Summary
Background and objectives
Delayed graft function (DGF) is associated with adverse long-term outcomes after deceased-donor kidney (DDK) transplantation. Ischemia-reperfusion injury plays a crucial role in the development of DGF. On the basis of promising animal data, this study evaluated any potential benefits of erythropoietin-alfa (EPO-α) given intra-arterially at the time of reperfusion of renal allograft on the degree of allograft function, as well as tubular cell injury measured by urinary biomarkers in the early post-transplant period.
Design, setting, participants, & measurements
A prospective, randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the influence of EPO-α administered intraoperatively on the outcomes of DDK transplantations performed at the study center between March 2007 and July 2009.
Results
Seventy-two patients were randomly assigned to EPO-α (n=36) or placebo (n=36). The incidences of DGF, slow graft function, and immediate graft function did not significantly differ between the treatment and control groups (41.7% versus 47.2%, 25.0% versus 36.1%, and 33.3% versus 16.7%, respectively; P=0.24). The groups had similar levels of urinary biomarkers, including neutrophil gelatinase-associated lipocalin and IL-18 at multiple times points soon after transplantation; urinary output during the first 3 postoperative days; 1-month renal function; and BP readings, hemoglobin, and adverse effects during the first month.
Conclusions
This study did not show any clinically demonstrable beneficial effects of high-dose EPO-α given intra-arterially during the early reperfusion phase in DDK transplant recipients in terms of reducing the incidence of DGF or improving short-term allograft function.
Introduction
Delayed graft function (DGF) negatively affects long-term outcome after deceased donor kidney (DDK) transplantation. A meta-analysis of 34 studies showed a 41% increase in long-term graft loss and a 38% relative increase in acute rejection in patients who developed DGF after renal transplantation (1). DDK transplant recipients with DGF were more likely to die with a functioning graft than were those without DGF (2). Data from United States scientific registries have shown an increase in the rate of DGF, from 14.7% during 1985–1992 to 23% for the 1998–2004 transplant cohorts (2,3). This increase has been contemporaneous with the use of expanded-criteria donor (ECD) and donation of kidneys after cardiac death. Risk factors for the development of DGF extend from the identification of a donor through the postoperative period and beyond (4). Among many factors, ischemia-reperfusion injury (IRI) plays a crucial role in the development of DGF. Studies in animal models and cultured human renal tubular cells have shown that erythropoietin may protect against experimentally induced renal IRI (5–11). Animal studies have also shown protective effect of erythropoietic agents in brain injury and cardiac IRI (12–15).
Currently, few options exist for avoiding or minimizing DGF, such as keeping patients euvolemic, avoiding hypotension and nephrotoxins, and minimizing warm and cold ischemia times. On the basis of the promising results from animal studies, and because kidney transplantation represents a classic model for AKI from IRI, it appears worthwhile to evaluate whether erythropoietin-alfa (EPO-α) given during early reperfusion would protect against IRI in DDK transplant recipients. EPO-α is a relatively safe medication used extensively in anemia of CKD.
Biomarkers have shown great promise in the early prediction of AKI with high sensitivity (16). Urinary neutrophil gelatinase-associated lipocalin (NGAL) and IL-18 predicted AKI soon after cardiac surgery and DGF very soon after kidney transplantation (17–19). NGAL is a 25-kD protein belonging to the lipocalin superfamily initially found in activated neutrophils. It is also found in certain epithelial cells, such as those in renal tubules, where its expression is dramatically increased after ischemic or nephrotoxic injury. IL-18 is a pro-inflammatory cytokine that is cleaved to mature IL-18 by caspase-1 in injured proximal tubules and is released into the urine (20).
We aimed to look at the effect of EPO-α given intra-arterially at the time of reperfusion of renal allograft on the degree of allograft function in the early post-transplant period. We also aimed to evaluate potential benefits of EPO-α on tubular cell damage by measuring levels of urinary biomarkers of kidney injury, including NGAL and IL-18.
Materials and Methods
We conducted a prospective, randomized, double-blind, placebo-controlled clinical trial to evaluate the influence of EPO-α administered intraoperatively on the outcomes of DDK transplantations performed at our center between March 2007 and July 2009. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was consistent with the International Conference on Harmonization of Good Clinical Practice. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The institutional review board approved the study protocol, and informed consent was obtained from each patient. The study is registered with ClinicalTrials.gov (NCT00425126).
Patients
Enrolled patients were ≥18 years of age and received a DDK transplant. Perioperative induction agents were rabbit-antithymocyte globulin (r-ATG), alemtuzumab, or basiliximab. The first dose of r-ATG (1.5 mg/kg) was given intraoperatively, followed by repeated doses during the next 3 days; the target cumulative dose was 6 mg/kg. Alemtuzumab was given as single 25-mg dose intraoperatively and basiliximab as two doses of 20 mg each, with the first dose given intraoperatively and the second dose given on postoperative day (POD) 3. Maintenance immunosuppression included a calcineurin inhibitor (microemulsion cyclosporine in two divided doses with a target trough level of 250–300 ng/ml or tacrolimus in two divided doses to maintain targeted trough level of 10–12 ng/ml) and mycophenolate mofetil at a dose of 1 g twice daily in r-ATG–induced and basiliximab-induced patients and 500 mg twice daily in alemtuzumab-induced patients. We used an early steroid-withdrawal protocol. Patients received 500 mg of intravenous methylprednisolone intraoperatively, followed by 250 mg on POD1, 125 mg on POD 2, prednisone 60 mg on POD 3, and 30 mg on POD 4, with subsequent discontinuation of steroids unless the patient were receiving long-term steroid treatment or were deemed to be at high immunologic risk. For those patients, steroids were continued. Patients were excluded from the study if they had a history of thrombosis (such as deep venous thrombosis, pulmonary embolism, or previous renal allograft thrombosis), hypercoagulable state, cerebrovascular accident, or acute coronary syndrome in the preceding 6 months. Patients undergoing live-donor or pediatric en bloc kidney and multiorgan transplantations were also excluded, as were those with preoperative hemoglobin level >12.5 g/dl, systolic BP >180 mmHg, seizure disorder, and allergic reaction to erythropoietin.
Randomization and Intervention
Patients who were admitted to our institution for kidney transplantation during the study period were approached for participation in the clinical trial. Study patients were randomly assigned to EPO-α (Procrit, Ortho Biotech, Titusville, NJ) or matched placebo. The hospital pharmacy created a randomization schedule using random assignments to a series of patient study numbers. The pharmacy filled syringes with 1 ml of normal saline or EPO-α (1 ml containing 40,000 units). The study medication was administered intraoperatively in a double-blinded manner into the ipsilateral iliac artery proximal to the graft anastomotic site immediately after the release of arterial clamp. The transplantation was otherwise performed in the standard manner.
Processing and Storage of Urinary Samples.
For each patient, approximately 10 ml of urine was collected at 0, 6, and 12 hours after transplantation, as well as POD 1 and 2. Time 0 was defined as the time surgery was completed, as documented in the anesthesia flow sheet. Each sample was placed in a centrifuge at 3300 RPM for 3 minutes. Evening samples were placed on ice overnight and were centrifuged the following morning. The urinary supernatant was frozen at −80°C and assayed in batches for NGAL, IL-18, and creatinine levels.
Measurement of Urinary NGAL and IL-18.
Urinary NGAL was measured using ELISA as described elsewhere (21).
Urinary IL-18 was quantified using a human IL-18 ELISA kit (Medical and Biologic Laboratories, Nagoya, Japan) that specifically detects the mature form of IL-18 as previously described (22). The measured values for the urinary biomarkers were normalized to urinary creatinine concentration in order to account for the differences in the relative amounts of water extracted along the nephron. Normalization to urinary creatinine concentration improves the prediction of incipient AKI and outcome (23).
Creatinine Determination.
Serum and urine creatinine concentrations were measured by the Jaffé assay using the P module of a Cobas analyzer (Roche Diagnostics, Indianapolis, IN) and the Beckman creatinine analyzer system (Beckman Instruments Inc., Fullerton, CA), respectively.
Outcomes
The primary end point was the level of graft function in the early post-transplant period, categorized as follows: DGF (defined as the need for dialysis within the first week of transplantation), slow graft function (SGF, ≤40% decrease in serum creatinine by POD 3), and immediate graft function (IGF, >40% decrease in serum creatinine by POD 3). Secondary end points included degree of AKI assessed using urinary NGAL and IL-18 levels measured at multiple time points as mentioned; incidence of allograft primary nonfunction; urine output during first 3 days after transplantation; 1-month serum creatinine and estimated GFR calculated according to the Modification of Diet in Renal Diseases equation; BP readings and hemoglobin levels at weeks 1, 2, and 4; and adverse events.
Statistical Analyses
A power analysis was performed with the following assumptions: α error of 0.05, β error of 0.2, and DGF incidences of 50% in the control group and 20% in the treatment group, resulting in the requirement for approximately 38 patients in each group. Continuous variables were expressed as the mean ± SD for parametric data (and compared between the groups using two-sided t test) or median with interquartile range for nonparametric data (and compared with Mann-Whitney U test). Variables such as biomarkers, BP, and hemoglobin that were expressed at multiple time points were compared between the groups using repeated-measures ANOVA. Categorical variables were expressed as percentages and were compared using chi-squared analysis. A P value <0.05 was considered to represent a statistically significant difference.
Results
Patient Characteristics
Patient enrollment into the study is shown in the flow chart (Figure 1). Seventy-nine patients were eligible for enrollment in the study and provided informed consent. However, 7 patients failed to receive the study drug in the operating room. The remaining 72 patients were enrolled in the study; 36 were randomly assigned to receive EPO-α and 36 to placebo (normal saline). Demographic features of the two groups are shown in Table 1. Overall, the groups were similar. A significantly higher number of donors in the EPO-α group were receiving pressors (86% versus 67%; P=0.05). There were higher prevalences of donor death from cerebrovascular accident (39% versus 9%; P=0.07) and pretransplant dialysis dependence (89% versus 72%; P=0.07) in the placebo than the intervention group, which neared statistical significance. The causes of renal failure in the EPO-α versus placebo groups were as follows: diabetes (14 versus 18), GN (7 versus 3), hypertension (3 versus 4), polycystic kidney disease (2 versus 4), and other causes (10 versus 7). Induction agents used in the EPO-α versus the placebo group were as follows: r-ATG (7 versus 9; P=0.78), alemtuzumab (12 versus 11; P=1.00), and basiliximab (17 versus 16; P=1.00). Maintenance immunosuppression included cyclosporine (control, 17; treatment, 15) or tacrolimus (control, 19; treatment, 21), along with mycophenolate mofetil in 34 patients in each group. Early steroid withdrawal was used in 33 of the control and 31 of the intervention recipients. Trough cyclosporine levels were similar for the control versus treatment groups on POD 3 (336±109 versus 360±97 ng/ml; P=0.53) and at week 1 (286±106 versus 290 ±80 ng/ml; P=0.92), week 2 (293±100 versus 296±108 ng/ml; P=0.93), and week 4 (244±65 versus 267±96 ng/ml; P=0.29). Trough tacrolimus levels were also similar for the control versus the treatment group on POD 3 (9.3±2.6 versus 10.0±4.3 ng/ml; P=0.57) and at week 1 (10.2±2.5 versus 10.0±3.7 ng/ml; P=0.89), week 2 (11.3±3.8 versus 10.7±3.2, P=0.60), and week 4 (11.8±3.5 versus 10.1±2.3 ng/ml, P=0.08).
Figure 1.
Flow chart showing patient enrollment into the study.
Table 1.
Demographic characteristics of the study groups
| Characteristic | Control Group (n=36) | EPO-α Group (n=36) | P Value |
|---|---|---|---|
| Donor factors | |||
| age (yr) | 41±17 | 39±17 | 0.63 |
| men/women (%) | 56/44 | 44/56 | 0.30 |
| ECD kidney (%) | 22 | 11 | 0.21 |
| death from CVA (%) | 39 | 19 | 0.07 |
| terminal urine output (ml/hr) | 159±151 | 167±147 | 0.83 |
| terminal serum creatinine (mg/dl) | 1.18±0.90 | 1.14±0.85 | 0.84 |
| pressor use (%) | 67 | 86 | 0.05 |
| Recipient factors | |||
| age (yr) | 56±13 | 58±11 | 0.53 |
| men/women % | 53/47 | 56/44 | 0.81 |
| African Americans (%) | 14 | 19 | 0.53 |
| body mass index (kg/m2) | 28.3±6.4 | 27.8±5.4 | 0.71 |
| diabetes mellitus (%) | 56 | 47 | 0.48 |
| hypertension (%) | 92 | 94 | 0.64 |
| coronary artery disease (%) | 36 | 25 | 0.31 |
| previous dialysis (%) | 89 | 72 | 0.07 |
| previous transplant (%) | 22 | 17 | 0.55 |
| previous ESA use (%) | 86 | 69 | 0.09 |
| recent PRA (%) | 1.7±8.8 | 3.3±15.3 | 0.66 |
| HLA mismatch | 3.6±1.4 | 3.2±1.6 | 0.36 |
| Transplant-related factors | |||
| cold ischemia time (hr) | 26.3±8.0 | 24.1±6.1 | 0.21 |
| warm ischemia time (min) | 14.2±13.2 | 16.3±16.3 | 0.62 |
| lowest systolic BP in operating room (mmHg) | 101±17 | 98±11 | 0.31 |
Data expressed with plus/minus sign are the mean ± SD. EPO, erythropoietin; ECD, expanded-criteria donor; CVA, cerebrovascular accident; ESA, erythropoietic stimulating agent; PRA, panel-reactive antibody.
Immediate Post-Transplant Allograft Function
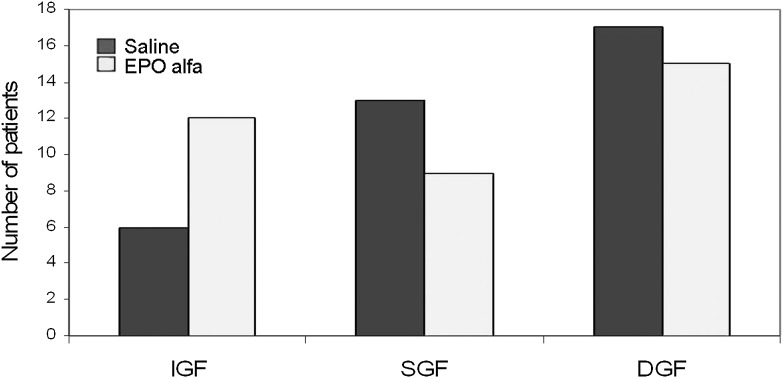
The incidences of DGF, SGF, and IGF after transplantation are shown in Figure 2. Of 72 patients enrolled in the study, 32 (44%) developed DGF. This includes 4 patients (2 in each group) who continued to have primary nonfunction of the graft. SGF was observed in 22 (31%) and IGF in 18 (25%) patients. There were no significant differences in the incidences of DGF, SGF, or IGF in the treatment versus control groups (41.7% versus 47.2%, 25.0% versus 36.1%, and 33.3% versus 16.7%, respectively; P=0.24). The number of dialysis treatments required per patient within the first week of transplantation was similar between treatment and control groups (1.3±2.0 versus 1.4±2.0; P=0.83). Five patients (3 in the treatment and 2 in the control group) underwent allograft biopsy within the first month of transplantation. Four biopsy samples showed no evidence of AR, and one biopsy sample (in the treatment group) showed evidence of severe antibody-mediated rejection with graft thrombosis that necessitated transplant nephrectomy.
Figure 2.
Incidences of delayed graft function (DGF), slow graft function (SGF), and immediate graft function (IGF) between the groups. There were no significant differences between the groups (P=0.24). EPO, erythropoietin.
Urinary Biomarkers of Kidney Injury
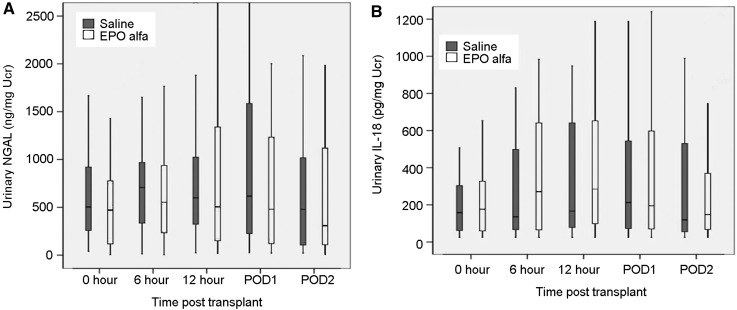
There were no significant differences between the groups in the levels of urinary NGAL (Figure 3A; P=0.35) or IL-18 (Figure 3B; P=0.53) at any time points.
Figure 3.
Comparison of urinary neutrophil gelatin-associated lipocalin (NGAL) and IL-18 levels between the groups at various time points in the early post-transplant period. The differences in NGAL (A) and IL-18 (B) were not statistically significant (P=0.35 and 0.53, respectively). EPO, erythropoietin.
Early Postoperative Urine Output
Urine output in the first 3 postoperative days was numerically higher in the treatment than the placebo group but did not reach statistical significance. Urine output (ml/d), expressed as median (interquartile range), was as follows: POD 1, 1700 (299–3701) versus 654 (255–2081) (P=0.42); POD 2, 1810 (470–3338) versus 1133 (412–2254) (P=0.29); and POD 3, 1675 (460–2965) versus 1125 (464–2845) (P=0.61).
Allograft Function, Patterns of BP, and Hemoglobin at 1 Month
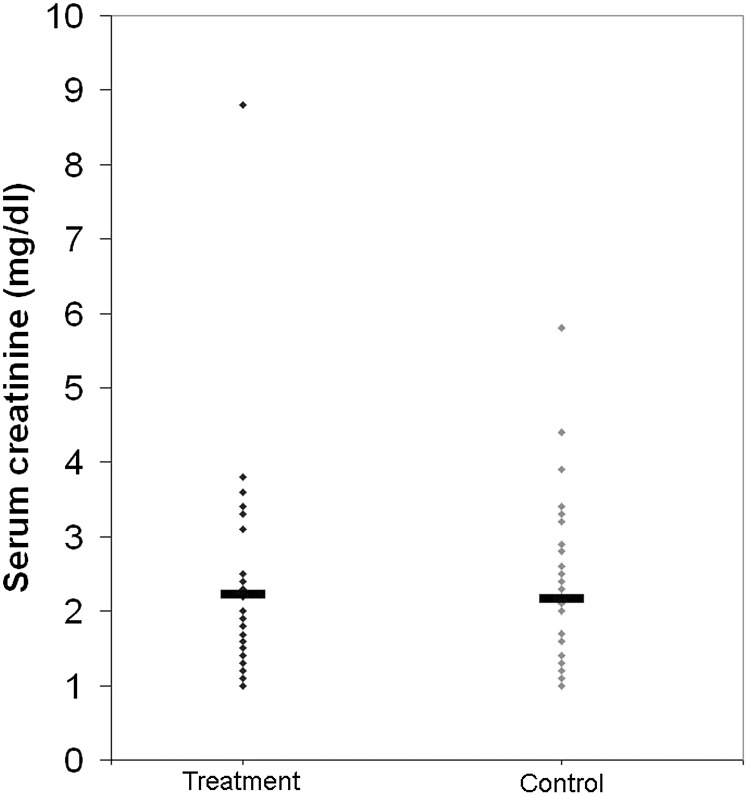
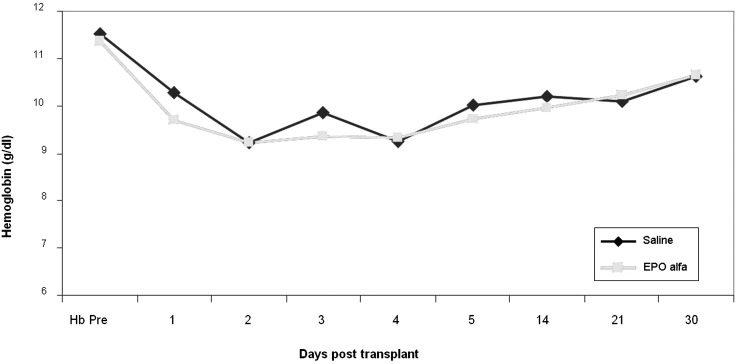
Serum creatinine levels at 4 weeks after transplantation were similar between the EPO-α and the control groups (2.23±1.38 versus 2.16±1.05 mg/dl; P=0.81 [Figure 4]). The estimated GFR at 4 weeks was also similar between the EPO-α and control groups (37.0±14.8 versus 36.7±14.1 ml/min per 1.73 m2; P=0.35). Systolic BP readings were numerically higher in the EPO-α group (P=0.05), and diastolic BP readings were similar between the groups before transplantation and at weeks 1, 2, and 4 after transplantation (Table 2). Hemoglobin levels were similar between the groups before transplantation, decreased after transplantation, and followed a similar pattern in both groups. No erythropoietic effect was evident in the EPO-α group (Figure 5).
Figure 4.
Serum creatinine levels in the treatment and control groups at 1 month. P=0.81.
Table 2.
BP patterns in the study groups
| Time Period | Systolic BPa | Diastolic BPa | ||||
|---|---|---|---|---|---|---|
| EPO-α Group | Control Group | P Value | EPO-α Group | Control Group | P Value | |
| Preop | 150±19 | 137±31 | 77±14 | 78±15 | ||
| Week 1 | 144±19 | 143±18 | 73±14 | 75±16 | ||
| Week 2 | 142±19 | 137±15 | 0.05 | 75±14 | 76±10 | 0.72 |
| Week 4 | 138±16 | 131±17 | 73±10 | 74±10 | ||
Data are the mean ± SD in mmHg. EPO, erythropoietin.
BP was measured after the patient rested for 10 minutes
Figure 5.
Comparison of hemoglobin levels before transplantation and several time points after transplantation between the groups. P=0.52. EPO, erythropoietin.
Safety
Adverse events during the study period are shown in Table 3 and were similar between the groups. No patients died during the study period.
Table 3.
Adverse events within first 30 days
| Adverse Event | Control Group | EPO-α Group |
|---|---|---|
| Intraoperative hypotension | 0 | 2 |
| Intraoperative bleeding | 3 | 0 |
| Postoperative bleeding | 1 | 2 |
| Deep venous thrombosis | 0 | 0 |
| Graft thrombosis | 0 | 1 |
| Lower-extremity ischemia | 0 | 1 |
| Death | 0 | 0 |
Data are number of patients with adverse event. EPO, erythropoietin.
Discussion
Our study did not show a significant reduction in the incidence of DGF or SGF or increase in IGF after the intra-arterial administration of high-dose EPO-α versus placebo at the time of surgical reperfusion of DDK transplants. Urinary biomarkers of AKI, including NGAL and IL-18, as well as urine output, were not significantly different between the two groups during the early post-transplant period. Systolic BP readings were numerically higher in the EPO-α group and diastolic BP readings were similar between the groups before transplantation as well as at weeks 1, 2, and 4 after surgery. Allograft function at 1 month was similar between the groups. High-dose EPO-α was tolerated, and there were no differences in the incidence of adverse events between the groups.
Animal studies have shown encouraging results regarding the ability of erythropoietin to protect the kidneys against IRI. The study by Sharples et al. showed a reduction in the increase of serum creatinine, improved urine flow rate, and a marked reduction of the histologic features of renal injury and the extent of apoptotic cell death when EPO-α was given intravenously 30 minutes before the commencement of ischemia and 5 minutes before the onset of reperfusion in male Wistar rats subjected to bilateral renal artery occlusion for 45 minutes (5). It was proposed that the protective effect of EPO on the proximal tubular epithelial cells is mediated through activation of EPO receptors. EPO receptors have been identified in rat and human kidney tissue (including cortex, medulla, and papilla), as well as in renal cell lines (including proximal tubular, mesangial, and collecting duct cells) (24). Activation of EPO receptors in turn leads to upregulation of Janus-activated kinase 2 (JAK2) signaling. JAK2 activation was shown to enhance PI3K and Akt (a serine/threonine protein kinase B) phosphorylation in endothelial and neuronal cells (25). Activated Akt works on multiple targets with antiapoptotic effects, including phosphorylation of caspase-9, maintenance of mitochondrial membrane potential, and preservation of glycolysis and ATP synthesis (26). The study by Vesey et al. demonstrated significant antiapoptotic and mitogenic effects of erythropoietin in isolated human proximal tubular cell lines exposed to hypoxia, with subsequent re-oxygenation in vitro (7). EPO administration also protected against experimental cisplatin-nephrotoxicity in rats (8,27). In the rat model of kidney transplantation, darbepoetin and carbamylated EPO given to donors and recipients protected the allograft from IRI by reducing oxidative stress and apoptosis. This cytoprotection was mediated through activation of tubular phosphorylated-Akt and peritubular capillary preservation (28).
A substantial body of animal data shows the neuroprotective effects of systemic EPO administered at 5000 U/kg for various brain injuries (12,13,29,30). Observed neurocytoprotective effects of EPO may be related to various mechanisms, including JAK2 phosphorylation of IκB and subsequent NF-κB–dependent transcription of neuroprotective genes, antioxidation, direct neurotrophic effects, angiogenesis, and inhibition of nitric oxide production (13,30,31). However, a recent study did not find any benefits of EPO in patients presenting with acute ischemic stroke and raised safety concern for its use, particularly in patients receiving thrombolytic therapy (32). Similarly, administration of high-dose intravenous EPO did not reduce infarct size in patients presenting with ST-segment elevation myocardial infarction who received thrombolytic therapy or percutaneous coronary intervention; the latter study found higher rates of adverse cardiovascular events in the EPO group (33,34).
To our knowledge, our study is the first randomized, double-blind trial that incorporated urinary biomarkers of AKI to test whether high-dose EPO-α administered directly into the iliac artery at the close proximity of the transplant renal artery anastomotic site soon after surgical reperfusion would positively influence immediate allograft outcome. An EPO-α dose of 40,000 U was chosen for the study on the basis of the previously used doses of 5000 U/kg in neuroprotective trials. By this, we aimed to achieve a relatively higher concentration of EPO-α molecules within the allograft in the very early stages of reperfusion. Measurement of urinary biomarkers of AKI, including NGAL and IL-18, at multiple time points within the early post-transplant period was included in the study in an attempt to evaluate any possible subtle attenuation of tubular damage conferred by EPO-α that may not manifest in other commonly used clinical tests. Our study failed to translate the promising experimental findings to the clinical setting despite a relatively higher incidence of DGF among the study population.
The recently published prospective study by Hafer et al. randomly assigned 88 patients to three doses of EPO-α at 40,000 units each (first dose given intra-arterially in the operating room, second and third doses given intravenously on POD 3 and 7) or placebo (35). The authors found no differences between the groups in terms of DGF, allograft function at week 6 or months 6 and 12, or histologic features (such as acute tubular injury and interstitial fibrosis at 6 weeks and 6 months). It should be noted that patients with higher risk for adverse outcomes, such as retransplant recipients with immunologic loss of their first transplant within the first year, those with cold ischemia time >24 hours, and those with panel-reactive antibody titer >25% were excluded from that study. High-dose EPO-β did not preserve renal allograft function or reduce the incidence of DGF or SGF among ECD kidney recipients in a randomized open-label study without a placebo group (36). EPO-β, 30,000 U, was administered intravenously 3–0.5 hours before transplantation, followed by subcutaneous injections of same dose at 12–24 hours and weeks 1 and 2 after transplantation. That study did not use urinary biomarkers of AKI or histology. Early administration of EPO did not reduce the incidence of AKI in intensive care unit patients at risk for AKI in a double-blind, placebo-controlled trial (37).
Our study had limitations. It is difficult to exclude a type 2 error due to sample size. This study aimed for 80% power to detect a relatively large difference in DGF, from 50% in the control group to 20% in the treatment group. It was not designed to detect smaller differences. There is a limitation of using DGF as a primary end point because the decision to dialyze is somewhat subjective and depends on the treating physician’s clinical judgment. One cannot exclude antiapoptotic and regenerative effects of EPO-α because of a lack of histologic and immunohistochemical data. Dosing of EPO-α is somewhat arbitrary, and it is possible that higher or frequent doses may be needed to demonstrate potential clinical benefits because we cannot exclude possible decrease in EPO-receptor density in the tubules resulting from ischemic damage. Most patients with advanced CKD are exposed to EPO agents before transplantation as part of anemia management, and it is difficult to exclude the residual effects of EPO agents, depending on the timing of the last dose received. However, this probably affected both groups in a similar manner. Because ischemic injury of the allograft begins at the time of organ procurement, it would be interesting to know whether earlier administration of EPO, such as during cold storage and machine perfusion, would result in any measurable beneficial effects.
In summary, this study did not show any clinically demonstrable beneficial effects of high-dose EPO-α given intra-arterially during the early reperfusion phase in DDK transplant recipients in terms of reducing the incidence of DGF or improving short-term allograft function.
Disclosures
None.
Acknowledgments
We thank Chirag R. Parikh for performing the assay for urinary biomarkers. We also thank Dai D. Nghiem and Carlos A. Vivas from the Division of Abdominal Transplantation, as well as Fellows from the Division of Nephrology and Hypertension at Allegheny General Hospital who assisted in the conduct of this study.
This study was supported by Ortho Biotech Clinical Affairs/Johnson & Johnson, Contract 06OBCA990002. Authors were solely responsible for the study design and manuscript writing.
This work was presented in part as a poster at the American Society of Nephrology Meeting and Scientific Exposition, November 2010, Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, Kim SJ: Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 21: 153–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL: Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 63: 968–974, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM: Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol 15: 2115–2124, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Ragolia L, Palaia T, Johnson B, Elzein H, Maesaka JK: Cytoprotection by darbepoetin/epoetin alfa in pig tubular and mouse mesangial cells. Kidney Int 65: 452–458, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Vesey DA, Cheung C, Pat B, Endre Z, Gobé G, Johnson DW: Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant 19: 348–355, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bagnis C, Beaufils H, Jacquiaud C, Adabra Y, Jouanneau C, Le Nahour G, Jaudon MC, Bourbouze R, Jacobs C, Deray G: Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol Dial Transplant 16: 932–938, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Patel NS, Sharples EJ, Cuzzocrea S, Chatterjee PK, Britti D, Yaqoob MM, Thiemermann C: Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int 66: 983–989, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC: Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int 69: 1806–1813, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, Tsakiris D: Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant 21: 330–336, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A: Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 97: 10526–10531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P: Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A 98: 4044–4049, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Semenza GL: Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation 109: 2050–2053, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ: A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 112: 999–1007, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soni SS, Pophale R, Ronco C: New biomarkers for acute renal injury. Clin Chem Lab Med 49: 1257–1263, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, Hewitt SM, Yuen PS, Star RA: Acute kidney injury biomarkers—needs, present status, and future promise. NephSAP 5: 63–71, 2006 [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ralib AM, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, Endre ZH: Test characteristics of urinary biomarkers depend on the quantitation method in acute kidney injury. J Am Soc Nephrol 23: 322–333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westenfelder C, Biddle DL, Baranowski RL: Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int 55: 808–820, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Chong ZZ, Kang JQ, Maiese K: Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation 106: 2973–2979, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Cantley LC: The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Salahudeen AK, Haider N, Jenkins J, Joshi M, Patel H, Huang H, Yang M, Zhe H: Antiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1354–F1365, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Cassis P, Azzollini N, Solini S, Mister M, Aiello S, Cugini D, Scudeletti P, Gagliardini E, Abbate M, Gallon L, Remuzzi G, Noris M: Both darbepoetin alfa and carbamylated erythropoietin prevent kidney graft dysfunction due to ischemia/reperfusion in rats. Transplantation 92: 271–279, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R: In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A 95: 4635–4640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M: Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol 401: 349–356, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Digicaylioglu M, Lipton SA: Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 412: 641–647, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C, EPO Stroke Trial Group : Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40: e647–e656, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Binbrek AS, Rao NS, Al Khaja N, Assaqqaf J, Sobel BE: Erythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST segment elevation acute myocardial infarction. Am J Cardiol 104: 1035–1040, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA, REVEAL Investigators : Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: A randomized controlled trial. JAMA 305: 1863–1872, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hafer C, Becker T, Kielstein JT, Bahlmann E, Schwarz A, Grinzoff N, Drzymala D, Bonnard I, Richter N, Lehner F, Klempnauer J, Haller H, Traeder J, Fliser D: High-dose erythropoietin has no effect on short- or long-term graft function following deceased donor kidney transplantation. Kidney Int 81: 314–320, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Martinez F, Kamar N, Pallet N, Lang P, Durrbach A, Lebranchu Y, Adem A, Barbier S, Cassuto-Viguier E, Glowaki F, Le Meur Y, Rostaing L, Legendre C, Hermine O, Choukroun G: NeoPDGF Study Investigators: High dose epoietin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo-PDGF Study. Am J Transplant 10: 1695– 1700, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JB, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM: Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 77: 1020–1030, 2010 [DOI] [PubMed] [Google Scholar]