Summary
Background and objectives
Nonlinear measures of heart rate variability (HRV) have gained recent interest as powerful risk predictors in various clinical settings. This study examined whether they improve risk stratification in hemodialysis patients.
Design, setting, participants, & measurements
To assess heart rate turbulence, deceleration capacity, fractal scaling exponent (α1), and other conventional HRV measures, 281 hemodialysis patients underwent 24-hour electrocardiography between January 2002 and May 2004 and were subsequently followed up.
Results
During a median 87-month follow-up, 77 patients (27%) died. Age, left ventricular ejection fraction, serum albumin, C-reactive protein, and calcium × phosphate independently predicted mortality. Whereas all nonlinear HRV measures predicted mortality, only decreased scaling exponent α1 remained significant after adjusting for clinical risk factors (hazard ratio per a 0.25 decrement, 1.46; 95% confidence interval [95% CI], 1.16–1.85). The inclusion of α1 into a prediction model composed of clinical risk factors increased the C statistic from 0.84 to 0.87 (P=0.03), with 50.8% (95% CI, 20.2–83.7) continuous net reclassification improvement for 5-year mortality. The predictive power of α1 showed an interaction with age (P=0.02) and was particularly strong in patients aged <70 years (n=208; hazard ratio, 1.87; 95% CI, 1.38–2.53), among whom α1 increased the C statistic from 0.85 to 0.89 (P=0.01), with a 93.1% (95% CI, 59.3–142.0) continuous net reclassification improvement.
Conclusions
Scaling exponent α1 that reflects fractal organization of short-term HRV improves risk stratification for mortality when added to the prediction model by conventional risk factors in hemodialysis patients, particularly those aged <70 years.
Introduction
The annual crude mortality rate in hemodialysis patients with ESRD in Japan is approximately 9% (1). Despite the progress of hemodialysis techniques, this number has remained unchanged over the last 15 years. Because the number of hemodialysis patients is increasing, better risk stratification and concentration of clinical resources are required to reduce the mortality.
Analysis of heart rate variability (HRV) by 24-hour electrocardiography has become a noninvasive tool for risk stratification in various clinical settings, including hemodialysis patients (2–5). Conventionally, two categories of measures have been used: time-domain HRV measures calculated as statistics of R-R intervals and frequency-domain HRV measures computed by spectrum analysis as R-R interval variances within specific frequency bands (6). Both of these measures reflect the magnitude of heart rate fluctuation and their decreases are associated with increased risk for adverse outcomes. Their prognostic associations have been attributed mainly to cardiac vagal dysfunction, because the magnitude of HRV is substantially dependent on the vagal modulation of heart rate (7). Recently, nonlinear measures of HRV have been developed as new risk predictors (8). These measures quantify various properties of heart rate dynamics, such as response patterns and self-correlations, which are caused by complex interplays between vagal and sympathetic regulations. Clinically, three measures have gained recent interest: heart rate turbulence (HRT) quantifying heart rate responses to premature beats (9), deceleration capacity (DC) estimating ability to decelerate heart rate on specific time scales (10), and fractal scaling exponents assessing fractal organization of heart rate regulation based on chaos theory (11). Several studies have indicated that these nonlinear HRV measures have greater predictive power than conventional HRV measures for mortality after myocardial infarction (MI) (12–14). In hemodialysis patients, the nonlinear measures might have greater discrimination ability than conventional measures, because cardiac vagal dysfunction is almost commonly observed among these patients (15–17). Although alterations in nonlinear HRV measures have been reported in hemodialysis patients (18,19), their prognostic value has not been examined with a sufficient sample size.
In this study, we sought to examine the independent predictive value of nonlinear HRV measures, including HRT, DC, and fractal scaling exponents, as well as conventional time- and frequency-domain HRV measures in hemodialysis patients who were subsequently followed up for 7.3 years. Our particular interest was whether the inclusion of nonlinear HRV measures improves risk stratification that can be achieved by conventional clinical risk factors in these patients.
Materials and Methods
Patients
Patients with ESRD who were on regular chronic hemodialysis therapy (a 4-hour hemodialysis session, three times a week) at the Hemodialysis Center of Nagoya Kyoritsu Hospital between January 2002 and May 2004 were eligible for this study. Patients were excluded if they had an implanted cardiac pacemaker or if their Holter electrocardiogram showed atrial fibrillation or sinus rhythm <75% of total beats.
The protocol was approved by the Ethics Review Board of Nagoya Kyoritsu Hospital. All participants gave their written informed consent to participate in this study.
Protocols
Baseline clinical data—including medical history, medications, blood cell count and chemistry, hemodialysis efficiency, and a chest radiograph and echocardiogram—were obtained at study entry. Within 1 month, patients underwent 24-hour Holter electrocardiography with a portable recorder (RAC-1202; Nihon Koden, Tokyo, Japan) under their usual daily activities at the middle of a week between hemodialysis sessions.
Data Analyses
Electrocardiograms were analyzed with a Holter scanner (DSC-3300; Nihon Koden, Tokyo, Japan), on which all R waves were detected and automatically labeled. QRS complexes were considered as sinus rhythm only when they had a narrow supraventricular morphology, R-R intervals were between 300 and 2000 ms and differed ≤20% from the average of five preceding sinus rhythm R-R intervals, and consecutive R-R interval differences were ≤200 ms. Results of the automatic analysis were reviewed, and any errors in R-wave detection and in QRS labeling were manually edited.
HRV Analyses
We calculated the conventional HRV measures recognized as mortality predictors by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (6), which included SDs of all normal-to-normal (NN) intervals (SDNN), SDs of the averages of NN intervals in all 5-minute segments of 24-hour recording (SDANN), square root of the mean square of differences between adjacent NN intervals (RMSSD), percentage of the number of pairs of adjacent NN intervals differing >50 ms in the total NN intervals (pNN50), HRV triangular index (HRVTI), as well as the variances corresponding to ultra-low frequency (ULF) (≤0.003 Hz), very-low frequency (VLF) (0.003–0.04 Hz), low frequency (LF) (0.04–0.15 Hz), and high frequency (HF) (0.15–0.40 Hz) bands. The variances were transformed in natural logarithmic (ln) value. The conventional HRV measures, except the LF/HF ratio, quantify the magnitude of HRV and substantially reflect vagal modulation of heart rate (6).
As nonlinear HRV measures, we calculated HRT (9), DC (10), and scaling exponents α1 and α2 (11). HRT estimates cardiac baroreceptor reflex function from the responses of NN intervals to putative transient arterial pressure reduction caused by ventricular premature contraction (VPC). The patterns of responses are classified into the following categories: normal (category 0), partially abnormal (category 1), and abnormal (category 2) (9,12,20). This measure is available only when >5 isolated VPCs suitable for the analysis are obtained from 24-hour electrocardiography. DC quantifies spontaneous increases in NN intervals averaged over 24 hours by the phase-rectified signal averaging method (21). DC calculated from short (four beats) overlapping segments is generally used. Currently, abnormal HRT and reduced DC are the most powerful predictors of post-MI mortality (9,10,12,13). Scaling exponents are calculated by detrended fluctuation analysis (11). This measure quantifies the correlation properties of fractal-like dynamics caused by complex interplay between vagal and sympathetic heart rate regulations (8). The scaling exponents were defined separately for the short term (4–11 beats) and long term (>11 beats) as α1 and α2, respectively. The exponents range between 0.5 and 1.5; values close to 0.5 indicate that the HRV is a white-noise–like uncorrelated random sequence, values close to 1.5 indicate a brown-noise–like strongly correlated inflexible sequence, and values around 1.0 indicate a fractal-noise–like moderately correlated and so-called regulated sequence. Decreased α1 has been reported to predict post-MI mortality independently of measures reflecting HRV magnitude (14).
Endpoint Analyses
All-cause mortality was the study endpoint. All patients were continuously followed up three times a week as part of their regular hemodialysis therapy at the Hemodialysis Center of Nagoya Kyoritsu Hospital. Patients were followed for outcomes until December 2009 unless they moved to other facilities or had a renal transplantation before that time. In those cases, patients were censored at that time.
Statistical Analyses
We used SAS version 9.2 software (SAS Institute, Cary, NC). Differences between survivors and nonsurvivors were evaluated by the chi-squared test for categorical data, the t test for normally distributed quantitative data, and the Wilcoxon two-sample test for those not normally distributed. Univariate and multivariable mortality risk were determined by Cox proportional hazards regression analysis with the following three steps. First, independent clinical risk factors were examined by stepwise variable selection from candidate variables including age, sex, hemodialysis duration, smoking, cardiothoracic ratio, left ventricular ejection fraction, diabetes, Kt/V, hematocrit, serum albumin, C-reactive protein, intact parathormone, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, calcium, phosphate, calcium × phosphate, history of stroke, coronary artery diseases, peripheral vascular diseases, 24-hour average heart rate, number of arrhythmias per 24 hours, and medications. Second, the hazard ratios (HRs) of HRV measures were determined and adjusted for the independent clinical risk factors. Finally, interactions of HRV measures by the clinical risk factors were evaluated. Survival curves were estimated by the Kaplan–Meier method and evaluated by the log-rank test. The cutoff value for HRV measures was determined by receiver-operating characteristic curve analysis. The improvement of risk prediction by adding HRV measures was evaluated by comparing C statistics for 5-year mortality between a baseline model consisting of only clinical risk factors and a new model including both clinical risk factors and HRV measures. We also evaluated the net reclassification improvement (NRI) without category, which reflects the difference between the ratios of improvement and deterioration in predicted probability caused by the inclusion of a new predictor (22,23). The 95% confidence intervals (95% CIs) of continuous NRI were estimated by the bootstrap method with 2000 random samplings. For all statistical analysis, P<0.05 was considered significant and corrected by the Bonferroni method for multiple comparisons.
Results
We screened 309 eligible patients, 28 (9.1%) of whom were excluded due to an implanted cardiac pacemaker (n=1), atrial fibrillation (n=21), and sinus beats <75% (n=6). Consequently, 281 patients (90.9%) were finally included. The median age of the 28 excluded patients was 71 years (interquartile range [IQR], 65–78), 17 (61%) were males, and the median hemodialysis duration was 4.4 years (IQR, 0.7–13.6). Twelve patients (nine with atrial fibrillation, two with sinus rhythm <75%, and one with a pacemaker) died during the follow-up.
Follow-Up Results
During a median follow-up of 87 (IQR, 78–90) months, there were 77 (27.4%) deaths. Although the follow-up of seven patients was censored before December 2009 due to hospital change (n=6) and renal transplantation (n=1), no survivors were censored during the first 5 years.
Baseline Characteristics
Table 1 shows the patient characteristics. All patients were Asian (Japanese). Compared with survivors, nonsurvivors were older; had a greater cardiothoracic ratio, lower serum albumin, higher C-reactive protein, and more frequent atrial premature contractions and VPCs; and were more likely to have a history of peripheral vascular diseases. There was no significant difference in medications. None of the patients took antiarrhythmic drugs except β-blockers and calcium channel blockers.
Table 1.
Baseline clinical features in patients grouped by survival state
| Characteristic | Survivors (n=204) | Nonsurvivors (n=77) | P |
|---|---|---|---|
| Age, yr | 61 (54–67) | 69 (64–73) | <0.001 |
| Male | 111 (54.4) | 42 (54.6) | 0.98 |
| Duration of hemodialysis, yr | 3.6 (1.1–8.7) | 4.5 (1.8–8.9) | 0.18 |
| Smoking | 44 (21.6) | 15 (19.5) | 0.70 |
| Cardiothoracic ratio | 49.0 (46.0–51.5) | 50.5 (48.5–55.0) | <0.001 |
| Left ventricular ejection fraction | 67.0 (58.0–72.0) | 64.0 (59.0–73.0) | 0.40 |
| Diabetes | 92 (45.1) | 44 (57.1) | 0.07 |
| Left ventricular hypertrophy | 96 (47.1) | 38 (49.4) | 0.73 |
| Kt/V | 1.39±0.30 | 1.36±0.32 | 0.38 |
| Hematocrit | 31.9 (30.1–34.0) | 32.4 (28.9–34.0) | 0.67 |
| Albumin, g/dl | 3.7 (3.5–3.9) | 3.4 (3.2–3.7) | <0.001 |
| C-reactive protein, mg/L | 1.3 (0.6–4.0) | 4.8 (1.2–15.5) | <0.001 |
| Intact parathyroid hormone, pg/ml | 83 (37–171) | 109 (33–159) | 0.83 |
| Triglyceride, mg/dl | 105 (75–150) | 92 (69–134) | 0.22 |
| Total cholesterol, mg/dl | 168 (143–190) | 162 (137–199) | 0.77 |
| HDL cholesterol, mg/dl | 42 (34–53) | 42 (34–50) | 0.54 |
| LDL cholesterol, mg/dl | 96 (77–116) | 98 (76–119) | 0.75 |
| Calcium, mg/L | 8.9 (8.4–9.5) | 8.9 (8.4–9.5) | 0.77 |
| Phosphate, mg/L | 5.3±1.2 | 5.3±1.3 | 0.82 |
| Calcium × phosphate, mg2/L2 | 47 (40–55) | 49 (41–56) | 0.61 |
| Clinical history | |||
| stroke | 16 (7.8) | 7 (9.1) | 0.73 |
| coronary artery disease | 52 (25.5) | 26 (33.8) | 0.16 |
| peripheral vascular disease | 9 (4.4) | 10 (13.0) | 0.01 |
| Medication | |||
| β blockers | 35 (17.2) | 15 (19.5) | 0.64 |
| angiotensin converting enzyme inhibitors | 56 (27.5) | 20 (26.0) | 0.80 |
| angiotensin receptor blockers | 57 (27.9) | 16 (20.8) | 0.22 |
| calcium channel blockers | 115 (56.4) | 50 (64.9) | 0.19 |
| Holter electrocardiography | |||
| heart rate per min | 70.2±11.1 | 71.2±13.0 | 0.42 |
| atrial premature contraction per 24 h | 29 (9–105) | 125 (29–768) | <0.001 |
| ventricular premature contraction per 24 h | 4 (1–41) | 12 (3–228) | 0.006 |
Data are n (%) for categorical data, median (interquartile range) for continuous normally distributed data, and mean ± SD for those not normally distributed.
Table 2 shows the HRV measures. HRT was analyzable only in 105 (37%) patients with >5 suitable VPCs. Among these patients, nonsurvivors were more likely to have abnormal HRT. The other HRV measures were analyzable in all 281 patients. Compared with survivors, nonsurvivors were lower in SDNN, SDANN, HRVTI, ULF, VLF, LF, LF/HF, scaling exponent α1, and DC and were higher in scaling exponent α2; however, they did not differ significantly in RMSSD, pNN50, or HF.
Table 2.
Measures of heart rate variability at baseline in patients grouped by survival state
| Survivors (n=204) | Nonsurvivors (n=77) | P | |
|---|---|---|---|
| SDNN, ms | 95.8 (70.8–120.9) | 86.5 (55.9–106.1) | 0.007 |
| SDANN, ms | 87.1 (66.9–111.4) | 79.8 (51.6–97.9) | 0.008 |
| RMSSD, ms | 13.9 (10.2–18.0) | 13.2 (10.0–17.6) | 0.60 |
| pNN50, ms | 0.40 (0.04–1.77) | 0.23 (0.02–1.63) | 0.65 |
| HRVTI, ms | 24.3 (18.1–31.4) | 19.8 (15.0–27.8) | 0.001a |
| ULF, ln (ms2) | 8.78 (8.03–9.35) | 8.29 (7.64–9.00) | <0.001a |
| VLF, ln (ms2) | 6.19 (5.41–6.78) | 5.56 (4.61–6.40) | <0.001a |
| LF, ln (ms2) | 4.51 (3.71–5.35) | 4.02 (2.75–4.84) | 0.004 |
| HF, ln (ms2) | 3.80 (3.08–4.46) | 3.69 (2.92–4.38) | 0.38 |
| LF/HF | 1.92 (1.17–3.52) | 1.18 (0.82–2.26) | <0.001a |
| Scaling exponent α1 | 1.17 (0.98–1.34) | 1.01 (0.77–1.15) | <0.001a |
| Scaling exponent α2 | 1.20 (1.16–1.25) | 1.24 (1.18–1.29) | <0.001a |
| HRT category, 0/1/2, n (%)b | 20 (29.4%)/29 (42.7%)/19 (27.9%) | 5 (13.5%)/12 (32.4%)/20 (54.1%) | 0.02 |
| DC, ms | 4.33 (3.31–5.68) | 3.81 (2.61–4.70) | <0.001a |
Data are the median (interquartile range) unless otherwise indicated. SDNN, SD of normal-to-normal intervals during 24 hours; SDANN, SD of the averages of NN intervals in all 5-minute segments during 24 hours; RMSSD, square root of the mean square of differences between adjacent NN intervals; pNN50, percentage of the number of pairs of adjacent NN R-R intervals differing >50 ms in the total NN intervals; HRVTI, heart rate variability triangular index; ULF, ultra-low frequency (≤0.003 Hz) power; VLF, very-low frequency (0.003–0.04 Hz) power; LF, low frequency (0.04–0.15 Hz) power; HF, high frequency (0.15–0.40 Hz) power; HRT, heart rate turbulence; DC, deceleration capacity.
Significant after correcting for multiple comparisons of 14 variables (P<0.003 for type 1 error level of 0.05).
Data were obtained only in patients with >5 ventricular ectopic beats (per day) suitable for HRT analysis.
There were modest (r<0.30) yet significant correlations between HRV measures and clinical variables. Particularly, positive correlations (r>0.20) were observed in LF/HF and scaling exponent α1 with age, SDNN, and SDANN with left ventricular ejection fraction, and SDNN, SDANN, HRVTI, ULF, VLF, LF/HF, and DC with serum albumin and negative correlations (r<−0.20) in SDNN, SDANN, and HRVTI with C-reactive protein level.
Survival Analyses
Through stepwise Cox hazards analysis, age, left ventricular ejection fraction, serum albumin, C-reactive protein, and calcium × phosphate were extracted as independent risk factors (Table 3). These variables were used as clinical risk factors in the following analyses.
Table 3.
Multivariable Cox proportional hazards model by conventional clinical risk factors
| Predictor | Unit | HR (95% CI)a | P |
|---|---|---|---|
| Age | 1 yr | 1.08 (1.05–1.11) | <0.001 |
| LVEF | −1% | 1.02 (1.00–1.04) | 0.03 |
| Albumin | −1 g/dl | 3.73 (2.02–6.87) | <0.001 |
| C-reactive protein | 1 mg/dl | 1.40 (1.24–1.57) | <0.001 |
| Calcium × phosphate | 1 mg2/L2 | 1.03 (1.01–1.05) | 0.003 |
The model was generated by stepwise variable selection from the candidate variables of age, sex, hemodialysis duration, smoking, cardiothoracic ratio, left ventricular ejection fraction, Kt/V, hematocrit, serum albumin, C-reactive protein, intact parathyroid hormone, triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, calcium, phosphate, calcium × phosphate, history of stroke, coronary artery diseases, and peripheral vascular diseases, 24-hour average heart rate, number of arrhythmias per 24 hours, and medications. HR, hazard ratio; 95% CI, 95% confidence interval; LVEF, left ventricular ejection fraction.
HR for 1 unit change in predictors.
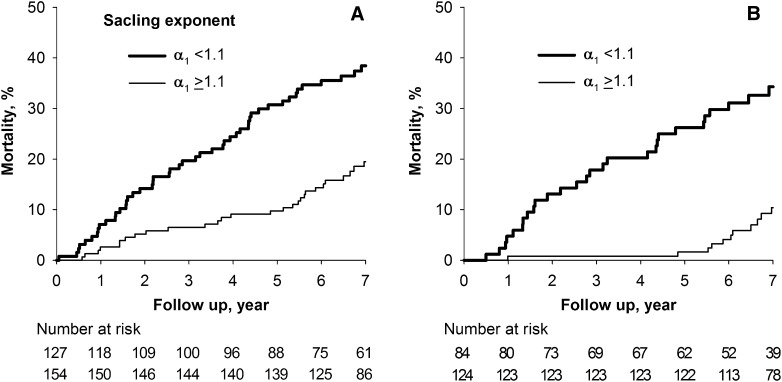
Table 4 shows unadjusted and adjusted hazard ratios of HRV measures. All HRV measures but RMSSD, pNN50, and HF predicted mortality. After adjusting for the independent clinical risk factors, only scaling exponent α1 was an independent predictor. Scaling exponent α1 showed a significant interaction by age (P=0.02); it predicted mortality only in patients aged <70 years (n=208; adjusted HR per a decrement of 0.25, 1.87; 95% CI, 1.38–2.53) but not in those aged ≥70 years (HR, 1.09; 95% CI, 0.75–1.60). No significant interaction was observed by the other clinical risk factors. Receiver-operating characteristic curve analysis revealed that the best cutoff value of scaling exponent α1 was 1.1. Figure 1 shows Kaplan–Meier curves with this cutoff. Among patients aged <70 years, mortality rates during the first 5 years were 1.6% and 26.2% for those above and below the cutoff, respectively.
Table 4.
Unadjusted and adjusted HRs of HRV measures for mortality risk
| Predictor | Unit | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| HR (95% CI)b | P | HR (95% CI)b | P | ||
| SDNN | −25 ms | 1.30 (1.09–1.55) | 0.004 | 1.08 (0.90–1.30) | 0.42 |
| SDANN | −25 ms | 1.32 (1.09–1.59) | 0.004 | 1.09 (0.89–1.33) | 0.40 |
| RMSSD | −1.5 ms | 1.00 (0.96–1.03) | 0.89 | 1.00 (0.97–1.04) | 0.78 |
| pNN50 | −1.5 ms | 0.99 (0.92–1.05) | 0.64 | 1.02 (0.95–1.09) | 0.63 |
| HRVTI | −10 | 1.24 (1.10–1.40) | <0.001c | 1.14 (0.85–1.52) | 0.39 |
| ULF | −0.5 ln (ms2) | 1.24 (1.10–1.40) | <0.001c | 1.09 (0.95–1.25) | 0.21 |
| VLF | −0.5 ln (ms2) | 1.18 (1.08–1.29) | <0.001c | 1.07 (0.96–1.18) | 0.22 |
| LF | −5 ln (ms2) | 3.55 (1.63–7.70) | 0.001c | 1.83 (0.77–4.31) | 0.16 |
| HF | −5 ln (ms2) | 1.85 (0.62–5.46) | 0.26 | 1.17 (0.41–3.29) | 0.76 |
| LF/HF | −5 | 5.17 (2.12–12.62) | <0.001c | 2.85 (1.10–7.35) | 0.03 |
| Scaling exponent α1 | −0.25 | 1.75 (1.41–2.16) | <0.001c | 1.46 (1.16–1.85) | 0.001c |
| Scaling exponent α2 | 0.25 | 3.73 (1.86–7.49) | <0.001c | 1.25 (0.61–2.53) | 0.54 |
| HRT categoryd | 0, 1, 2 | 1.95 (1.22–3.11) | 0.005 | 1.34 (0.81–2.22) | 0.26 |
| DC | −1 ms | 1.36 (1.15–1.60) | <0.001c | 1.13 (0.96–1.34) | 0.14 |
HR, hazard ratio; HRV, heart rate variability; SDNN, SD of normal-to-normal intervals during 24 hours; SDANN, SD of the averages of NN intervals in all 5-minute segments during 24 hours; RMSSD, square root of the mean square of differences between adjacent NN intervals; pNN50, percentage of the number of pairs of adjacent NN R-R intervals differing >50 ms in the total NN intervals; HRVTI, heart rate variability triangular index; ULF, ultra-low frequency (≤0.003 Hz) power; VLF, very-low frequency (0.003–0.04 Hz) power; LF, low frequency (0.04–0.15 Hz) power; HF, high frequency (0.15–0.40 Hz) power; HRT, heart rate turbulence; DC, deceleration capacity.
Adjusted for age, left ventricular ejection fraction, serum albumin, C-reactive protein, and calcium × phosphate.
HR for 1 unit change in continuous variables or for the presence of features.
Significant after correcting for multiple analyses of 14 variables (P<0.003 for type 1 error level of 0.05).
n=68 for survivors and n=37 for nonsurvivors.
Figure 1.
Decreased scaling exponent α1 predicts increased risk for mortality in hemodialysis patients, particularly those <70 years of age. Kaplan–Meier curves for mortality in (A) all 281 hemodialysis patients and (B) 201 patients aged <70 years. In both panels, patients were stratified by scaling exponent α1 <1.1 and ≥1.1. Log-rank test chi-squared statistics are 15.46 (P<0.001) for A and 23.7 (P<0.001) for B.
Added Predictive Value
The inclusion of scaling exponent α1 improved the prediction of 5-year mortality. Its addition to the clinical risk factors increased the C statistic from 0.84 (SEM 0.026) to 0.87 (SEM 0.023) (P=0.03), yielding a continuous NRI of 50.8% (95% CI, 20.2%–83.7%). Among patients aged <70 years, the C statistic increased from 0.85 (SEM 0.032) to 0.89 (SEM 0.027) (P=0.01), yielding a continuous NRI of 93.1% (95% CI, 59.3%–142.0%).
Discussion
To our knowledge, this is the first prospective cohort study to examine the predictive value of nonlinear HRV measures in chronic hemodialysis patients. We found that all HRV measures recognized as post-MI mortality risk (9,10,14) predicted death in hemodialysis patients, whereas after adjusting for independent clinical risk factors, only scaling exponent α1 remained a significant predictor. We observed that the addition of scaling exponent α1 to the clinical risk factors significantly improved the mortality prediction. Scaling exponent α1 had an interaction by age and the predictive value was substantial among patients aged <70 years. Scaling exponent α1 can be obtained by the automated analysis of 24-hour electrocardiography. It may contribute to the improvement of risk stratification in hemodialysis patients.
Several earlier studies have suggested the predictive value of 24-hour HRV in hemodialysis patients (2–5). We previously reported that reduced HRVTI and ULF were independent predictors of cardiac death in hemodialysis patients (3,4), but the number of events was insufficient as convincing evidence (24). Oikawa et al. (5) conducted a 5.8-year prospective study of 383 hemodialysis patients and found that SDNN <75 ms independently predicted all-cause and cardiovascular mortality. In both this study and previous studies (3,4,18), we observed no significant predictive power for SDNN. This difference may result from the different timing of 24-hour electrocardiography recordings (i.e., over the hemodialysis period in the study by Oikawa et al. and between hemodialysis sessions in our studies). Because 24-hour SDNN is substantially contributed by the day-night difference in heart rate (6), it could be influenced by hemodialysis session. In addition, SDNN is known to decrease with hemodialysis itself, particularly when the hemodialysis involves hemodynamic instability (25).
In this study, not only SDNN but all other conventional HRV measures showed no independent predictive power. This is in contrast to their independent predictive power in post-MI patients (10,14,20). In this study, we observed modest (r=0.2–0.3) yet significant correlations of these measures with serum albumin and C-reactive protein levels, which have not been adjusted in the post-MI studies. The predictive power of conventional HRV measures may be absorbed by the associations between these clinical risk factors and cardiac vagal dysfunction (6,7).
In contrast, scaling exponent α1 was a powerful mortality predictor even after adjusting for the clinical risk factors. This measure quantifies the correlation properties of short-term (4–11 beats) HRV caused by complex interplay between vagal and sympathetic heart rate regulations (8). Our observations suggest that aside from cardiac vagal dysfunctions, altered interaction or balance between vagal and sympathetic controls may be deleterious in chronic hemodialysis patients.
As a possible mechanism for the predictive power of scaling exponent α1, sympathetic overactivity may be relevant. Earlier studies have reported that sympathetic nerve activity is increased in hemodialysis patients (15,26) and an elevated plasma NE level independently predicts mortality (27). Scaling exponent α1 decreases with increased levels of circulating noradrenaline in healthy men (28) and increases with β-blocker therapy in patients with heart failure (29,30). Interestingly, it also increases with atropine and decreases with vagal activation (28,31). Because vagal activity is generally reduced in hemodialysis patients (15–17), those with and without sympathetic overactivity might be better discriminated by scaling exponent α1 than by the measures simply reflecting vagal modulation.
This study has several limitations. First, our findings may not be extended directly to hemodialysis patients in other countries. Crude mortality rates in Europe and the United States are higher than those in Japan (32). The spectrum of the causes and risk factors for death may also differ across regions. Second, we recorded Holter electrocardiography between hemodialysis sessions. In general, cardiac arrhythmias occur more frequently during and after hemodialysis. Their predictive value might have been underestimated in this study. Third, although autonomic function is variable overtime, we analyzed Holter electrocardiography recording only at baseline. The time course of HRV measures may have stronger predictive power. Fourth, because the study endpoint was all-cause mortality, it is unclear whether decreased α1 is associated with the specific pathologic causes of death or is simply a maker indicating poor clinical conditions. The latter seems unlikely, however, because the prognostic association of α1 was independent of age, left ventricular ejection fraction, serum albumin, C-reactive protein, and calcium × phosphate. Finally, this study suggests that the analysis of scaling exponent α1 may be useful for identifying high-risk patients, but it is unclear whether decreased α1 is treatable or, if treatable, whether the treatment reduces the mortality.
Decreased scaling exponent α1 of HRV detected by 24-hour electrocardiography is an independent predictor for mortality in chronic hemodialysis patients and the addition of this measure to clinical risk factors improves risk stratification in these patients, particularly those aged <70 years.
Disclosures
None.
Acknowledgments
The study was supported by grants from the Knowledge Hub of Aichi (the Priority Research Project P3-G1-S1 to J. H.), the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research 20590832 and 23591055 to J.H.), and the Japanese Ministry of Health, Labour, and Welfare (Research Grant for Nervous and Mental Disorders 20B-7, 23-2 to J.H.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Nakai S, Suzuki K, Masakane I, Wada A, Itami N, Ogata S, Kimata N, Shigematsu T, Shinoda T, Syouji T, Taniguchi M, Tsuchida K, Nakamoto H, Nishi S, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Marubayashi S, Morita O, Yamagata K, Wakai K, Watanabe Y, Iseki K, Tsubakihara Y: Overview of regular dialysis treatment in Japan (as of 31 December 2008). Ther Apher Dial 14: 505–540, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ranpuria R, Hall M, Chan CT, Unruh M: Heart rate variability (HRV) in kidney failure: Measurement and consequences of reduced HRV. Nephrol Dial Transplant 23: 444–449, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Hayano J, Takahashi H, Toriyama T, Mukai S, Okada A, Sakata S, Yamada A, Ohte N, Kawahara H: Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrol Dial Transplant 14: 1480–1488, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G: Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant 18: 318–325, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Oikawa K, Ishihara R, Maeda T, Yamaguchi K, Koike A, Kawaguchi H, Tabata Y, Murotani N, Itoh H: Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol 131: 370–377, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Camm AJ, Malik M, Bigger JT, Jr, Breithardt G, Cerutti S, Cohen RJ, Coumel P, Fallen EL, Kleiger RE, Lombardi F, Malliani A, Moss AJ, Rottman JN, Schmidt G, Schwartz PJ, Singer DH, Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology : Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 7.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, Camm AJ, Cappato R, Cobbe SM, Di Mario C, Maron BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza M, Vardas P, Wellens HJ, Zipes DP: Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J 22: 1374–1450, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Huikuri HV, Perkiömäki JS, Maestri R, Pinna GD: Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philos Transact A Math Phys Eng Sci 367: 1223–1238, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schömig A: Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 353: 1390–1396, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, Hnatkova K, Schömig A, Huikuri H, Bunde A, Malik M, Schmidt G: Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet 367: 1674–1681, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Peng CK, Havlin S, Stanley HE, Goldberger AL: Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5: 82–87, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Barthel P, Schneider R, Bauer A, Ulm K, Schmitt C, Schömig A, Schmidt G: Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation 108: 1221–1226, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Bauer A, Barthel P, Schneider R, Ulm K, Müller A, Joeinig A, Stich R, Kiviniemi A, Hnatkova K, Huikuri H, Schömig A, Malik M, Schmidt G: Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk). Eur Heart J 30: 576–583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huikuri HV, Mäkikallio TH, Peng CK, Goldberger AL, Hintze U, Møller M: Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101: 47–53, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kurata C, Uehara A, Sugi T, Ishikawa A, Fujita K, Yonemura K, Hishida A, Ishikawa K, Tawarahara K, Shouda S, Mikami T: Cardiac autonomic neuropathy in patients with chronic renal failure on hemodialysis. Nephron 84: 312–319, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Burgess ED: Cardiac vagal denervation in hemodialysis patients. Nephron 30: 228–230, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Rubinger D, Sapoznikov D, Pollak A, Popovtzer MM, Luria MH: Heart rate variability during chronic hemodialysis and after renal transplantation: Studies in patients without and with systemic amyloidosis. J Am Soc Nephrol 10: 1972–1981, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Fukuta H, Hayano J, Ishihara S, Sakata S, Ohte N, Takahashi H, Yokoya M, Toriyama T, Kawahara H, Yajima K, Kobayashi K, Kimura G: Prognostic value of nonlinear heart rate dynamics in hemodialysis patients with coronary artery disease. Kidney Int 64: 641–648, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Celik A, Melek M, Yuksel S, Onrat E, Avsar A: Cardiac autonomic dysfunction in hemodialysis patients: The value of heart rate turbulence. Hemodial Int 15: 193–199, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, Guzik P, Lombardi F, Müller A, Oto A, Schneider R, Watanabe M, Wichterle D, Zareba W: Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol 52: 1353–1365, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kantelhardt JW, Bauer A, Schumann AY, Barthel P, Schneider R, Malik M, Schmidt G: Phase-rectified signal averaging for the detection of quasi-periodicities and the prediction of cardiovascular risk. Chaos 17: 015112, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babyak MA: What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 66: 411–421, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rubinger D, Revis N, Pollak A, Luria MH, Sapoznikov D: Predictors of haemodynamic instability and heart rate variability during haemodialysis. Nephrol Dial Transplant 19: 2053–2060, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG: Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS: Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105: 1354–1359, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Tulppo MP, Mäkikallio TH, Seppänen T, Shoemaker K, Tutungi E, Hughson RL, Huikuri HV: Effects of pharmacological adrenergic and vagal modulation on fractal heart rate dynamics. Clin Physiol 21: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Lin LY, Lin JL, Du CC, Lai LP, Tseng YZ, Huang SK: Reversal of deteriorated fractal behavior of heart rate variability by beta-blocker therapy in patients with advanced congestive heart failure. J Cardiovasc Electrophysiol 12: 26–32, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Ridha M, Mäkikallio TH, Lopera G, Pastor J, de Marchena E, Chakko S, Huikuri HV, Castellanos A, Myerburg RJ: Effects of carvedilol on heart rate dynamics in patients with congestive heart failure. Ann Noninvasive Electrocardiol 7: 133–138, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppänen T, Mäkikallio TH, Huikuri HV: Physiological background of the loss of fractal heart rate dynamics. Circulation 112: 314–319, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Goodkin DA, Young EW, Kurokawa K, Prütz KG, Levin NW: Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis 44[Suppl 2]: 16–21, 2004 [DOI] [PubMed] [Google Scholar]