manufacturers' vials) as well as in the drug administration wards (the intravenous bags). The "notes" section of Table 1 shows that each facility is designed slightly differently from the others. In most instances, the pharmacy, where drug preparation takes place, is situated on a different floor from where the drug administration takes place.
Contact frequency of work surfaces/objects
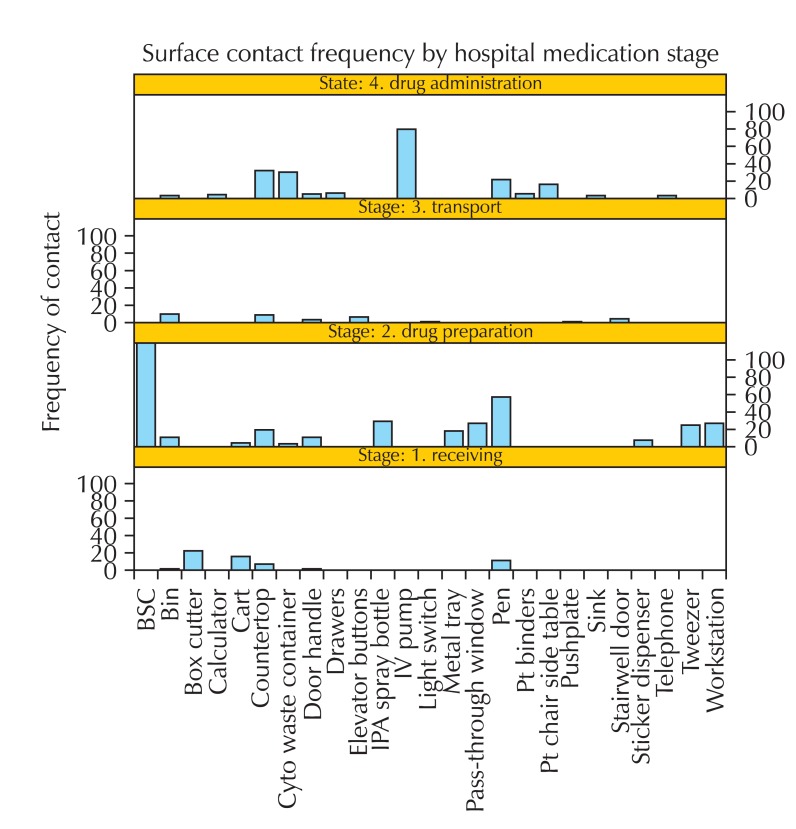
Fig. 2 displays the frequency of surfaces that came into contact with antineoplastic drugs and/or potentially drug-contaminated surfaces contacted by healthcare staff for each stage of the medication system. The box cutter is the most frequently contacted object during drug delivery as it is used to open packages of drug shipments.
Fig. 2.
Surface contact frequency by hospital medication system stage. BSC: biological safety cabinet, IPA: isopropyl alcohol, IV: intravenous, Pt: patient.
With respect to drug preparation, the biological safety cabinet is the most frequently contacted surface as antineoplastic drugs are prepared in these cabinets at all sites. The next most frequently contacted object during drug preparation is the writing instrument found inside the biological safety cabinet. The writing instrument is used by the Pharmacy Technician to label the prepared drugs and/or verify drug dosages. Following these two surfaces/objects, there is a great deal of variability with respect to what is contacted and how frequently they are contacted.
In the third stage of the medication circuit, drug transport within the facility, Fig. 2 shows that the bin where the prepared drugs are held for pick up is the most frequently contacted surface.
During drug administration the intravenous pump is the object that is most contacted by workers because virtually all of the drugs observed were in solution form and had to be administered intravenously using the mechanical pump. Similar to the drug preparation stage, there is a subsequent assortment of surfaces which are contacted across the sites during drug administration.
Surface contamination levels
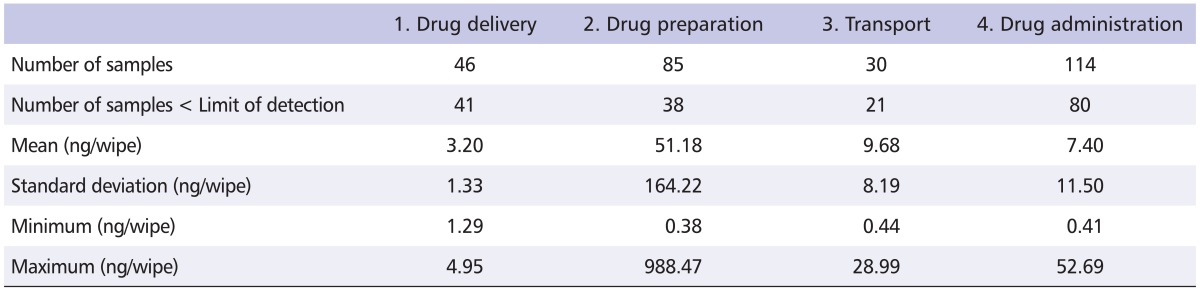
Of the 275 surface samples collected at the participating sites, 180 (65%) were less than the limit of detection. Table 2 summarizes the CP contamination levels and reveals detectable levels of residual drug contamination at each stage of the medication circuit.
Table 2.
Surface contamination levels at each stage of the hospital medication system
Job categories at risk of exposure
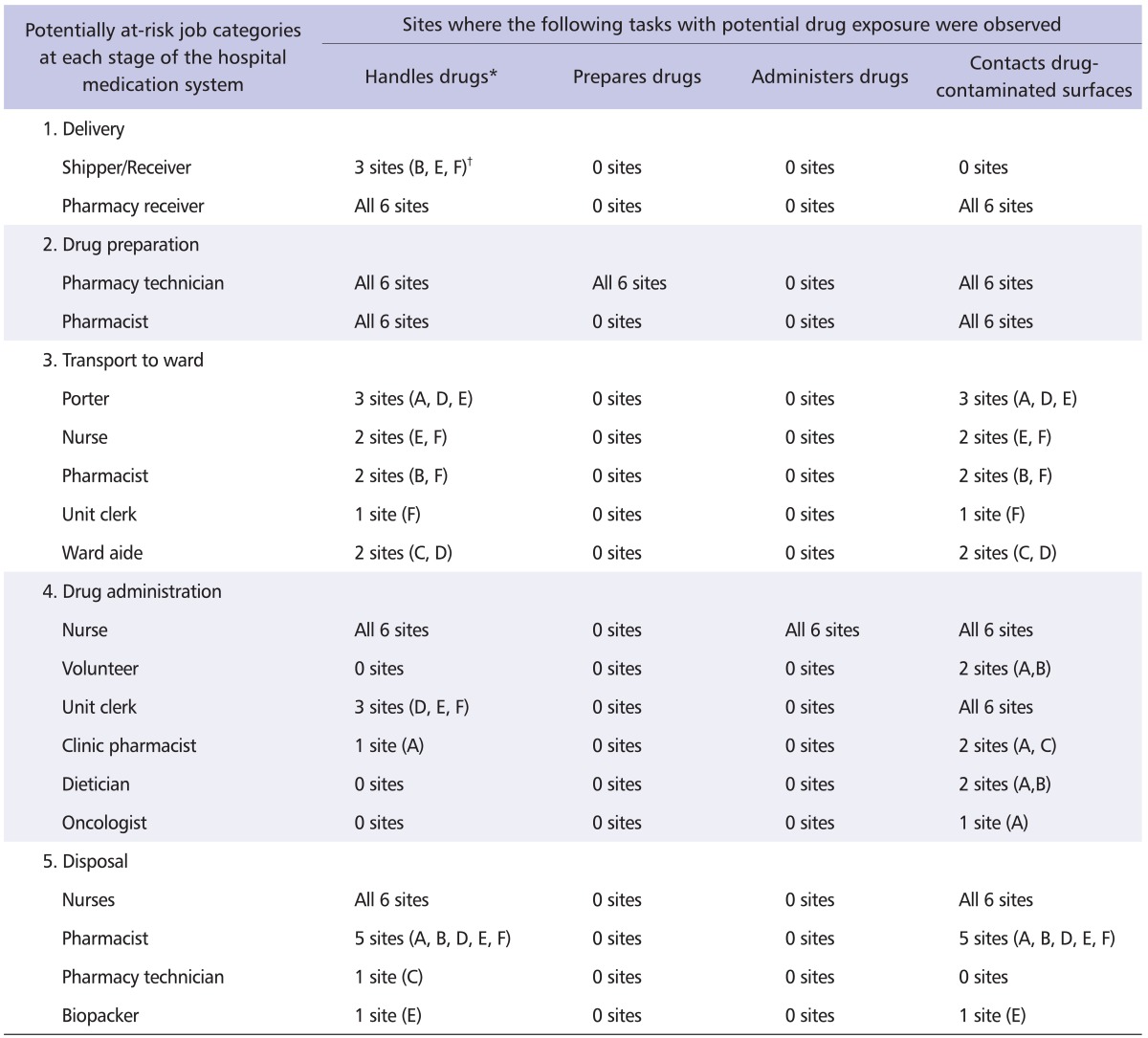
Table 3 summarizes the stages of the hospital medication system with the corresponding job categories that may be at risk of exposure at each of the participating sites. The pharmacy receiver, pharmacy technician, pharmacist, and nurse are potentially exposed to antineoplastic drugs at all sites. During transport of the prepared drugs to the ward, various job categories are at risk of exposure via handling of the antineoplastic drugs. Among the stages of the medication hospital medication system, drug administration has the most job categories (six) at potential risk of exposure.
Table 3.
Observed job categories and associated tasks with potential for dermal exposure at each healthcare facility
*Includes shipments, drug vials, intravenous bags and waste containers.
†Letters refers to participating sites as per Table 1.
With respect to the disposal of the drug products, Site E had a unique job category known as "biopackers" who are responsible for transferring the sealed waste containers from a holding area to a waste disposal room, where the containers would subsequently be picked-up by a waste disposal company.
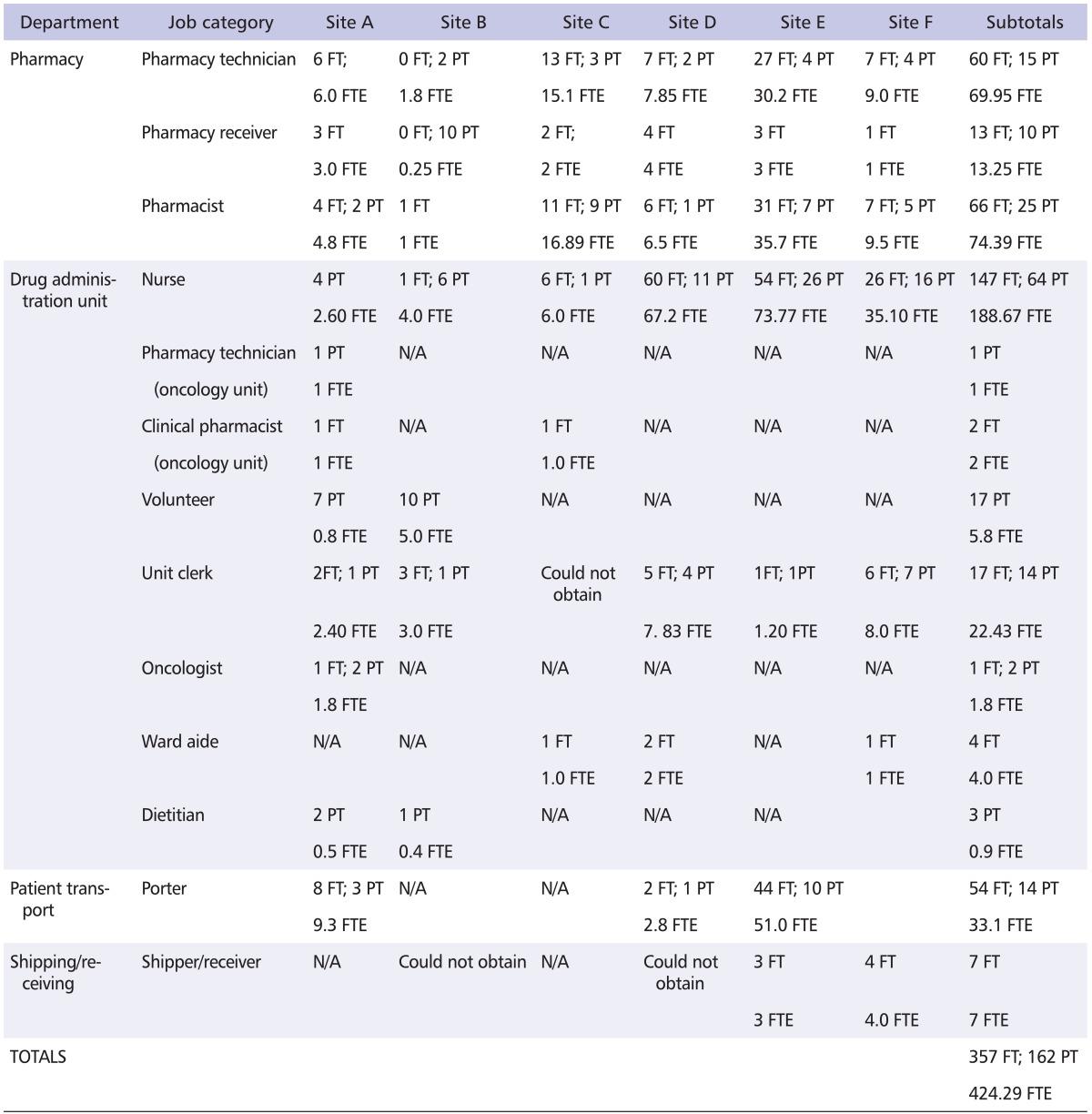
Based on the information provided by the key personnel during the interviews, it is estimated that over 500 workers at the six participating sites may have been occupationally exposed. The distribution of the various job categories at each site that are potentially exposed is shown in Table 4.
Table 4.
Number of workers per at-risk job category at participating sites
FT: full-time, PT: part-time, FTE: full-time equivalent, N/A: not applicable to the site.
Discussion
We hypothesized that contamination of surfaces with antineoplastic drugs occurs at every stage of the hospital medication system. Through observation and subsequent sampling of the most frequently contacted surfaces, our study results prove that surface contamination, and hence the potential for occupational exposure to antineoplastic drugs, does occur at every stage of the hospital medication system. Those job categories most likely to be exposed are pharmacy receiver, pharmacy technician, pharmacist, and nurse as these cohorts were consistently observed to be in contact with antineoplastic drugs at each of the 6 participating sites. Up to 11 job categories (not including housekeeping) per site are potentially at risk of exposure. The characteristics of a site that results in the most number of job categories exposed include: a) having the drugs initially delivered to the shipping/receiving department (as opposed to directly to the pharmacy), b) having multiple job categories responsible for transport of prepared drugs to the ward(s) and, c) having more than one drug administration unit.
Our study builds on the list of healthcare workers exposed to antineoplastic drugs due to handling activities developed by the National Institute for Occupational Safety and Health (NIOSH) [18]. In addition to the cohorts specified by NIOSH, our results suggest that it is reasonable to add the following personnel to the existing list: unit clerk, porter, ward aide, dietitian, oncologist, biopacker and shipper/receiver.
Although our study primarily focused on occupational exposure, it is conceivable that patients, family members, and friends are also at risk of exposure, as suggested by Sorsa et al [19]. This is because communal objects such as elevator buttons, door handles and patient chair side tables, which may be contacted by non-hospital personnel, were found to be contaminated in our study.
The finding of surface contamination throughout the entire hospital medication system implies that healthcare workers contacting these surfaces are at risk of exposure via the dermal route. Our calculations suggest that more than 500 workers at the 6 participating sites are at risk. If this figure were extrapolated to all sites where antineoplastic drugs are handled in the province of British Columbia, then a conservative estimate of the number of healthcare workers at risk is 3,500 [20]. Given the number of workers potentially exposed to these hazardous agents, members of our research team are currently conducting a quantitative exposure assessment of those job categories believed to be at risk.
Among the study limitations, we were unable to ascertain the frequency of contact associated with housekeeping personnel as they are employees of a company that declined to participate in our study. We can surmise that housekeepers would contact the cytotoxic waste containers but cannot estimate the contact risk for other surfaces. As waste containers were found to have detectable levels of drug residue, we can infer that housekeepers do indeed face an exposure potential. Another limitation is the possible absence of certain job categories and/or surfaces in our results despite repeated site observations. Nevertheless, since we performed the site observations on multiple days and observed different individuals, we are confident that we have captured a reasonably representative understanding of the hospital medication system, the job categories commonly at risk of exposure and the potentially contaminated work surfaces at each site.
To our knowledge, this is the first study of its kind to examine the occupational exposure potential to antineoplastic drugs throughout the entire hospital medication system of a healthcare facility. Based on the study results, we are now able to develop an appropriate sampling strategy for all job categories considered at risk of exposure. This is important in order to validate that existing control measures are not only appropriate in reducing occupational exposure to antineoplastic drugs but that they are comprehensive in scope to protect all at-risk job categories within a healthcare facility.
Acknowledgements
We are indebted to the participating hospitals as well as the personnel interviewed and/or observed. The authors would like to thank the WorkSafeBC Research Secretariat for funding this project. We would like to acknowledge the efforts of Claire Pitcher, Jennifer Shum, Pearl Siganporia, Sarah Chiarello and Alexandra Barzan who were responsible for conducting the site observations and/or collecting wipe samples.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sessink PJ, Bos RP. Drugs hazardous to healthcare workers. Evaluation of methods for monitoring occupational exposure to cytostatic drugs. Drug Saf. 1999;20:347–359. doi: 10.2165/00002018-199920040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sessink PJ, Boer KA, Scheefhals AP, Anzion RB, Bos RP. Occupational exposure to antineoplastic agents at several departments in a hospital. Environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workers. Int Arch Occup Environ Health. 1992;64:105–112. doi: 10.1007/BF00381477. [DOI] [PubMed] [Google Scholar]
- 3.Connor TH, Anderson RW, Sessink PJ, Broadfield L, Power LA. Surface contamination with antineoplastic agents in six cancer treatment centers in Canada and the United States. Am J Health Syst Pharm. 1999;56:1427–1432. doi: 10.1093/ajhp/56.14.1427. [DOI] [PubMed] [Google Scholar]
- 4.Siderov J, Kirsa S, McLauchlan R. Surface contamination of cytotoxic chemotherapy preparation areas in Australian Hospital Pharmacy Departments. J Pharm Pract Res. 2009;39:117–121. [Google Scholar]
- 5.Schmaus G, Schierl R, Funck S. Monitoring surface contamination by antineoplastic drugs using gas chromatography-mass spectrometry and voltammetry. Am J Health Syst Pharm. 2002;59:956–961. doi: 10.1093/ajhp/59.10.956. [DOI] [PubMed] [Google Scholar]
- 6.Acampora A, Castiglia L, Miraglia N, Pieri M, Soave C, Liotti F, Sannolo N. A case study: surface contamination of cyclophosphamide due to working practices and cleaning procedures in two Italian hospitals. Ann Occup Hyg. 2005;49:611–618. doi: 10.1093/annhyg/mei029. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo D, Ursini CL, Perniconi B, Francesco AD, Giglio M, Rubino FM, Marinaccio A, Iavicoli S. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat Res. 2005;587:45–51. doi: 10.1016/j.mrgentox.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Touzin K, Bussières JF, Langlois E, Lefebvre M. Evaluation of surface contamination in a hospital hematology--oncology pharmacy. J Oncol Pharm Pract. 2009;15:53–61. doi: 10.1177/1078155208096904. [DOI] [PubMed] [Google Scholar]
- 9.Connor TH, Sessink PJ, Harrison BR, Pretty JR, Peters BG, Alfaro RM, Bilos A, Beckmann G, Bing MR, Anderson LM, Dechristoforo R. Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: results of three studies. Am J Health Syst Pharm. 2005;62:475–484. doi: 10.1093/ajhp/62.5.475. [DOI] [PubMed] [Google Scholar]
- 10.Mason HJ, Morton J, Garfitt SJ, Iqbal S, Jones K. Cytotoxic drug contamination on the outside of vials delivered to a hospital pharmacy. Ann Occup Hyg. 2003;47:681–685. doi: 10.1093/annhyg/meg078. [DOI] [PubMed] [Google Scholar]
- 11.Hedmer M, Georgiadi A, Bremberg ER, Jönsson BA, Eksborg S. Surface contamination of cyclophosphamide packaging and surface contamination with antineoplastic drugs in a hospital pharmacy in Sweden. Ann Occup Hyg. 2005;49:629–637. doi: 10.1093/annhyg/mei042. [DOI] [PubMed] [Google Scholar]
- 12.Zeedijk M, Greijdanus B, Steenstra FB, Uges DR. Monitoring exposure of cytostatics on the hospital ward. Eur J Hosp Pharm Sci. 2005;11:18–22. [Google Scholar]
- 13.Shih TS, Lu PY, Chen CH, Soo JC, Tsai CL, Tsai PJ. Exposure profiles and source identifications for workers exposed to crystalline silica during a municipal waste incinerator relining period. J Hazard Mater. 2008;154:469–475. doi: 10.1016/j.jhazmat.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Chu WC, Hon CY, Danyluk Q, Chua PP, Astrakianakis G. Pilot assessment of the antineoplastic drug contamination levels in British Columbian hospitals pre- and post-cleaning. J Oncol Pharm Pract. 2011 doi: 10.1177/1078155211402106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health. 2005;78:403–412. doi: 10.1007/s00420-004-0595-1. [DOI] [PubMed] [Google Scholar]
- 16.Kromhout H, Hoek F, Uitterhoeve R, Huijbers R, Overmars RF, Anzion R, Vermeulen R. Postulating a dermal pathway for exposure to anti-neoplastic drugs among hospital workers. Applying a conceptual model to the results of three workplace surveys. Ann Occup Hyg. 2000;44:551–560. doi: 10.1016/s0003-4878(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 17.Sessink PJ, Van de Kerkhof MC, Anzion RB, Noordhoek J, Bos RP. Environmental contamination and assessment of exposure to antineoplastic agents by determination of cyclophosphamide in urine of exposed pharmacy technicians: is skin absorption an important exposure route? Arch Environ Health. 1994;49:165–169. doi: 10.1080/00039896.1994.9940377. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Occupational Safety and Health. NIOSH Alert: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. Cincinatti (OH): US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 2004. DHHS (NIOSH) Publication No. 2004-165. [Google Scholar]
- 19.Sorsa M, Hameila M, Jarviluoma E. Handling anticancer drugs. Ann NY Acad Sci. 2006;1076:628–634. doi: 10.1196/annals.1371.008. [DOI] [PubMed] [Google Scholar]
- 20.Occupational Exposure Estimates - Antineoplastic Agents [Internet] Vancouver (BC): CAREX Canada; 2011. [cited 30 May 2011]. Available from: http://www.carexcanada.ca/en/antineoplastic_agents/occupational_exposure_estimates/phase_2/ [Google Scholar]