Abstract
Objectives
This study was designed to investigate whether long-term, low-level exposure to monocyclic aromatic hydrocarbons (MAHs) induced insulin resistance.
Methods
The subjects were 110 male workers who were occupationally exposed to styrene, toluene, and xylene. One hundred and ten age-matched male workers who had never been occupationally exposed to organic solvents were selected as a control group. Cytokines, which have played a key role in the pathogenesis of insulin resistance, and oxidative stress indices were measured. Assessment of exposure to MAHs was performed by measuring their ambient levels and their urinary metabolites in exposed workers, and the resulting parameters between the exposed group and non-exposed control groups were compared.
Results
There was no significant difference in general characteristics and anthropometric parameters between the two groups; however, total cholesterol, fasting glucose, fasting insulin, and homeostasis model assessment of insulin resistance levels were significantly higher in the exposed group. Phenylglyoxylic acid levels showed significant association with tumor necrosis factor-α, total oxidative status, and oxidative stress index via multiple linear regression analysis. Further, there was a negative correlation between methylhippuric acid levels and total anti-oxidative capacity, and there was a significant relationship between MAHs exposure and fasting glucose levels, as found by multiple logistic regression analysis (odds ratio = 3.95, 95% confidence interval = 1.074-14.530).
Conclusion
This study indicated that MAHs increase fasting glucose level and insulin resistance. Furthermore, these results suggested that absorbing the organic solvent itself and active metabolic intermediates can increase oxidative stress and cytokine levels, resulting in the changes in glucose metabolism and the induction of insulin resistance.
Keywords: Aromatic hydrocarbons, Insulin resistance, Cytokines, Oxidative stress
Introduction
Organic solvents are widely used at home as well as in workplaces, and their health hazards have been known for a long time. Diverse hazardous chemicals, including organic solvents that humans are occupationally or non-occupationally exposed to, have their own physicochemical characteristics with various absorption routes and target organs. They have been demonstrated to cause toxicity in various organs [1] including the liver [2,3], hematopoietic organs [4], nervous system [5], immunoendocrinological system [6], kidney, cardiovascular system, skin, mucosa, and reproductive organs, and have been reported to decrease or damage the body homeostasis maintenance function [7].
Some studies have reported that exposure to organic solvents may be related to insulin resistance (IR) or associated metabolic syndrome (MetS). Kaukiainen et al. [8] reported that workers exposed to organic solvent mixtures had higher fasting glucose levels, and Hong et al. [9] mentioned that environmental contaminants including organic solvents may result in IR. Although the relationship between organic solvent exposure and IR has not been directly described, it has been reported that exposure to organic solvents increased cytokines including tumor necrosis factor-alpha (TNF-α) which are involved in inflammatory responses [6,10] and increased oxidative stress while reducing the activities of antioxidant enzymes [11,12]. It has also been reported that increased cytokines such as TNF-α, interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) play a critical role in the development of IR as well as being involved in immunological reactions and signal transductions [13,14]; and that increased reactive oxygen species (ROS) are involved in blood glucose elevation and development of IR through activating c-Jun NH2-terminal kinase (JNK) and Iκβ kinase B (IKKβ), which are stress kinases, leading to accelerated phosphorylation of insulin receptor substrate-1 (IRS-1) serine [15,16]. However, studies on the relationship between exposure to low-level monocyclic aromatic hydrocarbons (MAHs) and IR and the pathogenesis of IR have been exceedingly insufficient in both Korea and other countries and thus, such studies are increasingly needed.
In this study, IL-1β, IL-6, and TNF-α, which are cytokines playing key roles in the pathogenesis of IR, and oxidative stress indices were measured in the subjects. Exposure to styrene, toluene, and xylene, which were representative MAHs, was assessed through measuring ambient levels and urinary metabolites in exposed workers. Finally, these parameters were compared between the exposed group and non-exposed control group to identify the effects of chronic exposure to low-level MAHs and IR in Korean workers.
Materials and Methods
This paper was a cross-sectional study aimed at inspecting the effect of low-level organic solvent exposure on insulin resistance. The subjects in the exposed group were workers who had been exposed to MAHs in paint manufacturing factories. Female subjects were excluded from this study as the number of female workers was not large enough to use in statistical calculations. The subjects in the control group were randomly selected among workers with a similar range in age as the subjects in the exposed group, in order to control the effects of aging. The level of organic solvent in the air and urinary metabolites in urine were set as exposure variables, and cytokines and oxidative stress levels were compared between the exposed and control groups. This study was reviewed and approved by the Institutional Review Board of the Occupational Safety and Health Research Institute, and informed consent was obtained from all the workers who volunteered to participate in the study.
Study subjects
One hundred and ten male workers (mean age of 38.9) who were occupationally exposed to mixed organic solvents including styrene, toluene, and xylene in three paint manufacturing factories were selected as test subjects in the exposed group. The control group was composed of 110 age-matched (mean age of 38.6) male manufacturing workers and office workers at the same factories who had never been occupationally exposed to organic solvents. Workers with clinically diagnosed diabetes, those taking medication for a medical condition, and those with acute infections and/or diseases that may have suppressed their immune systems were excluded from the study.
Questionnaire survey
Self-administered questionnaires were distributed and in-person interviews were conducted to obtain information on the subjects' general characteristics including age, smoking, drinking, physical activity, job characteristics including daily working hours and working duration, and eating habits.
Measuring ambient organic solvent levels and urinary metabolites
Ambient monitoring and biological monitoring was carried out for all the subjects. Ambient organic solvent levels were analyzed according to 'Method 1501 Aromatic Hydrocarbons' in National Institute of Occupational Safety and Health [17]. Organic solvents in the air at the workplace were sampled using low-flow active samplers (Low Flow Sampler 113 D, Gilian, USA) and analyzed using gas chromatography (CP-3800 GC/FID, Varian Ltd., USA). The urine used to measure the levels of urinary metabolites was obtained at around 18:00-19:00 after most workers had completed work. Urinary excretion of hippuric acid (HA) and methyl hippuric acid (mHA), which are metabolites of toluene and xylene, respectively, were analyzed using gas chromatography (CP-3800 GC/FID, Varian Ltd., USA), and the results were adjusted to urinary creatinine [18]. Phenylglyoxylic acid (PGA) and mandelic acid (MA), metabolites of styrene, were analyzed using gas chromatography according to the method by Ogata and Taguchi [19], and the results were adjusted to urinary creatinine.
Blood collection and analysis
Venous blood was collected between 07:00 and 09:00 after the subjects had fasted for more than 10-hours, and centrifuged for 15 minutes at 2,500 rpm within 30 minutes after sampling to separate plasma and serum. Separated plasma and serum were divided into 100 µL vials and frozen, and transported to the laboratory for analysis where biochemical tests were performed using a biochemistry analyzer (COBAS Integra 400, Roche Diagnostics Ltd., Switzerland). Fasting insulin levels were analyzed using a luminescence immunoassay system (ACCESS, Sanofi Diagnostics Pasteur, Inc., Minnesota, USA). IL-1β, IL-6 and TNF-α were analyzed using a sandwich ELISA kit (R&D systems, Minnesota, USA) by the method suggested by the manufacturer. All results are presented as mean values of duplicate measurements.
Evaluation of oxidative stress
Total oxidative status (TOS) and total anti-oxidative capacity (TAC) were measured using a colorimetric test kit (Immunodiagnostik AG, Bensheim, Germany). For TOS, total lipid peroxides, which are directly correlated with oxygen radicals, were measured in the plasma and adjusted to reference material to calculate the concentration as a H2O2-equivalent (µmol/L). For TAC, H2O2 remaining in the plasma after reaction with antioxidants for a specific time was measured to identify the reactivity of antioxidants (presented as H2O2-equivalent [µmol/L]). The oxidative stress index (OSI) which was the TOS-to-TAC ratio was calculated using the equation, OSI (arbitrary unit) = TOS (µmol H2O2 equivalent/L) / TAC (µmol H2O2 equivalent/L).
Evaluation of insulin resistance
Insulin resistance was evaluated using a homeostasis model assessment of insulin resistance (HOMA-IR). The HOMA-IR was calculated using the equation, [HOMA-IR = fasting insulin (µU/mL) × fasting glucose (mg/dL) / 405].
Data analysis
The data was analyzed using SPSS software version 12.0.1 (SPSS Inc., Chicago, IL, USA). T-tests were performed to compare the exposed and control groups, and multiple linear regression analysis and multiple logistic regression analysis was used to analyze the relationship between parameters. In the multiple logistic regression analysis, subjects with results in the upper 25% of HOMA-IR were considered part of the group having insulin resistance, and the lower 75% was considered part of normal group. Subjects with fasting plasma glucose levlevels greater than or equal to 100 mg/dL were considered part of the high-glucose group and those with lower levels were considered part of the normal group [20]. All results were presented as mean ± standard deviation, and p-values less than 0.05 were considered significant.
Results
Characteristics of the study subjects
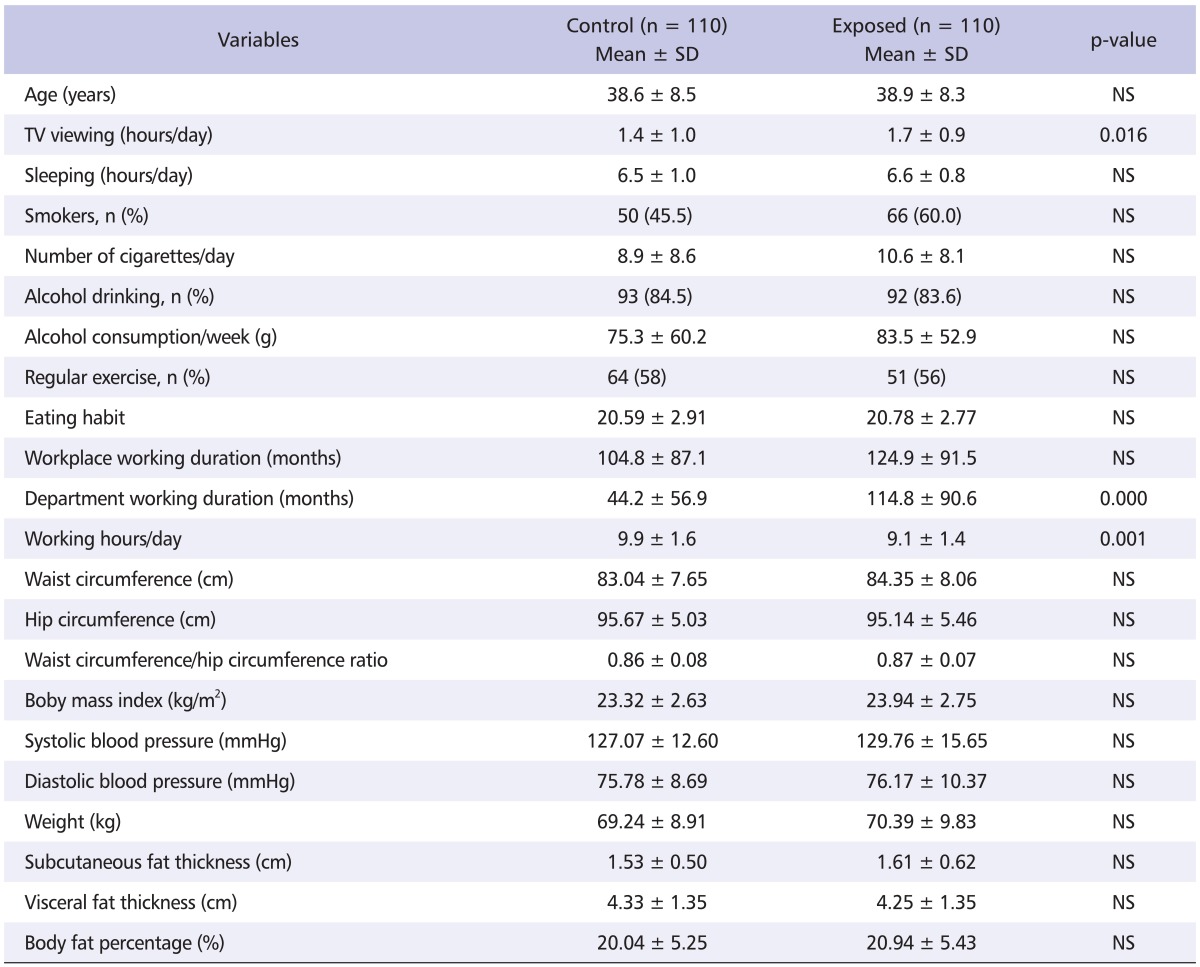
The mean age of workers was 38.6 ± 8.5 and 38.9 ± 8.3 years in the control and exposed group, respectively. There were no significant differences in age, smoking and drinking rate, eating habits, and exercise habits between the two groups. TV viewing hours (1.4 ± 1.0 vs. 1.7 ± 0.9 hours per day) and department working duration (44.2 ± 56.9 vs. 114.8 ± 90.6 months) were significantly longer in the exposed group (p < 0.05). The subjects in the exposed group worked on average, 9.1 ± 1.4 hours, during which they were exposed to organic solvents while subjects in the control group worked for longer hours at an average of 9.9 ± 1.6 hours (p < 0.001). Additionally, there was no significant difference with respect to any anthropometric parameters between the control and exposed groups (Table 1).
Table 1.
General and job characteristics of study subjects
SD: standard deviation, NS: non significant.
Level of exposure to MAHs and its correlation with metabolites in the exposed group
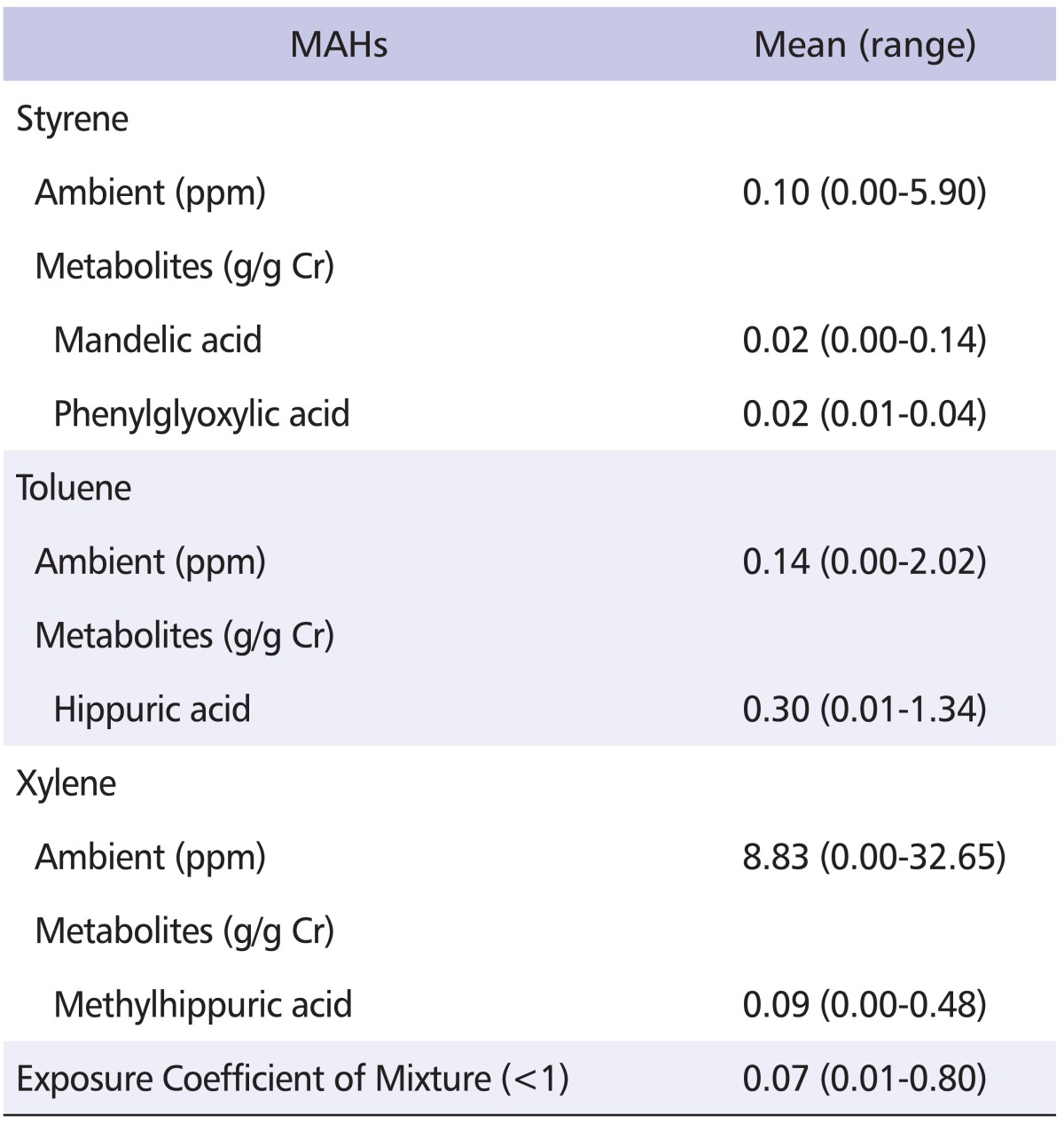
Workers in the exposed group were exposed to average styrene levels of 0.10 ppm (range, 0.00-5.90) with mean levels of urinary PGA, a metabolite of styrene, of 0.02 g/g creatinine (range, 0.01-0.04). The correlation coefficient (r) between ambient styrene and urinary PGA level was 0.625 (p < 0.05). Workers in the exposed group were exposed to average toluene levels of 0.14 ppm (range, 0.00-2.02) while HA, a urinary metabolite of toluene, was 0.30 g/g creatinine (range, 0.01-1.34). There was no significant correlation between ambient toluene and urinary HA levels. Workers in the exposed group were exposed to average xylene levels of 8.83 ppm (range, 0.00-32.65) while mHA, a urinary metabolite of xylene, was 0.09 g/g creatinine (range, 0.00-0.48). The correlation coefficient between ambient xylene and urinary mHA level was 0.316 (p < 0.01). Ambient exposure levels of styrene, toluene, and xylene and concentrations of urinary excretion of their metabolites MA, PGA, HA, and mHA were all below the threshold limit value-time weighted average (TLV-TWA) and biological exposure index (BEI) of Korea, respectively (Table 2).
Table 2.
Concentration of ambient MAHs and their urinary metabolites in 110 exposed workers
Threshold limit value - time weighted average: Styrene: 20 ppm, Toluene: 50 ppm, Xylene: 100 ppm.
Biological exposure index: Mandelic acid: 0.8 g/g creatinine, Phenylglyoxylic acid: 0.24 g/g creatinine, Hippuric acid: 2.5 g/g creatinine, Methylhippuric acid: 1.5 g/g creatinine.
MAHs: monocyclic aromatic hydrocarbons.
Serum clinical parameters of the study subjects
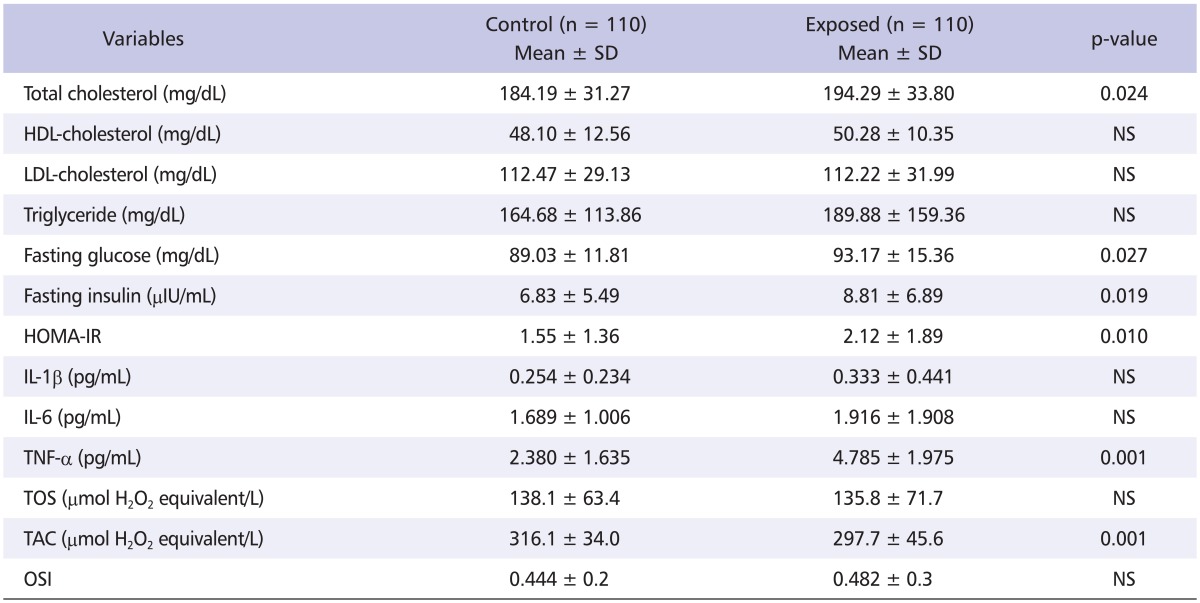
Total cholesterol, fasting glucose, fasting insulin, and HOMA-IR levels were statistically significantly higher in the exposed group (p < 0.05), while HDL-cholesterol, LDL-cholesterol, triglyceride were not significantly different between groups. Further, TNF-α levels in the exposed group were significantly higher compared to levels in the control group (4.785 ± 1.975 pg/mL vs. 2.380 ± 1.635 pg/mL, p < 0.001). IL-1β and IL-6 levels were higher in the exposed group, although the difference compared to those in the control group was not statistically significant. TOS, TAC and OSI were measured to examine the changes in oxidative stress by exposure to MAHs. TAC, which indicates anti-oxidative capacity, was significantly lower in the exposed group compared to levels in control group, while TOS and OSI were not statistically different between groups (Table 3).
Table 3.
Comparison of serum clinical parameters between control and exposed workers
SD: standard deviation, NS: Non-significant, HOMA-IR: homeostasis model assessment of insulin resistance, IL-1β: interleukin-1 beta, IL-6: interleukin-6, TNF-α: tumor necrosis factor-alpha, TOS: total oxidative status, TAC: total anti-oxidative capacity, OSI: oxidative stress index.
Relationship between MAHs metabolites and cytokines or oxidative stress indices
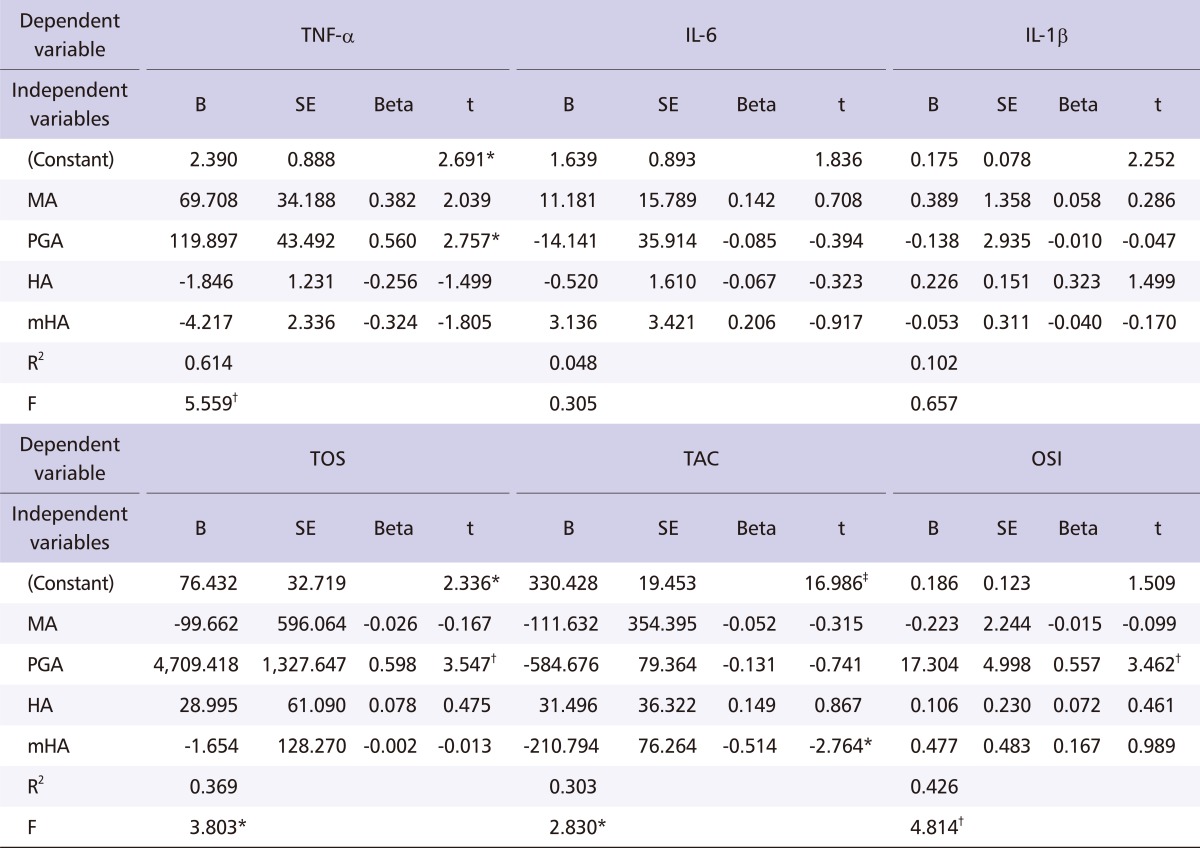
The results from the multiple linear regression analysis for the 110 exposed workers have been provided in Table 4. Only the test items that had statistical significance have been listed. TNF-α was significantly affected by PGA (p < 0.05), but other cytokines such as IL-6 and IL-1α were not significantly affected by independent variables (such as MA, PGA, HA and mHA). TOS (p < 0.01) and OSI (p < 0.01) were significantly affected by PGA positively, and TAC was negatively affected by mHA (p < 0.05).
Table 4.
Results of a multiple linear regression model for the association among MAHs metabolites, cytokines, and oxidative stress indices
MA: mandelic acid, PGA: phenylglyoxylic acid, HA: hippuric acid, mHA: methylhippuric acid, TNF-α: tumor necrosis factor-alpha, IL-6: interleukin-6, IL-1β: interleukin-1 beta, TOS: total oxidative status, TAC: total anti-oxidative capacity, OSI: oxidative stress index.
*p < 0.05.
†p < 0.01.
‡p < 0.001.
Relationship between exposure to MAHs and insulin resistance
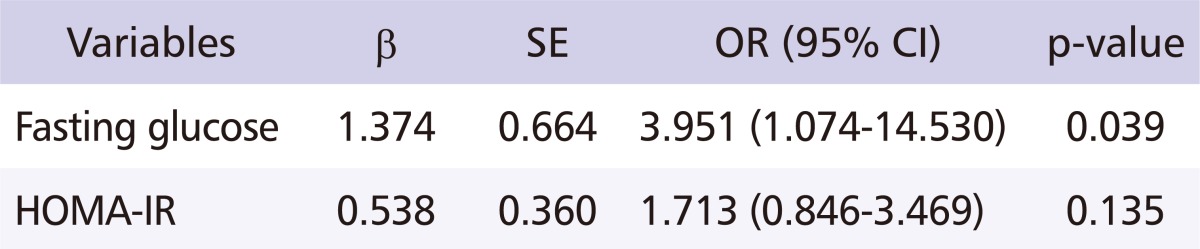
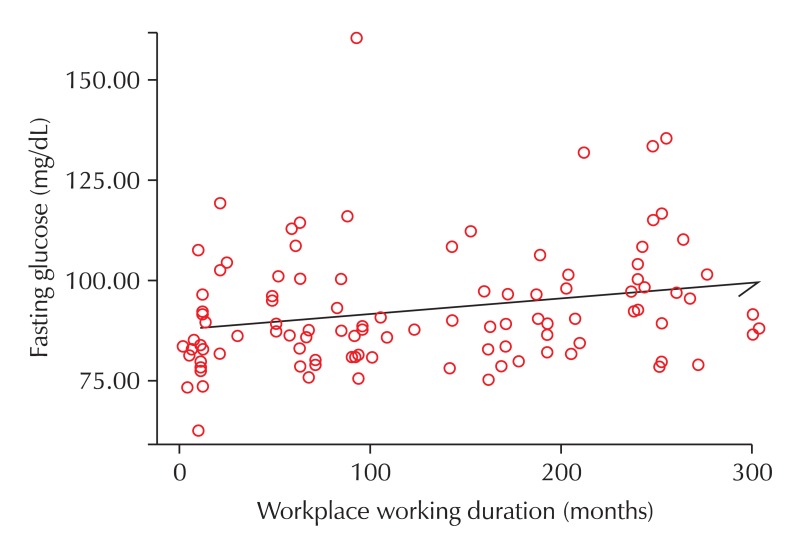
After adjusting for the workers' age, smoking habits, drinking habits, waist and hip circumference, weight, and regular exercise habits, a multiple logistic regression analysis was performed (with exposure levels of MAHs as the independent variable, and fasting glucose and HOMA-IR levels as the dependent variable) in order to identify the relationship between exposure to MAHs and IR. The odds ratio (OR) between exposure levels of MAHs and fasting glucose was 3.951 (95% cofidence interval [CI] = 1.074-14.530, p = 0.039), indicating a significant relationship. The OR between exposure levels of MAHs and HOMA-IR was 1.713 (95% CI = 0.846-3.469, p = 0.135), which did not indicate a significant relationship (Table 5). Also, after adjusting for age, increase in working duration to MAHs was related to increased blood glucose levels (Fig. 1).
Table 5.
Multiple logistic regression analysis for fasting glucose and HOMA-IR
Independent variable: exposure levels of monocyclic aromatic hydrocarbons.
Adjustment to age, smoking, drinking habit, waist and hip circumference, weight and regular exercise.
SE: standard error, OR: odds ratio, CI: confidence interval, HOMA-IR: homeostasis model assessment of insulin resistance.
Fig. 1.
Scatter-plot graph between workplace working duration and fasting glucose. Adjustment to age, smoking, drinking habit, waist and hip circumference, weight and regular exercise (r = 0.242, p = 0.014).
Discussion
There have been many reports on the correlation between IR and lifestyle, but there are only a few studies regarding exposure to hazardous chemicals, despite the fact that many have suggested the relationship between exposure to organic solvents and IR. Therefore, we carried out this study to analyze the characteristics of working environments in manufacturing factories in Korea and analyzed their relationship with IR to compare the results with those from other countries and clearly identify the relationship.
We studied styrene, toluene, and xylene, which are MAHs widely used in industries in paint, plastic and rubber manufacturing as thinner, and attempted to understand the effect of exposure to these chemicals on the development of IR with a specific focus on cytokines and oxidative stress. With regard to lifestyle, Pardee et al. [21] reported that television viewing time was associated with hypertension and obesity, and that TV viewing time was reported to be positively correlated with BMI [22]. Mummery et al. [23] also reported that time spent sitting was positively correlated with BMI. In addition, De León et al. [24] reported that there were direct associations between a sedentary lifestyle and triglyceride, IR, and MetS. Thus, these results suggest that induction of hypertension, obesity, IR, and MetS is associated with sedentary behaviors. However, in this study, a comparison of anthropometric results between the control and exposed groups showed no statistically significant difference. This result may be due to the lack of a significant difference in general characteristics that may have affected the anthropometric results between the groups. Therefore, we can induce that the effects of lifestyle and so on are ignorable in the various results of the analysis and in the the interpretation of the analysis in these two groups.
Mokuda et al. [25] reported that liver damage induced by carbon tetrachloride increased glucose and insulin levels in blood, and Kaukiainen et al. [8] reported that fasting blood glucose levels were higher in those exposed to mixed organic solvents than in the control group who were not exposed to hazardous chemicals: organic solvent exposure was positively correlated with glucose, cholesterol and triglyceride though these were within reference ranges. In the present study, total cholesterol, fasting glucose, fasting insulin, and HOMA-IR were significantly higher in the exposed group. This result was comparable to other reports, and thought to be the effect of exposure to MAHs.
Styrene exposure has been reported to change the physiological levels of serotonin and TNF-α which are neurotransmitters [26], and TNF-α inhibits signaling by insulin through inhibiting IRS-1 function by serine phosphorylation [27]. TNF-α is known to have a biological action opposite to adiponectin, even though its three-dimensional structure is very similar to that of adiponectin. These two are known to inhibit the production and action of each other [28]. TNF-α also elevates blood fatty acids by promoting lipolysis in adipocytes, which may cause a decrease in insulin [29]. IL-1β may suppress IRS-1 expression ERK-dependently during transcription and ERK-independently during post-transcription, which may damage insulin signaling [14]. Among cytokines, IL-1β, IL-6 and TNF-α were measured and compared between the exposed and control groups in this study. Only TNF-α levels were significantly higher in the exposed group compared to controls, and IL-1β and IL-6 levels were not significantly different, though somewhat higher in the exposed group. Multiple linear regression analysis between these cytokines and organic solvents did not show any significance; however, cytokines and metabolites of organic solvents showed a significant relationship. Further, PGA, a metabolite of styrene, affected TNF-α positively, meaning that there was an increase in TNF-α levels in the exposed group, and positively correlated with PGA. It is likely that TNF-α induced an initial immune reaction and diseases such as IR with a larger quantity than required for maintaining normal physiological function. In this study, IL-6 was not significantly high; however, others have reported that it increases IR by increasing free fatty acid levels in blood [30] and partly induces IR by stimulating the degradation of body fat, interrupting glucose metabolism, and decreasing the adiponectin [31].
With respect to oxidative stress, TOS and OSI were not significantly different between groups while TAC was significantly lower in the exposed group. Multiple linear regression analysis between oxidative stress indices and ambient organic solvents or their metabolites showed significant positive association between PGA and TOS, or PGA and OSI, and showed a negative association between mHA and TAC. Like cytokines, oxidative stress indices were not associated with ambient organic solvents levels but were associated with urinary metabolites. Absorbed dose seems to have stronger effect than ambient exposure level of organic solvents on the induction of physiological changes.
Most absorbed hazardous chemicals including MAHs are excreted from the body by the metabolic action of xenobiotics-metabolizing enzymes. During this process, highly active and reactive metabolic intermediates and ROS are generated, and may induce in-vivo toxicity through covalent bonding with body macromolecules [32]. Such ROS toxicity in humans has been known to affect cell signaling as well as the endocrine and immune systems [33]. Karagözler et al. [34] reported that TNF-α was significantly increased in house painters, and the activities of superoxide dismutase and glutathione peroxidase, which are antioxidant enzymes, were significantly lower, and malondialdehyde, a measure of lipid peroxidation, was found to be significantly elevated. Styrene oxide, a metabolic intermediate of styrene, was found to decrease reduced glutathione levels, and ROS increases were observed in clara cells incubated with styrene and its metabolites [35]. In addition, styrene was reported to induce an inflammatory response by expressing glutathione S-transferase, an oxidative stress index, and expressing monocyte chemoattractant protein-1 (MCP-1), a chemotactic cytokine, through NF-κB activation [10].
NF-κB is a redox-sensitive transcription factor which reacts to cytokines, hormones, and metabolites, and plays a central role in diverse responses associated with protection via rapid gene expression induction. TNF-α, IL-1β, mitogen, oxidative stress, and inflammatory cytokines are related to its activity [36]. While oxidative stress inducers including ROS have been known to result in insulin resistance by activating kinases such as JNK and IKKβ [37], hyperglycemia is known to result in oxidative stress through glucose auto-oxidation, formation of advanced glycation end products, arachidonic acid metabolism abnormality, and activation of protein kinase C [38], indicating that increases in blood glucose or oxidative stress by any reason affects reciprocally, possibly leading to a vicious circle.
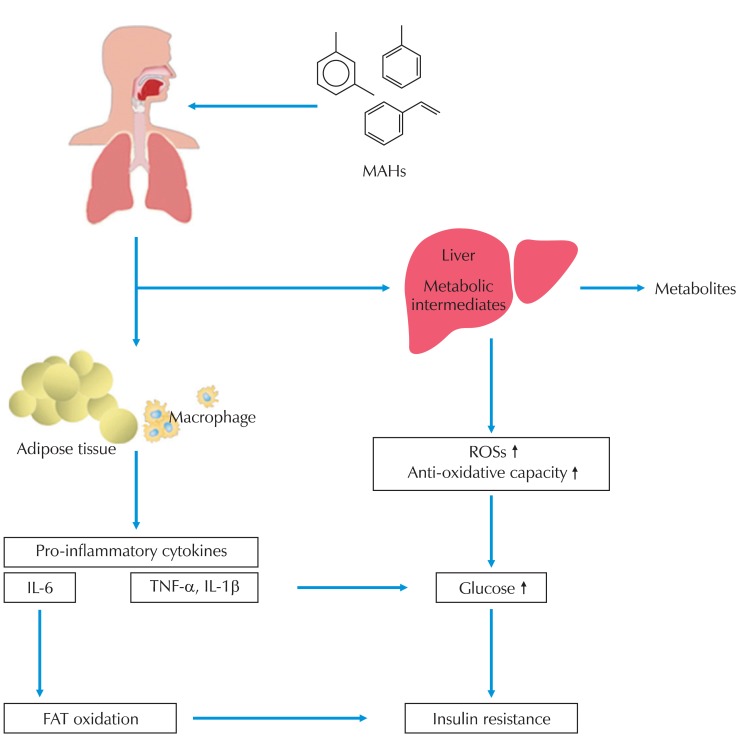
In this study, multiple logistic regression analysis (with fasting glucose and HOMA-IR levels as the dependent variables and MAHs exposure, cytokines, and oxidative stress as the independent variables) showed a relationship between MAHs exposure and fasting glucose. From the results above, we can confirm that MAHs exposure increases fasting glucose and IR. This high level of IR in the exposed group is thought to be due to the effect of oxidative stress and changes in physiological levels of cytokines that are generated by unchanged organic solvents and the active metabolic intermediate produced during glucose metabolism and degradation (Fig. 2).
Fig. 2.
Possible mechanism of insulin resistance by MAHs. MAHs: monocyclic aromatic hydrocarbons, ROSs: reactive oxygen species.
In conclusion, the results of the current study demonstrated that exposure to organic solvents may induce IR, though the study population were exposed to organic solvents at as low as 10% of exposure limit. Considering the recent increase of cerebro and cardiovascular diseases at workplaces and the high association between IR and cardiovascular diseases, this relationship between IR and exposure to chemical indicates that this study can be useful as basic data for the health management of workers exposed to chemicals.
Despite its relevant findings, this study has several limitations. First, the number of subjects for both the test and control group was insufficient to make clear conclusions. Second, the subjects were exposed to MAHs at low levels, and physiological levels of cytokines and levels of oxidative stress could not be compared according to different exposure levels. Third, active metabolic intermediates, transcription factors such as NF-κB, and kinases that affect cytokines and oxidative stress were not measured but interpreted by citing the results from other researchers. Therefore, studies investigating clear mechanisms obtained via evaluating the levels of metabolic intermediates and transcription factors are needed in future research.
Acknowledgments
This study was supported by the intramural research fund of the Occupational Safety and Health Research Institute (OSHRI).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Xiao JQ, Levin SM. The diagnosis and management of solvent-related disorders. Am J Ind Med. 2000;37:44–61. doi: 10.1002/(sici)1097-0274(200001)37:1<44::aid-ajim5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Warnes TW, Jain SK, Smith A. Hepatotoxic effects of workplace exposures. In: Baxter PJ, Adams PH, Tar-Ching A, Cockcroft A, Harrington JM, editors. Hunter's diseases of occupations. 9th ed. London (UK): Hodder Arnold; 1999. pp. 881–900. [Google Scholar]
- 3.Brautbar N, Williams J., 2nd Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health. 2002;205:479–491. doi: 10.1078/1438-4639-00175. [DOI] [PubMed] [Google Scholar]
- 4.Natelson EA. Benzene-induced acute myeloid leukemia: a clinician's perspective. Am J Hematol. 2007;82:826–830. doi: 10.1002/ajh.20934. [DOI] [PubMed] [Google Scholar]
- 5.White RF, Proctor SP. Solvents and neurotoxicity. Lancet. 1997;349:1239–1243. doi: 10.1016/S0140-6736(96)07218-2. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann G, Mühlenberg J, Fischäder G, Kulla C, Rehwagen M, Herbarth O, Lehmann I. An experimental model for the determination of immunomodulating effects by volatile compounds. Toxicol In Vitro. 2005;19:685–693. doi: 10.1016/j.tiv.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Merker GH, Metzner G, Raabe F. T(H1)-directed modulation of in vitro cytokine production in human peripheral blood mononuclear cells by styrene-7.8-oxide. Toxicol Lett. 2006;160:105–111. doi: 10.1016/j.toxlet.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Kaukiainen A, Vehmas T, Rantala K, Nurminen M, Martikainen R, Taskinen H. Results of common laboratory tests in solvent-exposed workers. Int Arch Occup Environ Health. 2004;77:39–46. doi: 10.1007/s00420-003-0476-z. [DOI] [PubMed] [Google Scholar]
- 9.Hong YC, Park EY, Park MS, Ko JA, Oh SY, Kim H, Lee KH, Leem JH, Ha EH. Community level exposure to chemicals and oxidative stress in adult population. Toxicol Lett. 2009;184:139–144. doi: 10.1016/j.toxlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Röder-Stolinski C, Fischäder G, Oostingh GJ, Feltens R, Kohse F, von Bergen M, Mörbt N, Eder K, Duschl A, Lehmann I. Styrene induces an inflammatory response in human lung epithelial cells via oxidative stress and NF-kappaB activation. Toxicol Appl Pharmacol. 2008;231:241–247. doi: 10.1016/j.taap.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Kappus H. Oxidative stress in chemical toxicity. Arch Toxicol. 1987;60:144–149. doi: 10.1007/BF00296968. [DOI] [PubMed] [Google Scholar]
- 12.Hussein AS, Abdalla MS, Hussein JS, Shousha WG, Mohame AH. Antioxidants in of shoe-makers exposed to organic solvents. J Appl Sci Res. 2008;4:1107–1117. [Google Scholar]
- 13.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 14.Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan M, Lee J, Konstantopoulos N, Hansen L, Shoelson SE. Salicylate inhibition of IKK (IkB kinase) reverses insulin resistance in Zucker (fa/fa) rats. Diabetes. 2000;49(Suppl 1):A292. [Google Scholar]
- 16.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 17.National Institute of Occupational Safety and Health. Method 1501. In: Eller PM, Cassinelli ME, editors. NIOSH manual of analytical methods. 4th ed. Cincinnati (OH): National Institute of Occupational Safety and Health; 1994. [Google Scholar]
- 18.de Carvalho D, Lanchote VL, Bonato PS, Queiroz RH, Santos AC, Dreossi SA. A new derivatization procedure for the analysis of hippuric acid and m-methyl-hippuric acid by gas chromatography. Int Arch Occup Environ Health. 1991;63:33–37. doi: 10.1007/BF00406195. [DOI] [PubMed] [Google Scholar]
- 19.Ogata M, Taguchi T. Simultaneous determination of urinary creatinine and metabolites of toluene, xylene, styrene, ethylbenzene and phenol by automated high performance liquid chromatography. Int Arch Occup Environ Health. 1988;61:131–140. doi: 10.1007/BF00381617. [DOI] [PubMed] [Google Scholar]
- 20.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 21.Pardee PE, Norman GJ, Lustig RH, Preud'homme D, Schwimmer JB. Television viewing and hypertension in obese children. Am J Prev Med. 2007;33:439–443. doi: 10.1016/j.amepre.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Henderson VR. Longitudinal associations between television viewing and body mass index among white and black girls. J Adolesc Health. 2007;41:544–550. doi: 10.1016/j.jadohealth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mummery WK, Schofield GM, Steele R, Eakin EG, Brown WJ. Occupational sitting time and overweight and obesity in Australian workers. Am J Prev Med. 2005;29:91–97. doi: 10.1016/j.amepre.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera de León A, Rodríguez-Pérez Mdel C, Rodríguez-Benjumeda LM, Anía-Lafuente B, Brito-Díaz B, Muros de Fuentes M, Almeida-González D, Batista-Medina M, Aguirre-Jaime A. Sedentary lifestyle: physical activity duration versus percentage of energy expenditure. Rev Esp Cardiol. 2007;60:244–250. [PubMed] [Google Scholar]
- 25.Mokuda O, Ubukata E, Sakamoto Y. Impaired glucose uptake and intact gluconeogenesis in perfused rat liver after carbon tetrachloride injury. Biochem Mol Med. 1995;54:38–42. doi: 10.1006/bmme.1995.1006. [DOI] [PubMed] [Google Scholar]
- 26.Kim KW, Lee MY, Ryu HW, Ko KS, Kim TG, Won YL, Heo KH, Kim MG. Report No.: OSHRI 2008-25-36. Incheon (Korea): Occupational Safety and Health Research Institute; 2008. Exposure of hazardous chemicals and metabolic syndrome (I) p. 49. Korean. [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesitylinked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 29.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 30.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Arner P. Insulin resistance in type 2 diabetes -- role of the adipokines. Curr Mol Med. 2005;5:333–339. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- 32.Hinson JA, Pumford NR, Roberts DW. Mechanisms of acetaminophen toxicity: immunochemical detection of drug-protein adducts. Drug Metab Rev. 1995;27:73–92. doi: 10.3109/03602539509029816. [DOI] [PubMed] [Google Scholar]
- 33.Erdamar H, Demirci H, Yaman H, Erbil MK, Yakar T, Sancak B, Elbeg S, Biberoğlu G, Yetkin I. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med. 2008;46:1004–1010. doi: 10.1515/CCLM.2008.183. [DOI] [PubMed] [Google Scholar]
- 34.Karagözler AA, Mehmet N, Batçioglu K. Effects of long-term solvent exposure on blood cytokine levels and antioxidant enzyme activities in house painters. J Toxicol Environ Health A. 2002;65:1237–1246. doi: 10.1080/152873902760125723. [DOI] [PubMed] [Google Scholar]
- 35.Carlson GP, Turner M, Mantick NA. Effects of styrene and styrene oxide on glutathione-related antioxidant enzymes. Toxicology. 2006;227:217–226. doi: 10.1016/j.tox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br J Nutr. 2001;86(Suppl 1):S121–S127. doi: 10.1079/bjn2001340. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson JW. Metabolic stress in insulin's target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]