Abstract
Objectives
Various cytokines induced by inhalation of coal dust may mediate inflammation and lead to tissue damage or fibrosis, such as coal workers' pneumoconiosis (CWP).
Methods
To investigate the relevance of serum cytokines in CWP, the levels of serum interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α) as CWP biomarkers in 110 retired coal miners (22 controls and 88 CWP subjects) were related to cross sectional findings and 1-year progressive changes of the pneumoconiosis. Progressive changes of CWP were evaluated by paired comparison of chest radiographs. Analysis by a receiver operating characteristic curve assessed the biomarker potential of each cytokine.
Results
The mean serum IL-8 level was significantly higher in CWP compared to controls and IL-8 levels correlated with the degree of CWP. The median serum TNF-α level was significantly higher in subjects with progressive CWP compared to subjects without CWP progression. The area under the ROC curve for IL-8 (0.70) and TNF-α (0.72) for CWP identification and progression, respectively, indicated the biomarker potential of the two cytokines. Serum cutoff values of IL-8 and TNF-α were 11.63 pg/mL (sensitivity, 69%; specificity, 64%) and 4.52 pg/mL (sensitivity, 67%; specificity, 79%), respectively.
Conclusion
The results suggest that high levels of serum IL-8 are associated with the presence of CWP and those of serum TNF-α are associated with the progression of CWP.
Keywords: Anthracosis, Interleukin-8, Tumor necrosis factor-α, Receiver operating characteristic, Follow-up
Introduction
Of the various occupational lung diseases, those induced by inhalation of dusts such as asbestos, crystalline silica, and coal are most prevalent. Inhalation of dusts may cause a variety of lung diseases such as coal workers' pneumoconiosis (CWP), progressive massive fibrosis, chronic alveolitis, and emphysema [1]. Notably, crystalline silica has been classified as a class I carcinogen by the International Agency for Research on Cancer [2]. CWP is a lung disease caused by inhalation of coal dust. Once a silica threshold has been exceeded, silica-induced pulmonary disease may progress without further exposure to silica. Clinical detection of CWP, however, is currently dependent on radiological and lung function abnormalities, which are both late diagnostic tools. Identification of accurate and reliable biomarkers would enable earlier detection before irreversible radiological changes in the lung occur [3,4].
Cytokines influence various biological events such as inflammation, metabolic mechanism, cell growth and proliferation, morphogenesis, fibrosis, and homeostasis. Major sources of cytokines in the lung are epithelial cells, endothelial cells, fibroblasts, and inflammatory cells [5]. In previous reports, the relationship between pulmonary inflammation and dusts, and cytokines has been demonstrated for mediators of various toxicological and pathological effects, and several cytokines related with coal dust [6-10]. In one study, the initial concentrations of tumor necrosis factor-alpha (TNF-α) were related to later progression of CWP. Miners who showed abnormally high dust-stimulated release of TNF-α had an increased risk of progression in CWP. TNF-α in pneumoconiosis induced by coal dust was reported to be a powerful tool to estimate individual prognosis of pneumoconiotic disease, even after the end of occupational exposure [11]. Interleukin-8 (IL-8) is a chemokine secreted by a variety of cells types including fibroblasts in response to IL-1 and TNF-α [12]. IL-8 is an important activator and chemoattractant for neutrophils, and has been implicated in a variety of inflammatory diseases [13]. IL-8 is important in the lung inflammation produced by crystalline silica. Both TNF-α and IL-8 were reported to be increased in the supernatant of spontaneous or dust-stimulated monocytes isolated from peripheral blood and in sera of CWP, which did not include progressive CWP [14].
While various studies have addressed the increased production of IL-8 following exposure crystalline silica or coal mine dust in macrophage and fibroblasts, only one human validation study has been published [1]. The available data suggest the importance of IL-8 and TNF-α in CWP. However, little information exists concerning the in vivo relevance of predictive discrimination between the levels of cytokines and progression of CWP. To determine the significance of serum cytokines regard to progression of CWP, a longitudinal design is necessary. However, follow-up studies dealing with the prognostic usefulness of cytokines are scarce in Korea.
The present study examined the relationship between serum levels of IL-8 and TNF-α, and CWP findings in retired coal miners with previous cross sectional findings, and examined the relationship between initial levels of same cytokines and 1-year progressive changes of CWP.
Materials and Methods
Study design
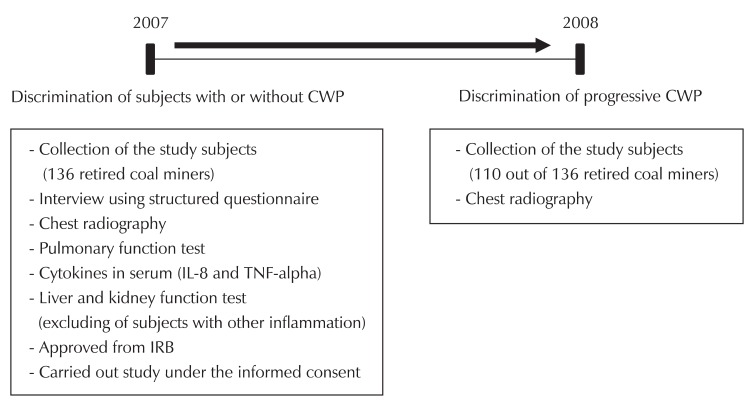
Fig. 1 shows the design of the study. A group of 136 male retired coal miners, who were randomly selected from the examinee for diagnosis of pneumoconiosis at Ansan Workers' Compensation Insurance Hospital, Korea Workers' Compensation & Welfare Service, were recruited for follow-up. The study was performed using one-year prospective data and cross-sectional findings. One-year progression of CWP was evaluated by the paired comparison of chest-radiographs made in 2007 and 2008. Chest radiographs in 2008 were measured only in 110 subjects. Ultimately, 110 out of 136 subjects previously screened for serum cytokines (81% of initial subjects) participated in the follow-up.
Fig. 1.
Design of the study. Serum cytokines (IL-8 and TNF-α) of retired coal miners were related to presence of coal workers pneumoconiosis (CWP) and to progression of CWP. Pulmonary function was determined spirometry data (FVC and FEV1) in 2007. Progression of CWP was evaluated by paired comparison of chest-radiographs made in 2007 and 2008. General characteristics were surveyed from structured questionnaire and interviews. IL-8: interleukin-8, TNF-α: tumor necrosis factor-alpha, IRB: institutional review board, FVC: forced vital capacity, FEV1: forced expiratory volume in one second.
Retired coal miners who did not have CWP (ILO classification of 0/0 or 0/1) were chosen as controls and CWP patients were identified from physician lists in the pneumoconiosis review committee. CWP patients were divided into two groups according to the presence and the absence of progressive changes on chest radiographs.
Pulmonary function was determined in 2007 by spirometry data that included results of forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). Pulmonary function test (PFT) and personal information in cluding age, body mass index (BMI), and various personal histories (job and smoking status) were obtained by a structured questionnaire. All subjects provided informed consent and the study was approved by the Research Ethics Committee of our institute (Fig. 1).
Analysis of serum cytokines
Serum was separated from whole blood samples, and stored at -80℃ until assay. Analysis of serum cytokines was measured by an EV 3513 cytokine biochip array (Randox Laboratories, Crumlin, UK) using sandwich and competitive chemiluminescence immunoassays, as previously described [15,16]. Detection limits of IL-8 and TNF-α were 1.5 pg/mL and 2.6 pg/mL, respectively.
PFT
PFT was measured in accordance with recommended guideline of ATS/ERS Task Force [17] using a Vmax22 spirometer (SensorMedics, San Diego, CA, USA). Measured parameters were FVC (the volume delivered during an expiration made as forcefully and completely as possible starting from full inspiration), FEV1 (the volume delivered in the first second of an FVC maneuver), and the FEV1/FVC (%FEV1/FVC) ratio. The predicted volumes of FVC and FEV1 were calculated by a by previously reported equation [18]: predicted FVC volume (L) = 0.148 × height (in) -0.025 × age (yr) -4.241; predicted FEV1 volume (L) = 0.092 × height (in) -0.032 × age (yr) -1.26. The predicted percentages (%) of FVC (%FVC) and FEV1 (%FEV1) were calculated as: %predicted = measured volume (L) / predicted volume (L) × 100. Test of pulmonary function was performed in the sitting position via closed circuit method, measuring inhaled and exhaled air at the same test cycle. Tests were carried out until gaining three adequate data. The criteria levels of %FVC predicted, %FEV1 predicted, and %FEV1/FVC ratio were 80%, 80%, and 70%, respectively.
Chest radiography
Chest radiographs were obtained and scored according to the classification rules of the International Labor Office (ILO) [19] by an experienced panel of physicians in the pneumoconiosis review committee of Korea Worker's Compensation & Welfare Service.
Statistical analyses
Levels of serum IL-8 and TNF-α were log-normally distributed. Therefore, cytokine data was log-transformed for parametric statistical tests, and the parametric unpaired student's t-test and ANOVA test were used to determine the magnitudes of between-group differences. If the sample sizes between control and test groups were also different or numbers of case each group were incompatible for parametric statistical tests, the nonparametric Mann-Whitney U test and Kruskal-Wallis one-way analysis based on rank sums were used to determine differences among the study groups. A multiple logistic regression model was constructed to compare controls with subjects with CWP, adjusted for age, BMI, exposure period, smoking status, and pulmonary function. Analysis by receiver operating characteristic (ROC) curve assessed the biomarker potential of each cytokine for the discrimination of the presence and progression of CWP. The best statistical levels of "cutoff" were calculated by minimizing the distance between the point with specificity = 1 and sensitivity = 1 and the points on the ROC curve. In the ROC analysis, an area under the curve (AUC) of 1.0 indicates perfect discrimination, whereas an area of 0.5 indicates that the test discriminates no better than chance. Values of p < 0.05 were considered statistically significant. All statistical evaluations were done with SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Study subjects
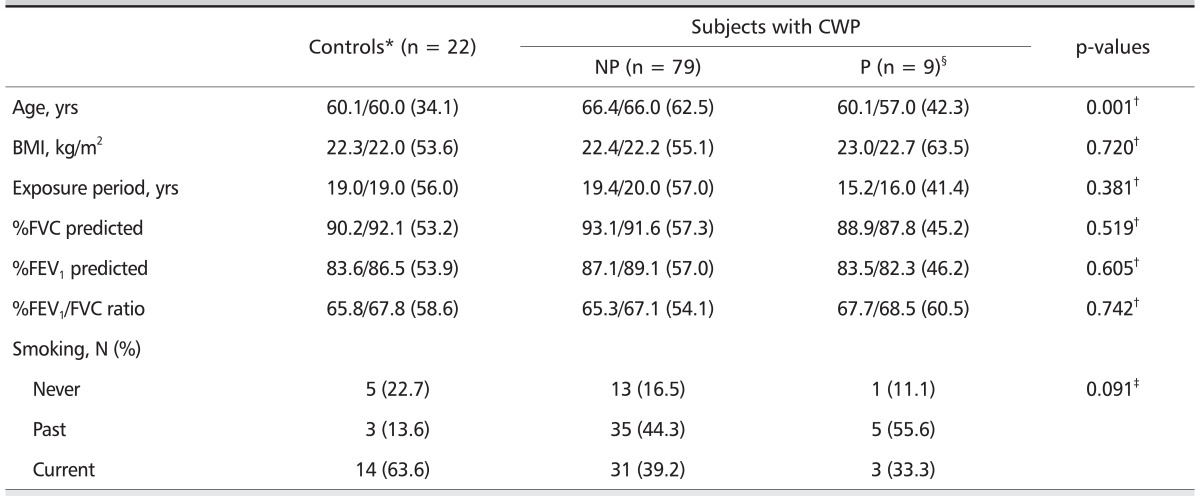
General characteristics of study subjects are shown in Table 1. In this study, 22 subjects were classified as controls and 88 were classified as CWP patients. Of the CWP patients, 79 and nine subjects were classified as non-progressive and progressive CWP, respectively, and 64 and 24 subjects were small and large opacity, respectively. The mean age was significantly higher in CWP without progression group compared with the other two groups. Other general characteristics including averages of BMI, exposure period, and pulmonary function (%FVC predicted, %FEV1 predicted, %FEV1/FVC ratio), and smoking status did not show statistical differences among the study groups.
Table 1.
General characteristics and 1-year progression of pneumoconiosis in retired coal miners with and without CWP
Values are presented as arithmetic mean/median (mean rank) or number (%).
CWP: coal workers' pneumoconiosis, NP: without progression, P: with progression, BMI: body mass index, FVC: forced vital capacity, FEV1: forced expiratory volume in one second.
*International Labor Office classification 0/0 or 0/1.
†Calculated by Kruskal-Wallis H test.
‡Calculated by χ2-test.
§One-year progression of CWP was evaluated by paired comparison of chest-radiographs: made in 2007 and 2008. Category I (4 subjects): 1/0 (1) → 1/1, 1/1 (2) → 1/2, 1/1 (1) → 2/1, Category II (3 subjects): 2/1 (2) → 2/3, 2/2 (1) → 4A, Large opacity (2 subjects): 4A (2) → 4B.
Concentrations of serum IL-8 and TNF-α
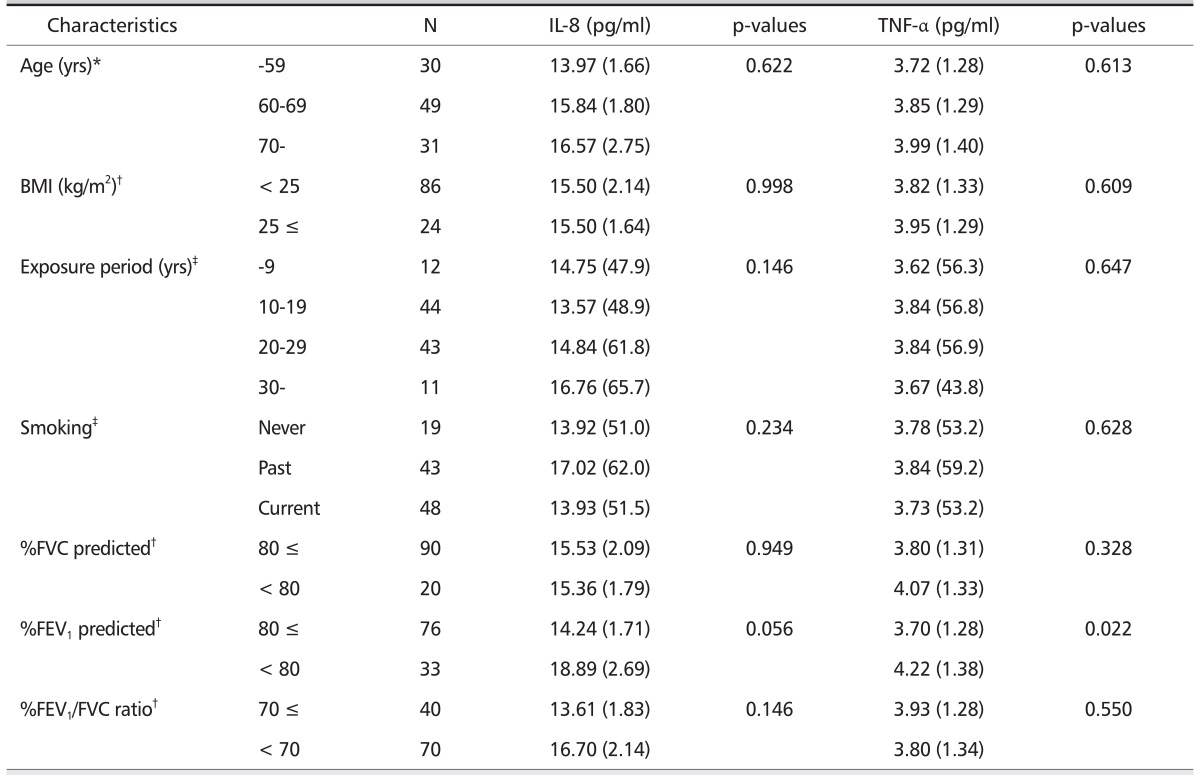
The mean serum TNF-α level was significantly higher (p = 0.022) in the subjects with low %FEV1 predicted (< 80%) (4.22 pg/mL) vs. with normal %FEV1 predicted (3.70 pg/mL) (Table 2). Although there was no statistical significance, the mean serum IL-8 level tended to increase with increasing age, and the mean serum IL-8 level in the subjects with low %FEV1 predicted (< 80%) tended to increase compared with normal %FEV1 predicted. No differences were observed in the serum levels of IL-8 and TNF-α according to BMI, exposure period, smoking status, %FVC predicted, and %FEV1/FVC ratio.
Table 2.
Concentrations of serum cytokines according to general characteristics
*Calculated by ANOVA test, geometric mean (geometric standard deviation).
†Calculated by t-test, geometric mean (geometric standard devia tion).
‡Calculated by Kruskal-Wallis H test, median (mean rank).
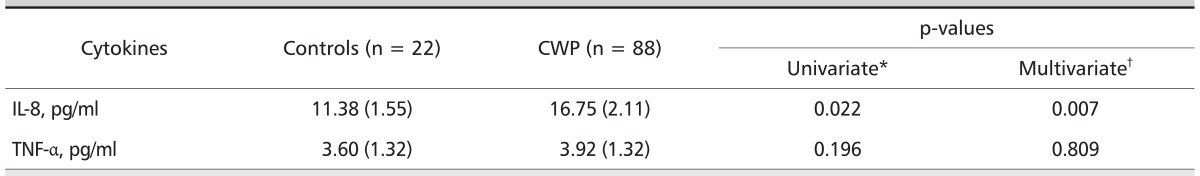
Serum cytokine levels according to CWP are shown in Table 3. The mean serum IL-8 level was significantly higher in subjects with CWP (16.75 pg/mL) vs. controls (11.38 pg/mL) in crude analysis (p = 0.022), and was both significantly and independently higher in adjusted logistic regression model (p = 0.007 for all comparisons). However, no differences were evident in the mean serum TNF-α level in subjects with CWP compared with controls (p = 0.379 for all comparisons).
Table 3.
Mean concentration of serum cytokines according to CWP
Values are presented as geometric mean (standard deviation). ILO classification 0/0 or 0/1.
CWP: coal workers' pneumoconiosis, IL-8: interleukin-8, TNF-α: tumor necrosis factor-alpha, BMI: body mass index, RCM: reliability centered maintenance, FVC: forced vital capaity, FEV1: forced expiratory volume in on second.
*Calculated by student's t-test (healthy RCM vs. RCM with CWP).
†Calculated from a multiple logistic regression model with serum level of each cytokines, adjusted for age, BMI, exposure period, smoking status, and pulmonary function (%FVC predicted, %FEV1 predicted, and %FEV1/FVC ratio).
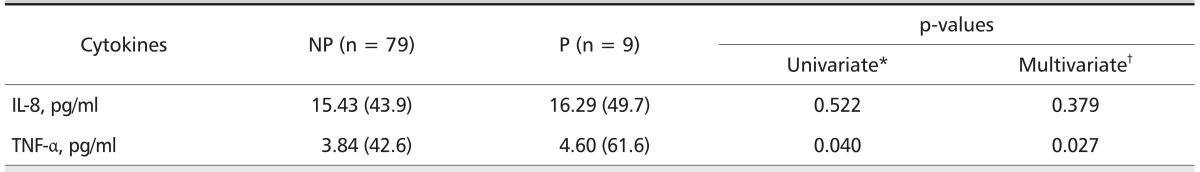
In subjects with CWP, the median serum TNF-α level was significantly higher in subjects with progressive CWP (4.60 pg/mL) than in those without progressive CWP (median, 3.84 pg/mL) in crude analysis (p = 0.040), was and both significantly and independently higher in adjusted logistic regression model (p = 0.027 for all comparisons). However, no difference was evident in the median serum IL-8 level in subjects with and without progressive CWP (p = 0.809 for all comparisons) (Table 4).
Table 4.
Median levels of serum cytokines according to progression of CWP
Values are presented as median (mean rank). ILO classification 0/0 or 0/1
CWP: coal workers' pneumoconiosis, NP: without progression, P: with progression, IL: interleukin, TNF-α: tumor necrosis factor-alpha, BMI: body mass index, FVC: forced vital capacity, FEV1: forced expiratory volume in on second.
*Calculated by Mann-Withney U test.
†Calculated from a multiple logistic regression model with serum level of each cytokines, adjusted for age, BMI, exposure period, smoking status, and pulmonary function (%FVC predicted, %FEV1 predicted, and %FEV1/FVC ratio).
ROC analysis of serum IL-8 and TNF-α
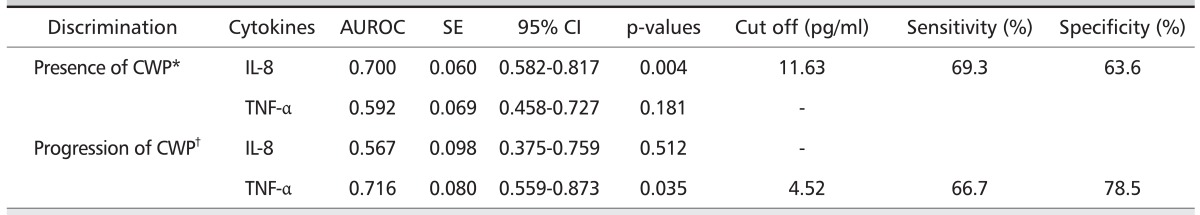
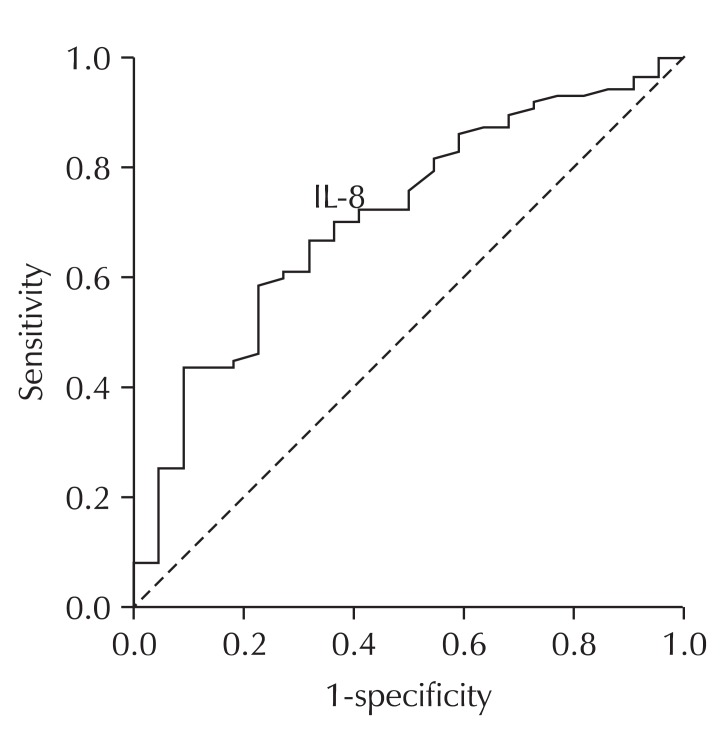
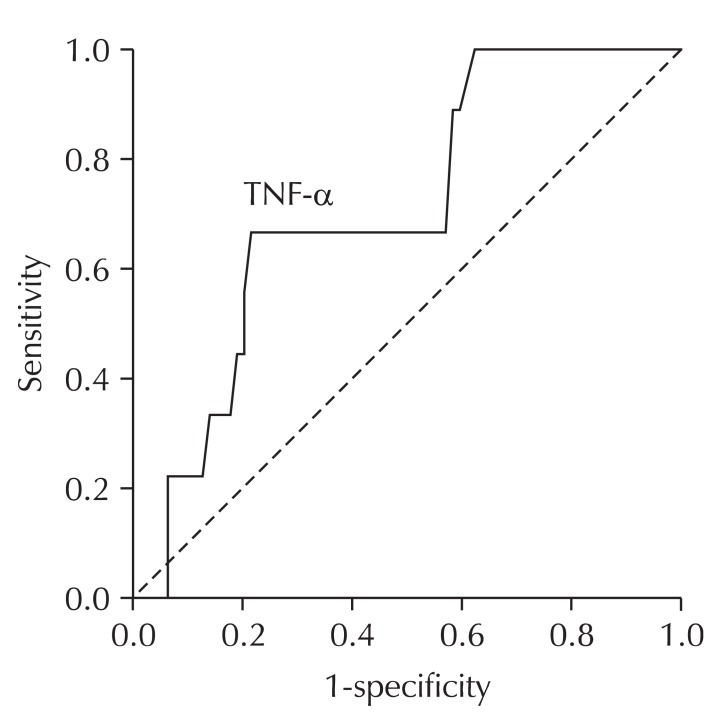
The AUC of the ROC curve for serum IL-8 level was 0.70 (95% confidence interval [CI], 0.58 to 0.82). The serum IL-8 level of 11.63 pg/ml was determined to be the optimal cutoff value with resulting sensitivity and specificity of 69.3% and 63.6%, respectively, for the presence of CWP. The AUC of the ROC curve for serum TNF-α level was 0.72 (95% CI, 0.56 to 0.87). The serum TNF-α level of 4.52 pg/mL was determined as the optimal cutoff value with resulting sensitivity and specificity of 66.7% and 78.5%, respectively, for the progression of CWP (Table 5, Figs. 2 and 3).
Table 5.
Area under the ROC curve for serum cytokines
Fig. 2.
Receiver operating characteristics (ROC) curve of serum IL-8 for the diagnostic discrimination of coal workers' pneumoconiosis (CWP) in all subjects (n = 110). The area under the ROC curve for serum interleukin-8 (IL-8) level was 0.70 (95% confidence interval, 0.58 to 0.82). The serum IL-8 level at 11.63 pg/mL was determined as the most optimal cutoff value with resulting sensitivity and specifi city of 69.3% and 63.6% for the presence of CWP.
Fig. 3.
Receiver operating characteristics (ROC) curve of serum tumor necrosis factor-alpha (TNF-α) for the discrimination of progression of peumoconiosis in the subjects with coal workers' pneumoconiosis (CWP) (n = 88). The area under the ROC curve for serum TNF-α level was 0.72 (95% confidence interval, 0.56 to 0.87). The serum TNF-α level at 4.52 pg/mL was determined as the most optimal cutoff value with resulting sensitivity and specifi city of 66.7% and 78.5% for the progression of pneumoconiosis.
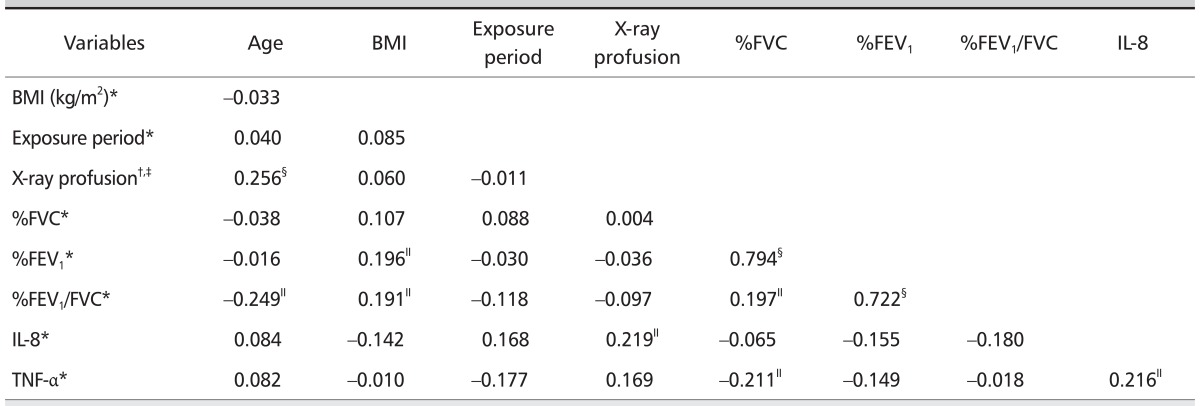
Relationships between serum cytokines levels and associated variables
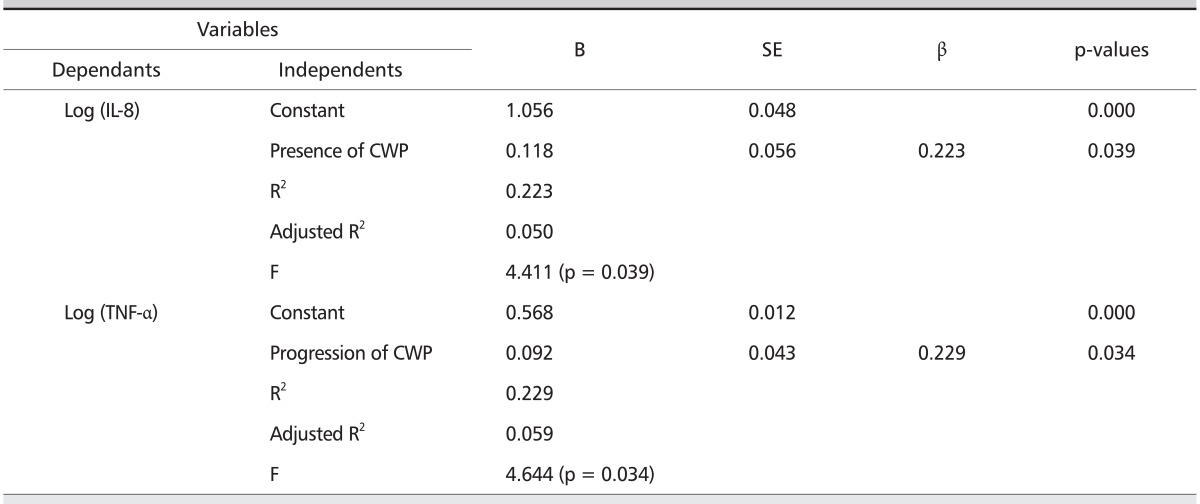
Serum IL-8 levels correlated with pneumoconiosis classifications in the CWP subjects with small opacity (rho = 0.219, p < 0.05) and serum TNF-α levels (r = 0.216, p < 0.05) (Table 6). Serum TNF-α levels correlated with %FVC predicted (r = 0.211, p < 0.05). Stepwise multiple regression analysis (Table 7) revealed that the serum IL-8 levels were positively related with the presence of CWP (adjusted R2 = 0.050, β = 0.223, p = 0.039), and a positive relation of serum TNF-α levels with the progression of CWP (adjusted R2 = 0.059, β = 0.229, p = 0.034).
Table 6.
Correlation coefficient between concentration of serum cytokines and independent variables (n = 110)
Cytokines were log transferred data.
BMI: body mass index, FVC: forced vital capacity, FEV1: forced expiratory volume in one second, IL-8: interleukin-8, TNF-α: tumor necrosis factor-alpha.
*Pearson's product moment correlation coefficient (r), §p < 0.01, ∥p < 0.05.
†Spearman's rank correlation coefficient (rho), §p < 0.01, ∥p < 0.05.
‡Severity (ILO stage)of pneumoconiosis with small opacity (n = 86).
Table 7.
Stepwise multiple regression analysis of serum cytokines against associated variables
Subjects: controls and CWP patients with small opacity (n = 86). Unit: pg/mL
B: regression coefficients, SE: standard error, β: standardized B, IL-8: interleukin-8, CWP: coal workers' pneumoconiosis, TNF-α: tumor necrosis factor-alpha, BMI: body mass inedx, FVC: forced vital capacity, FEV1: forced expiratory volume in on second.
Independent variables: age, BMI, exposure period, smoking status (yes/no), presence of CWP (yes/no), progression of CWP (yes/no), %FVC predicted, %FEV1 predicted, %FEV1/FVC ratio, and serum cytokines (IL-8, and TNF-α).
Discussion
CWP results from exposure to coal mine dust and is characterized by a progressive fibrotic reaction in the lung that can cause functional damage and irreversible change [1]. Fibrosis is a disorder characterized by a qualitative and quantitative alteration of the deposition of extracellular matrix with accumulation of mesenchymal cells in replacement of normal tissue. The sequence of events leading to fibrosis of an organ involves the subsequent processes of injury with inflammation and disruption of the normal tissue architecture, followed by tissue repair with accumulation of mesenchymal cells in this area [20]. Reactive oxygen species and cytokines may play important roles in mineral dust exposure and related lung disorders. However, the exact consequences of the mechanisms that occur in the lungs of subjects chronically exposed to coal dust are still unclear [1]. Castranova and Vallyathan [21] proposed that the development and progression of CWP occurs with four basic mechanisms: a) direct cytotoxicity of coal dust or silica; b) activation of oxidant production by pulmonary phagocytes; c) activation of mediator release from alveolar macrophages and epithelial cells, which leads to recruitment of polymorphonuclear leukocytes and macrophages, resulting in the production of proinflammatory cytokines and reactive species and in further lung injury and scarring; and d) secretion of growth factors from alveolar macrophages and epithelial cells, stimulating fibroblast proliferation and eventual scarring. The risk of CWP depends on the concentration and duration of exposure to coal dust that usually contains relatively small amounts of free crystalline silica (quartz). McCunney et al. [22] proposed that the active agent within coal is iron rather than quartz. Therefore, by identifying components of coal before mining activities, the risk of developing CWP might be reduced.
Alcohol alters cytokine levels in the blood and in a vari ety of tissues [23]. Acute ethanol intoxication may inhibit the production of IL-8 and TNF-α [24], and the abnormalities of production of inflammatory cytokines in chronic alcoholic patients observed depending on both the status of ethanol intake and the existence of alcoholic liver disease [25]. Although we presently lacked information on the alcohol consumption of the study subjects, the demonstrated physiologic and pathologic coordination of serum cytokines [26-28] prompted the exclusion of subjects who had exceed the criteria level related to the function of liver in serum, including aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase, because measurement of liver function is a standard diagnostic tool for the identification of chronic alcohol exposure [29]. We also excluded the subjects who had exceed the criteria level related to the function of kidney in serum, including blood urea nitrogen and creatinine [30,31].
Identification of biomarkers that are accurate and reliable in the prediction and early detection of CWP are imperative for the implementation of timely intervention strategies [3]. The present study was performed using one-year (2007-2008) prospective data and cross-sectional findings. The alveolar macrophage may be the first cell encountered by the invading organism and become activated. The activated alveolar macrophage will produce not only IL-8, but also early response cytokines such as TNF and IL-1. With the developing inflammatory response and increased permeability of the alveolar capillary membrane, TNF and IL-1 could stimulate other cells of membrane (pulmonary epithelial cells, interstitial fibroblasts, smooth-muscle cells, and endothelial cells) to establish a significant IL-8 chemotactic gradient, culminating in the recruitment of neutrophils into the lung parenchyma [32]. Useful biomarkers for crystalline silica- and CWP-pneumoconiosis that have been identified include IL-8 and TNF-α released from monocytes, Clara cell protein in serum, reactive oxygen species, and various antioxidants [3].
IL-8 is a member of a structurally similar family of cytokines designated chemokines, which display chemotactic activity for neutrophils. IL-8 has been implicated in a variety of inflammatory diseases. Various cells express IL-8 mRNA and rapidly produce IL-8 protein, including monocytes, T lymphocytes, neutrophils, fibroblasts, endothelial cells, and epithelial cells. IL-8 affects the adhesion of neutrophils to the endothelium and induces the transendothelial migration of neutrophils [13]. IL-8 is produced in response to proinflammatory stimuli. The accumulation of inflammatory leukocytes in the lung is a hallmark of either acute or chronic pulmonary inflammation [32]. Strieter et al. [12] reported the central role of the alveolar macrophage in the elicitation of polymorphonuclear cells into the lung via the production of IL-8. Although TNF has no direct neutrophil chemoattractant activity, which is potent inducer of IL-8 production in a variety of cell types, including alveolar macrophages, epithelial cells, lung fibroblasts, and endothelial cells [33,34]. Presently, serum IL-8 levels weakly correlated with serum TNF-α levels (p < 0.05). IL-8 is important in crystalline silica-induced lung inflammation [11] and its level is increased in the supernatant of spontaneous or coal-stimulated monocytes from peripheral blood and in sera of CWP patients [14]. Presently, the mean serum IL-8 level was significantly higher in CWP patients compared to controls (p = 0.007 for all comparisons). Serum IL-8 levels correlated with pneumoconiosis classifications in the subjects with small opacity (p < 0.05) and were positively related with the presence of CWP (p = 0.039). IL-8 is a chemoattractant cytokine having distinct target specificity for the neutrophil. However, it is also active on primed eosinophils and is involved in neutrophilic inflammation in asthma and chronic bronchitis [35], and IL-8 levels may be relevant to asthma pathogenesis [36]. Keatings et al. [37] suggested that IL-8, but not TNF-α, is significantly higher in patients with chronic obstructive pulmonary disease (COPD) than asthmatics, and that the TNF-α and IL-8 cytokines may be involved in COPD-related inflammation. In this study, serum IL-8 levels, but not TNF-α, tended to increase with severities of COPD (data not shown).
TNF-α is a proinflammatory cytokine that is important in the early onset of inflammation, development, and progression of several diseases including pulmonary fibrosis. TNF-α can be released by a number of cell types including activated macrophages, monocytes, and polymorphonuclear cells [1]. They are initiators of cytokine networks and lead to neutrophil recruitment and chemotaxis [38]. TNF-α has various roles that include synergistic effects in inflammatory and immune responses [3]. TNF-α would be responsible for the initiation and perpetuation of the inflammatory reaction observed in the lung of patients with progressive massive fibrosis. TNF-α, which can directly induce fibroblast proliferation, can also trigger the production of mediators [39]. The serum level of TNF-α level tends to increase in CWP [40]. TNF-α has been reported as a predicted biomarker for progressive pneumoconiosis, with a level that correlates with severity of pneumoconiosis [11], and as a useful index for coal dust exposure and a biomarker for pneumoconiosis with progressive fibrosis in the lung [3]. In the present study, the median serum TNF-α level was significantly higher in subjects with progression of CWP compared with subjects without progression (p = 0.027 for all comparisons). The serum TNF-α levels were positively related with the progression of CWP (p = 0.034).
Applying ROC curve analyses, AUC for IL-8 (0.70) values for the identification of CWP and TNF-α (0.72) for the pre diction of progression of CWP indicated the reasonable potential of IL-8 and TNF-α as biomarkers. The cutoff values of serum IL-8 and TNF-α were 11.63 pg/mL (sensitivity, 69%; specificity, 64%) and 4.52 pg/mL (sensitivity, 67%; specificity, 79%) res pectively. These results suggest that levels of serum IL-8 and TNF-α might serve as biomarkers for the presence of CWP and for the progression of CWP, respectively.
Although pneumoconiosis is the most prevalent lung disease that produces decreased pulmonary function and emphysema [1], the present difference between measured cytokines and PFT was not compelling, unless TNF-α showed a significant difference according to criteria level (80%) of %FEV1 predicted. The best explanation for these observations is that decreased PFT is the result of inflammation or fibrosis in the lung but cytokines influence the current inflammatory response.
The study has several limitations. One is that the follow-up period of 1 year was insufficient, especially in the absence of control data on subjects not exposed to mine dust. Another limitation concerns the lack of data of co-affective factors such as serum leptin related with TNF-α [41,42] and neurotrophic factor related with IL-8 [43]. Serum cytokines can be measured by various techniques that include enzyme-linked immunosorbent assay (ELISA), cytokine flow cytometry, and biochip array [44]. The mean serum TNF-α level determined using a biochip array was higher in controls and subjects with CWP compared with those reported by Zhai et al. [45] (1.7 and 1.4 pg/mL in affected subjects and controls, respectively), in coal miners with pneumoconiosis using ELISA. Optimal cutoff values may, therefore, differ depending on the method of analysis. However, presently, the serum levels of IL-8 and TNF-α significantly differed according to the presence or the progression of CWP, and the data in ROC analysis for the presence or the progression of CWP indicated the potential of IL-8 and TNF-α as biomarkers.
In conclusion, high levels of serum IL-8 are associated with the presence of CWP and those of serum TNF-α are associated with the progression of CWP. Further longitudinal follow-up studies are needed to investigate the potential of serum cytokines as useful biomarker of CWP, and future studies will be required to ascertain the cytokine profile in pneumoconiosis patients using lung specific specimens such as bronchoalveolar lavage fluid, exhaled breath condensate, or lung tissue.
Acknowledgments
The authors thank all retired coal miners who participated in this study. This study was conducted by the financial contribution of Korea Workers' Compensation & Welfare Service.
References
- 1.Schins RP, Borm PJ. Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg. 1999;43:7–33. doi: 10.1016/s0003-4878(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Silica, some silicates, coal dust and para-aramid fibrils. Lyon [France]: International Agency for Research on Cancer; 1997. p. 506. [Google Scholar]
- 3.Gulumian M, Borm PJ, Vallyathan V, Castranova V, Donaldson K, Nelson G, Murray J. Mechanistically identified suitable biomarkers of exposure, effect, and susceptibility for silicosis and coal-worker's pneumoconiosis: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2006;9:357–395. doi: 10.1080/15287390500196537. [DOI] [PubMed] [Google Scholar]
- 4.Porter DW, Hubbs AF, Mercer R, Robinson VA, Ramsey D, McLaurin J, Khan A, Battelli L, Brumbaugh K, Teass A, Castranova V. Progression of lung inflammation and damage in rats after cessation of silica inhalation. Toxicol Sci. 2004;79:370–380. doi: 10.1093/toxsci/kfh110. [DOI] [PubMed] [Google Scholar]
- 5.Elias JA, Zitnik RJ. Cytokine-cytokine interactions in the context of cytokine networking. Am J Respir Cell Mol Biol. 1992;7:365–367. doi: 10.1165/ajrcmb/7.4.365. [DOI] [PubMed] [Google Scholar]
- 6.Ates I, Suzen HS, Yucesoy B, Tekin IO, Karakaya A. Association of cytokine gene polymorphisms in CWP and its severity in Turkish coal workers. Am J Ind Med. 2008;51:741–747. doi: 10.1002/ajim.20632. [DOI] [PubMed] [Google Scholar]
- 7.Griwatz U, Seemayer NH. 29. P. 13 Release of cytokines by quartz and coal mine dust exposed macrophages. J Aerosol Sci. 1994;25(Suppl 1):495–496. [Google Scholar]
- 8.Lee JS, Shin JH, Lee JO, Lee WJ, Hwang JW, Kim JH, Choi BS. Blood levels of IL-l beta, IL-6, IL-8, TNF-alpha, and MCP-1 in pneumoconiosis patients exposed to inorganic dusts. Toxicol Res. 2009;25:217–224. doi: 10.5487/TR.2009.25.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prince P, Boulay ME, Pagé N, Desmeules M, Boulet LP. Induced sputum markers of fibrosis and decline in pulmonary function in asbestosis and silicosis: a pilot study. Int J Tuberc Lung Dis. 2008;12:813–819. [PubMed] [Google Scholar]
- 10.Ulker O, Yucesoy B, Demir O, Tekin I, Karakaya A. Serum and BAL cytokine and antioxidant enzyme levels at different stages of pneumoconiosis in coal workers. Hum Exp Toxicol. 2008;27:871–877. doi: 10.1177/0960327108098332. [DOI] [PubMed] [Google Scholar]
- 11.Schins RP, Borm PJ. Epidemiological evaluation of release of monocyte TNF-alpha as an exposure and effect marker in pneumoconiosis: a five year follow up study of coal workers. Occup Environ Med. 1995;52:441–450. doi: 10.1136/oem.52.7.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strieter RM, Chensue SW, Basha MA, Standiford TJ, Lynch JP, Baggiolini M, Kunkel SL. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factoralpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990;2:321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Chen H. The study on the interleukin-8 (IL-8) Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2002;19:697–702. [PubMed] [Google Scholar]
- 14.Kim KA, Lim Y, Kim JH, Kim EK, Chang HS, Park YM, Ahn BY. Potential biomarker of coal workers' pneumoconiosis. Toxicol Lett. 1999;108:297–302. doi: 10.1016/s0378-4274(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald SP, Lamont JV, McConnell RI, Benchikh el O. Development of a high-throughput automated analyzer using biochip array technology. Clin Chem. 2005;51:1165–1176. doi: 10.1373/clinchem.2005.049429. [DOI] [PubMed] [Google Scholar]
- 16.Molloy RM, Mc Connell RI, Lamont JV, FitzGerald SP. Automation of biochip array technology for quality results. Clin Chem Lab Med. 2005;43:1303–1313. doi: 10.1515/CCLM.2005.224. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 19.International Labour Office (ILO) Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Revised ed. Geneva (Switzerland): International Labor Organization; 2002. p. 51. [Google Scholar]
- 20.Vaillant P, Menard O, Vignaud JM, Martinet N, Martinet Y. The role of cytokines in human lung fibrosis. Monaldi Arch Chest Dis. 1996;51:145–152. [PubMed] [Google Scholar]
- 21.Castranova V, Vallyathan V. Silicosis and coal workers' pneumoconiosis. Environ Health Perspect. 2000;108(Suppl 4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCunney RJ, Morfeld P, Payne S. What component of coal causes coal workers' pneumoconiosis? J Occup Environ Med. 2009;51:462–471. doi: 10.1097/JOM.0b013e3181a01ada. [DOI] [PubMed] [Google Scholar]
- 23.Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- 25.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom. 2007;72:408–415. doi: 10.1002/cyto.b.20169. [DOI] [PubMed] [Google Scholar]
- 26.Avrămescu CS, Comănescu V, Popescu SN, Turculeanu A, Bălăoiu M, Popescu CF, Lungulescu M. Correlations among the serum levels of some interleukins and the histopathological aspects in chronic viral hepatitis C. Rom J Morphol Embryol. 2008;49:57–62. [PubMed] [Google Scholar]
- 27.Missale G, Ferrari C, Fiaccadori F. Cytokine mediators in acute inflammation and chronic course of viral hepatitis. Ann Ital Med Int. 1995;10:14–18. [PubMed] [Google Scholar]
- 28.Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 29.Helander A. Biological markers in alcoholism. J Neural Transm Suppl. 2003;66:15–32. doi: 10.1007/978-3-7091-0541-2_2. [DOI] [PubMed] [Google Scholar]
- 30.Brunzel NA. Renal function-nonprotein, nitrogen compounds, function tests, and renal disease. In: Anderson SC, Cockayne S, editors. Clinical chemistry: concepts and applications. New York (NY): McGraw-Hill Medical; 2003. pp. 373–399. [Google Scholar]
- 31.Ingram LR. Liver function. In: Anderson SC, Cockayne S, editors. Clinical chemistry: concepts and applications. New York (NY): McGraw-Hill Medical; 2003. pp. 285–321. [Google Scholar]
- 32.Strieter RM, Standiford TJ, Rolfe MW, Kunkel SL. Interleukine-8. In: Kelly J, editor. Cytokines of lung. New York (NY): Marcel Deckker; 1993. pp. 281–305. [Google Scholar]
- 33.Smart SJ, Casale TB. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994;266:L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- 34.Bittleman DB, Casale TB. Interleukin-8 mediates interleukin-1 alpha-induced neutrophil transcellular migration. Am J Respir Cell Mol Biol. 1995;13:323–329. doi: 10.1165/ajrcmb.13.3.7654388. [DOI] [PubMed] [Google Scholar]
- 35.Chanez P, Enander I, Jones I, Godard P, Bousquet J. Interleukin 8 in bronchoalveolar lavage of asthmatic and chronic bronchitis patients. Int Arch Allergy Immunol. 1996;111:83–88. doi: 10.1159/000237350. [DOI] [PubMed] [Google Scholar]
- 36.Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, Walters EH. Possible anti-inflammatory effect of salmeterol against interleukin-8 and neutrophil activation in asthma in vivo. Eur Respir J. 2003;21:994–999. doi: 10.1183/09031936.03.00109702. [DOI] [PubMed] [Google Scholar]
- 37.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced spu tum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 38.Lukacs NW, Ward PA. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–304. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 39.Vanhee D, Gosset P, Boitelle A, Wallaert B, Tonnel AB. Cytokines and cytokine network in silicosis and coal workers' pneumoconiosis. Eur Respir J. 1995;8:834–842. [PubMed] [Google Scholar]
- 40.Vallyathan V, Goins M, Lapp LN, Pack D, Leonard S, Shi X, Castranova V. Changes in bronchoalveolar lavage indices associated with radiographic classification in coal miners. Am J Respir Crit Care Med. 2000;162:958–965. doi: 10.1164/ajrccm.162.3.9909074. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ. Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocytederived macrophages. J Periodontal Implant Sci. 2010;40:119–124. doi: 10.5051/jpis.2010.40.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kythreotis P, Kokkini A, Avgeropoulou S, Hadjioannou A, Anastasakou E, Rasidakis A, Bakakos P. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2009;9:11. doi: 10.1186/1471-2466-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langford D, Masliah E. Role of trophic factors on neuroimmunity in neurodegenerative infectious diseases. J Neurovirol. 2002;8:625–638. doi: 10.1080/13550280290100996. [DOI] [PubMed] [Google Scholar]
- 44.Rostaing L, Peres C, Tkaczuk J, Charlet JP, Bories P, Durand D, Ohayon E, de Preval C, Abbal M. Ex vivo flow cytometry deter mi na tion of intracytoplasmic expression of IL-2, IL-6, IFN-gamma, and TNF-alpha in monocytes and T lymphocytes, in chronic hemodialysis patients. Am J Nephrol. 2000;20:18–26. doi: 10.1159/000013550. [DOI] [PubMed] [Google Scholar]
- 45.Zhai R, Liu G, Ge X, Bao W, Wu C, Yang C, Liang D. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin 6 (IL-6), and their soluble receptors in coal workers' pneumoconiosis. Respir Med. 2002;96:829–834. doi: 10.1053/rmed.2002.1367. [DOI] [PubMed] [Google Scholar]