Summary
Background
Acidosis and transplantation are associated with increased risk of bone disturbances. This study aimed to assess bone morphology and metabolism in acidotic patients with a renal graft, and to ameliorate bone characteristics by restoration of acid/base homeostasis with potassium citrate.
Methods
This was a 12-month controlled, randomized, interventional trial that included 30 renal transplant patients with metabolic acidosis (S-[HCO3−] <24 mmol/L) undergoing treatment with either potassium citrate to maintain S-[HCO3−] >24 mmol/L, or potassium chloride (control group). Iliac crest bone biopsies and dual-energy X-ray absorptiometry were performed at baseline and after 12 months of treatment. Bone biopsies were analyzed by in vitro micro-computed tomography and histomorphometry, including tetracycline double labeling. Serum biomarkers of bone turnover were measured at baseline and study end. Twenty-three healthy participants with normal kidney function comprised the reference group.
Results
Administration of potassium citrate resulted in persisting normalization of S-[HCO3−] versus potassium chloride. At 12 months, bone surface, connectivity density, cortical thickness, and cortical porosity were better preserved with potassium citrate than with potassium chloride, respectively. Serological biomarkers and bone tetracycline labeling indicate higher bone turnover with potassium citrate versus potassium chloride. In contrast, no relevant changes in bone mineral density were detected by dual-energy X-ray absorptiometry.
Conclusions
Treatment with potassium citrate in renal transplant patients is efficient and well tolerated for correction of metabolic acidosis and may be associated with improvement in bone quality. This study is limited by the heterogeneity of the investigated population with regard to age, sex, and transplant vintage.
Introduction
Renal transplant patients are at risk for developing bone abnormalities and fractures (1) due to various conditions including underlying renal osteodystrophy, immunosuppressive therapy, and persisting disturbances in acid/base homeostasis. A variety of structural bone abnormalities are associated with ESRD, covering the whole range from low- to high-turnover bone disorder (2–4). In patients with a kidney graft, pre-existing osteopenia may deteriorate under immunosuppressive therapy with calcineurin inhibitors (CNIs) (5) or corticosteroids (6). Consequently, bone mineral density (BMD) can be diminished by 6.8% and 8.8% at 6 and 18 months, respectively, after successful renal transplantation (7).
Metabolic acidosis may be an important contributor because it has been shown to be highly prevalent in renal transplant patients and to be associated with disturbances in mineral metabolism (8). The resorptive activity of osteoclasts in vitro is inversely related with systemic pH (9,10). In contrast, higher bicarbonate concentrations inhibit osteoclast function via soluble adenylyl cyclase and were shown to be associated with elevated bone volume density in a 1-week mouse calvaria culture system (11). Under a diet rich in acid, rats were found to develop increased bone resorption versus control animals (12). Classic human studies, experimental and in the setting of CKD, demonstrate the dramatic effect of acidosis on mineral bone loss (13–15). In addition, a reduction in BMD and in bone formation rate has been found by dynamic histomorphometry techniques (16,17). As a result, chronic metabolic acidosis contributes to metabolic bone disease in CKD patients (18). Similarly, patients with chronic metabolic acidosis frequently develop bone fractures, which may occur without concomitant renal insufficiency (19). Finally, the homeostatic relationships between blood ionized calcium, parathyroid hormone (PTH), and 1,25(OH)2D3 may be affected by chronic metabolic acidosis, exaggerating bone dissolution (20,21). Published experiences on the inter-relationship between acidosis and bone disorders in patients after kidney transplantation are lacking. Thus, we designed a study to prospectively investigate the efficacy of potassium citrate with regard to normalization of acid/base derangements and to examine associated alterations in mineral and bone metabolism in renal transplant patients.
Materials and Methods
Participants and Methods
The efficacy and safety of potassium citrate versus potassium chloride in renal transplant patients was investigated in a prospective randomized clinical trial. Participants were recruited between June 2007 and January 2009. This study adheres to the Declaration of Helsinki and was approved by the local ethics board as well as the governmental Swiss agency for pharmaceutical products (Swissmedic). This study was registered at ClinicalTrails.gov (NCT00913796).
This study included adult patients (aged 18–65 years) of any sex or ethnicity with a renal graft having been transplanted within the previous 8 years and being at least 3 months after transplantation, or patients scheduled to undergo transplantation from a living organ donor, and having a venous serum bicarbonate concentration <24 mmol/L. Stable renal graft function with a calculated estimated GFR (eGFR) >30 ml/min per 1.73 m2 (according to the Chronic Kidney Disease Epidemiology Collaboration equation) was required. Immunosuppressive therapy included a CNI (cyclosporine A or tacrolimus), mycophenolate mofetil (MMF), and prednisone. Exclusion criteria were acute rejection episodes, severe physical limitation, psychiatric disorder, malignancy, catabolic state due to systemic illness, acute systemic infection, and pregnancy. The original enrollment target was 60 study participants; however, recruitment was stopped after enrollment of 30 transplant patients. Healthy volunteers with normal eGFR and uninephrectomized patients (living donors) served as the reference group. All participants gave informed consent for study participation. Renal transplant patients were randomized 2:1 to receive potassium citrate or potassium chloride to achieve a bicarbonate level >24 mmol/L over 12 months of treatment. Both drugs were started in equimolar quantities. Thereafter, each compound was individually titrated in order to achieve the serum [HCO3−] goal and to avoid hyperkalemia. Safety laboratory assessments to control for serum potassium were done at week 4 and months 2, 6, 9, and 12. Baseline and 1-year-follow-up assessments were performed in renal transplant patients, whereas controls were only investigated once.
Blood and Urine Analyses
Serum calcium, phosphate, intact PTH (iPTH), alkaline phosphatase, and vitamin D (25-OH vitamin D, 1,25-OH vitamin D) were measured routinely. Biomarkers of bone metabolism were determined in morning fasting serum. Bone-specific alkaline phosphatase (BAP) was determined by the Alkphase-B ELISA Kit (Metra-Biosystems). The parameters β-CrossLaps (CTX), N-MID-osteocalcin (OC), and N-terminal propeptide of type I collagen (PINP) were measured in serum with electrochemiluminescence immunoassays on the automated analyzer Elecsys 2010 (Roche Diagnostics, Rotkreuz, Switzerland). The intra- and interassay variations were 2.4%–7.2% for CTX, 1.1%–5.9% for OC, and 1.7%–4.0% for PINP, respectively. Reference values were as follows: BAP, 3.4–19.8 µg/L for women and 3.7–21.1 µg/L for men; osteocalcin, 7.7–32 ng/ml; PINP, 20–100 ng/ml; and CTX, 0.13–0.46 ng/ml. Twenty-four–hour urine was collected under oil and thymol.
Assessment of BMD, Bone Mineral Content, and Whole Body Composition Analyses
Both BMD (g/cm2) and bone mineral content (BMC) (g) from lumbar spine (L1–L4), total hip, femoral neck, and nondominant forearm (total and one-third radius) were measured using dual-energy x-ray absorptiometry (DXA) on an Hologic QDR 4500 A device (Hologic Inc, Bedford, MA), and whole body composition (WBC) was calculated from these data. Measurements of lumbar spine, total hip, and femoral neck were used for analysis of areal BMD.
Bone Biopsy and Tetracycline Labeling
Iliac crest bone biopsies in renal transplant patients were performed at baseline and follow-up. In some renal transplant patients, the biopsy was taken intraoperatively during living kidney transplantation (n=4). Living kidney donors were biopsied once either during kidney donation or at any other time point during study duration. Bone was labeled with tetracycline according to the following schedule: 2-day oral administration of tetracycline hydrochloride (500 mg twice daily) 3 weeks and 1 week before bone biopsy. Biopsies were performed as described previously (22).
Micro-Computed Tomography
Bone biopsies were stored in 70% ethanol until analysis by high-resolution micro-computed tomography (µCT) using a μCT20 system (Scanco Medical, Bassersdorf, Switzerland) with a spatial resolution of 28 µm. The measurement of cancellous bone microarchitecture included the following parameters: bone volume density (BV/TV), bone surface density (BS/BV), connectivity density, trabecular number, trabecular separation, and trabecular thickness. Parameters of bone cortex involved cortical thickness, cortical porosity, and cortical volume density (BV/TV).
Histologic Preparation and Evaluation of Bone Biopsies
Biopsies were fixated in 4% buffered formalin, washed, dehydrated in an ascending series of ethanol (50%–100%), defatted in xylene, and infiltrated in methylmethacrylate and embedded using special Teflon molds according to standard protocols (23). Ground sections (30–40 mm) and thin sections (5 mm) were prepared and analyzed separately and were surface stained with toluidine blue and with toluidine blue and von Kossa/McNeil, respectively. For quantitative histology, the ground sections were read in a computer at a magnification of 15.6 by means of a microscope (Leica DFC 420C; Leica Microsystems, Heerbrugg, Switzerland) equipped with a digital camera (Leica DFC 420; Leica Microsystems). For histomorphometric measurements, three different phases were colored using specialized software (Adobe Photoshop 7; Adobe Systems Inc, San Jose, CA). An image analysis program (Leica Qwin; Leica Microsystems) was used for the quantitative determination of cortical structures as well as pores/cortex proportion. A fluorescence image analysis program (Leica Application Suite, Advanced Fluorescence; Leica Microsystems) was used to analyze tetracycline labeling of bone sections. A semiquantitative analysis of the intensity of trabecular connectivity was performed using the following scores: 0, no connectivity; 1, mild; 2, moderate; and 3, severe.
Statistical Analyses
Differences between the treatment groups were tested with the two-sided t test and the comparison between baseline and follow-up assessments was evaluated with the paired-samples t test. Significance was accepted at P≤0.05. Data are reported as mean ± SD. All statistical analyses were performed using SPSS software (version 16.0; SPSS Inc, Somer, NY).
Results
Patient Characteristics
Thirty renal transplant patients underwent randomization to receive either potassium citrate (n=19) or potassium chloride (n=11). The potassium citrate and the potassium chloride groups were comparable for demographic and biochemical characteristics shown in Tables 1 and 2, respectively. Significant differences at baseline between groups were found for urinary phosphate excretion (Table 2) and parameters measured by DXA (Table 3).
Table 1.
Immunosuppressive therapy
| Potassium Citrate (n=19) | Potassium Chloride (n=11) | |
|---|---|---|
| Age, yr | 48±12 | 48±8 |
| Sex, female/male | 4/15 | 0/11 |
| Transplant vintage, mo | 68±78 | 45±38 |
| Immunosuppression, yes/no (%) | ||
| tacrolimus | 10/9 (52.6) | 6/11 (54.5) |
| cyclosporine A | 9/10 (47.4) | 3/8 (27.3) |
| mycophenolate | 16/3 (84.2) | 9/11 (81.9) |
| azathioprine | 2/17 (10.5) | 2/9 (18.2) |
| everolimus | 0/19 (0) | 2/9 (18.2) |
| prednisone | 7/12 (36.8) | 2/9 (18.2) |
Table 2.
Serum and urinary parameters of mineral metabolism
| Baseline | Follow-Up | |||
|---|---|---|---|---|
| Potassium Citrate | Potassium Chloride | Potassium Citrate | Potassium Chloride | |
| Serum calcium, mM/L (2.10–2.60) | 2.39±0.10 | 2.38±0.07 | 2.36±0.12 | 2.33±0.11 |
| Serum phosphate, mM/L (0.80–1.45) | 1.02±0.22 | 0.96±0.19 | 0.98±0.22 | 0.92±0.18 |
| Intact parathyroid hormone, ng/L (15–65) | 81±49 | 80±33 | 85±37 | 78±29 |
| Bone-specific alkaline phosphatase, U/L (9–90) | 14±6 | 16±5 | 14±7 | 12±3 |
| 25-OH vitamin D, µg/L (>20) | 22.5±12.0 | 24.4±9.3 | 22.7±13.4 | 22.0±9.2 |
| 1,25-diOH vitamin D, ng/L (18.0–70.5) | 39.2±13.1 | 39.6±17.6 | 41.6±18.3 | 42.4±17.8 |
| Urinary calcium, mmol/d | 1.5±1.1 | 2.3±2.5 | 2.0±1.7 | 2.7±2.5 |
| Urinary phosphate, mmol/d | 20±7a | 29±6 | 24±9 | 27±7 |
Reference values are shown in parentheses.
P=0.001 versus potassium chloride.
Table 3.
Baseline DXA measurements and whole body composition
| Potassium Citrate (n=19) | Potassium Chloride (n=11) | |
|---|---|---|
| Lumbar spine | ||
| BMD (g/cm2) | 0.96±0.15 | 0.96±0.10 |
| T score | −1.15±1.29 | −1.17±0.97 |
| Z score | −0.67±1.41 | −0.92±1.05 |
| Hip | ||
| BMD (g/cm2) | 0.90±0.13 | 0.94±0.12 |
| T score | −0.77±0.86 | −0.59±0.78 |
| Z score | −0.43±0.95 | −0.34±0.71 |
| Femoral neck | ||
| BMD (g/cm2) | 0.76±0.12 | 0.78±0.08 |
| T score | −1.13±0.87 | −1.09±0.56 |
| Z score | −0.50±0.95 | −0.44±0.46 |
| Body weight, kg | 71.0±11.6a | 86.2±14.6 |
| Total body lean mass, g | 52,587±8374a | 61,900±6686 |
| Total body fat mass, g | 17,489±7778 | 23,812±9410 |
| Total BMC, g | 2340±404 | 2695±294 |
| Total body fat, % | 23.6±8.7 | 26.1±5.7 |
| BMI, kg/m2 | 23.9±3.1a | 26.8±4.3 |
DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density; BMC, bone mineral content; BMI, body mass index.
P≤0.01 versus potassium chloride.
Correction of Serum Bicarbonate Concentration with Potassium Citrate Treatment
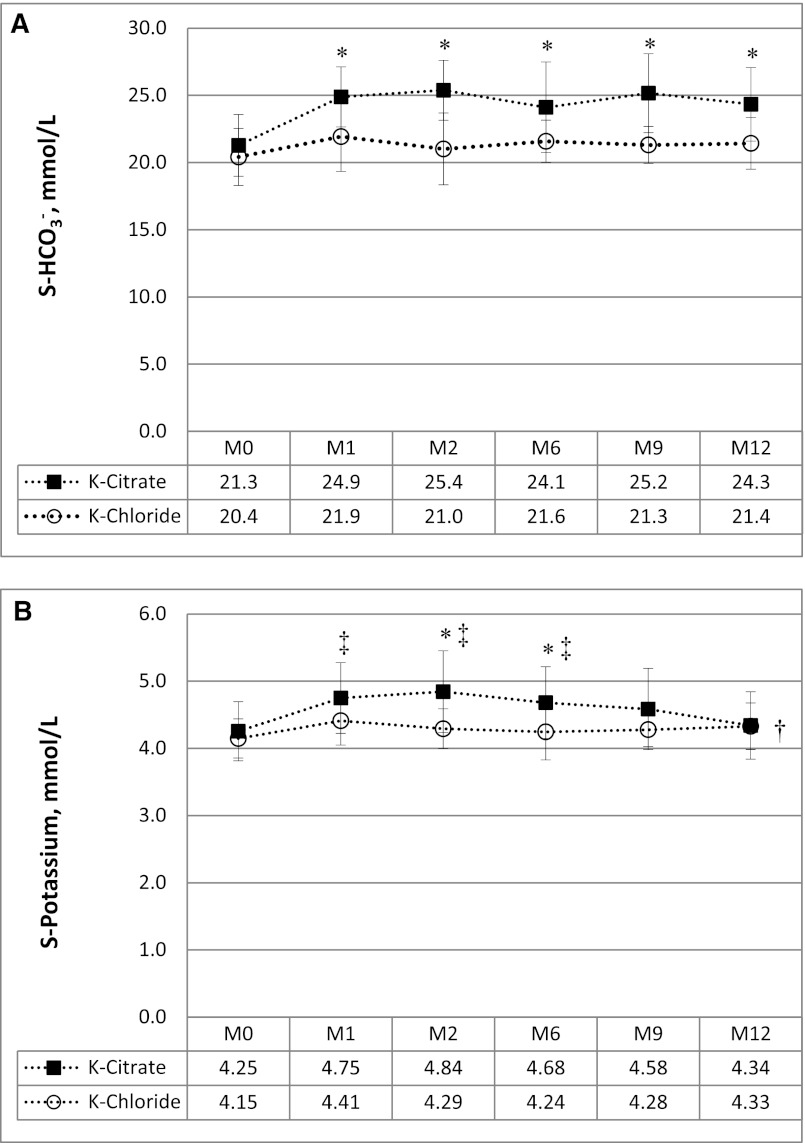
Potassium citrate increased serum bicarbonate significantly from 21.3±2.3 mmol/L at baseline to 24.9±2.0 mmol/l in the first month and maintained it constantly >24 mmol/L during the whole study period, whereas serum bicarbonate levels did not rise in the potassium chloride control group (Figure 1A). Mean citrate dosage and potassium intake are given in Table 4. The latter was significantly greater in the potassium citrate group versus the potassium chloride group. Despite a significant increase of S-[K+] with potassium citrate versus potassium chloride at months 2 and 6 (Figure 1B), it did not reach critical concentrations at any time point in either treatment group. Adverse effects consisted mainly of mild gastrointestinal symptoms in both groups.
Figure 1.
Correction of metabolic acidosis from potassium citrate versus potassium chloride administration and associated effects on serum potassium concentration. (A) Changes in serum bicarbonate concentration (S-HCO3−) over 12 months in relation to potassium citrate or potassium chloride intake. (B) Changes in serum potassium concentration over 12 months in relation to potassium citrate or potassium chloride intake. *P≤0.05 versus potassium chloride; †P≤0.05 versus baseline for potassium chloride; ‡P≤0.01 versus baseline for potassium citrate. S, serum; M, month.
Table 4.
Intake of potassium citrate or potassium chloride, along with changes in renal graft function, serum bicarbonate concentration, and serum pH
| Baseline | 2 mo | 6 mo | 12 mo | |||||
|---|---|---|---|---|---|---|---|---|
| Potassium Citrate | Potassium Chloride | Potassium Citrate | Potassium Chloride | Potassium Citrate | Potassium Chloride | Potassium Citrate | Potassium Chloride | |
| Citrate dosage, mmol | 0 | 0 | 32±11 | 0 | 29±16 | 0 | 34±15 | 0 |
| K+ dosage, mmol | 0 | 0 | 77±27a | 27±10 | 71±41a | 27±10 | 83±37a | 28±10 |
| Estimated GFR, ml/min per 1.73 m2 | 56±19 | 51±17 | – | – | – | – | 53±16b | 46±11b |
| Serum pH | 7.36 | 7.34 | 7.37a | 7.32 | 7.38 | 7.34 | 7.37 | 7.35 |
| Serum HCO3−, mmol/L | 21.3±2.3 | 20.4±2.1 | 25.4±2.2a | 21.0±2.7 | 24.1±3.4a | 21.6±1.6 | 24.3±2.7a | 21.4±1.9 |
P<0.05 versus potassium chloride.
P<0.05 versus baseline.
Effect of Treatment on Graft Function
Graft function was not significantly different between treatment arms at baseline with a mean eGFR of 56±19 ml/min per 1.73 m2 in the potassium citrate group versus 51±17 ml/min per 1.73 m2 in the potassium chloride group (Table 4). Excretory graft function changed significantly in both groups during the study period. However, only 44% of the patients in the potassium citrate group compared with 64% in the potassium chloride group had deterioration in graft function, with an overall mean decrease in eGFR from baseline of only 5% in the potassium citrate group versus 10% in the potassium chloride group (P=0.22).
Effect of Treatment on Mineral Metabolism
Treatment with either potassium citrate or potassium chloride did not change serum calcium, phosphate, alkaline phosphatase, 25-OH-vitamin D, 1,25-OH-vitamin D, and iPTH within the 1-year study period. In addition, there were no significant differences in serum concentrations of these parameters between the potassium citrate and potassium chloride groups at follow-up (Table 2). Both treatments had no effect on total urinary and fractional excretion of both calcium and phosphate.
Effect of Treatment on Areal BMD Assessed by DXA
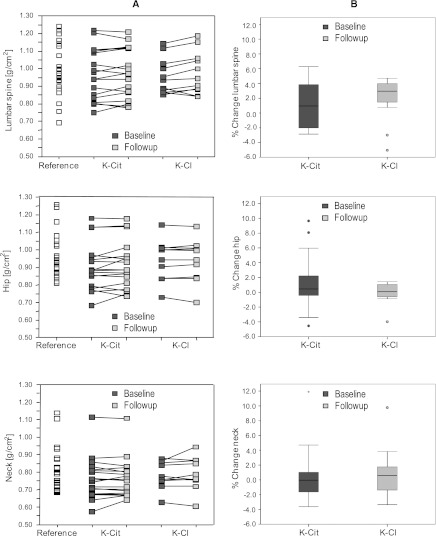
The reference group had a wide range in BMD at all measured sites, with a mean BMD of 0.99±0.15 g/cm2 at the lumbar spine, 0.97±0.12 g/cm2 at the total hip, and 0.81±0.12 g/cm2 at the femoral neck (Figure 2), and did not differ significantly from the combined renal transplant patient group for all parameters measured by DXA (BMD, T and Z scores, BMC, and body lean mass and total body fat mass [data not shown]). Similarly, BMD revealed no significant differences at all sites between treatment groups at baseline (Table 3). Follow-up DXA measurements could be performed in 29 renal transplant patients (Figure 2). Except for a slight but significant increase in Z scores at the lumbar spine for both the potassium citrate group (from −0.67±1.41 to −0.54±1.41; P<0.05) and the potassium chloride group (from −0.92±1.05 to −0.72±1.21; P=0.03), no significant differences were found between groups and versus baseline. The potassium citrate group showed a very heterogeneous pattern in respect to changes in areal BMD within 1 year of follow-up.
Figure 2.
Bone mineral density (BMD) measurements by dual x-ray absorptiometry at the lumbar spine, total hip, and femoral neck in g/cm2. (A) Results are given in absolute terms for healthy reference participants (Reference) and renal transplant patients before (Baseline) and after 12 months of treatment (Follow-up) with potassium citrate (K-Cit) or potassium chloride (K-Cl). (B) Results are given for renal transplant patients as percentage of change between baseline and 12 months of treatment with potassium citrate (K-Cit) or potassium chloride (K-Cl).
Effect of Treatment on Bone Microarchitecture
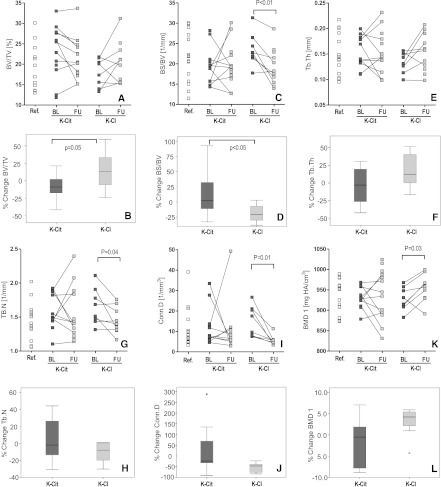
At baseline, 20 biopsies (potassium citrate group, n=12, potassium chloride group, n=8) were suitable for analysis of trabecular microarchitecture by µCT. Biopsies of 15 living kidney donors served as the reference group. At baseline, no significant differences in any parameter were found between the combined renal transplant patients and the reference group (Figure 3) and between treatment groups (Table 5). However, the potassium citrate group showed a tendency toward higher bone volume/total volume (BV/TV), lower bone surface/bone volume (BS/BV), and higher BMD.
Figure 3.
Bone spongiosa microarchitecture (µCT) of iliac crest samples from patients taking potassium citrate or potassium chloride at baseline and after 12 months of treatment (follow-up), as well as of living organ donors (reference value). Results are given as absolute values (A, C, E, G, I, and K) or percentage changes (B, D, F, H, J, and L) for the respective variables. B, D, F, H, J, and L are boxplots, representing the median, 25th and 75th percentiles, and 2 SDs of the mean percentage changes for potassium citrate and potassium chloride, respectively, between follow-up and baseline. Ref., reference; BL, baseline; FU, follow-up; K-Cit, potassium citrate; K-Cl, potassium chloride; BV, bone volume; TV, total volume; BS, bone surface; Tb.Th, trabecular thickness; Tb.N, trabecular number; Conn.D, connectivity density; BMD, bone mineral density.
Table 5.
Baseline µCT parameters of treatment groups
| Potassium Citrate (n=12) | Potassium Chloride (n=8) | P | |
|---|---|---|---|
| BV/TV, % | 22.9±3.2 | 17.9±3.4 | 0.07 |
| BS/BV, 1/mm | 19.5±4.5 | 23.5±4.0 | 0.06 |
| Trabecular thickness, 1/mm | 0.15±0.31 | 0.13±0.22 | 0.13 |
| Trabecular separation, mm | 0.64±0.12 | 0.61±0.09 | 0.71 |
| Trabecular number, 1/mm3 | 1.57±0.25 | 1.65±0.27 | 0.51 |
| Connectivity density, 1/mm | 11.9±9.2 | 14.7±7.5 | 0.49 |
| BMD, mg/HA cm3 | 261±72 | 204±40 | 0.06 |
| BMD1, mg/HA cm3 | 932±27 | 927±28 | 0.71 |
| Time between biopsies, mo | 14.5±3.8 | 13.1±1.0 | 0.31 |
µCT, micro-computed tomography; BV, bone volume; TV, total volume; BS, bone surface; BMD, bone mineral density.
Eighteen renal transplant patient biopsy pairs could be analyzed at baseline and follow-up, showing large scatter of alterations in µCT parameters (Figure 3). In the potassium citrate group, 7 of 10 patients lost BV/TV, whereas in the potassium chloride group, 5 patients exhibited increased BV/TV and 3 decreased BV/TV (Figure 3A), with a borderline significant difference between treatment groups (Figure 4; P=0.05). Mean BS/BV increased in the potassium citrate group, whereas all patients in the potassium chloride group demonstrated a significant decrease in BS/BV versus baseline (Figure 3C; P<0.01). The number of trabeculae was unaffected in the potassium citrate group, but decreased in the potassium chloride group (Figure 3G; P<0.05). Consequently, trabecular spacing increased in the potassium chloride group (not shown). Except for three patients in the potassium citrate group, all renal transplant patients had reduced connectivity density compared with baseline (Figure 3, I and J; P=0.01 in the potassium chloride group). BMD (P<0.05) and BMD1 decreased in the potassium citrate group but significantly increased in the potassium chloride group (Figure 3K; P<0.04), reflecting their observed increase in trabecular thickness (Figure 3E). In summary, trabecular bone microarchitecture remained mostly unaffected in the potassium chloride group. In contrast, bone of patients taking potassium citrate showed indirect signs of active bone remodeling. Finally, cortical thickness and cortical porosity were scattered in all patients (Figure 4) with no significant effects of either treatment. However, 8 of 12 patients (66.7%) taking potassium citrate, but only 6 of 11 patients (54.5%) taking potassium chloride, had an increase in cortical thickness at follow-up. Similarly, cortical porosity was reduced in only 5 of 12 patients taking potassium citrate but in 7 of 11 patients taking potassium chloride.
Figure 4.
Bone corticalis microarchitecture (µCT) of iliac crest samples from patients taking potassium citrate or potassium chloride at baseline and after 12 months of treatment, as well as of living organ donors (reference value). Ref., reference; K-Cit, potassium citrate; K-Cl, potassium chloride; Ct.Th, cortical thickness; BV, bone volume; TV, total volume; CT.Po, cortical porosity.
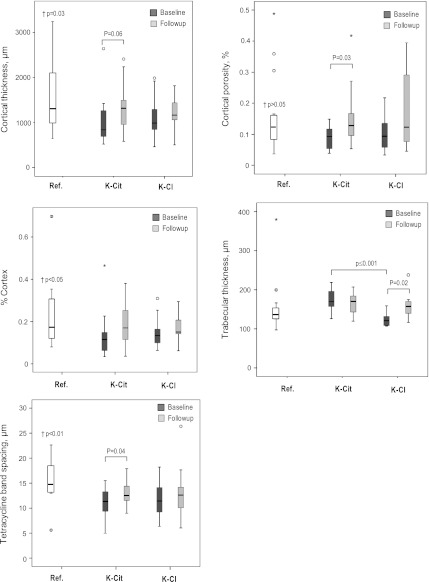
Bone microarchitecture was further analyzed by histomorphometry (Figure 5). No significant differences were found in trabecular thickness between the combined renal transplant patients and the reference group. In contrast, baseline cortical thickness (P=0.03), cortical porosity (P=0.05), and cortex/bone ratio (P=0.05), as well as fluorescence from tetracycline double labeling (P=0.003), were all significantly lower in combined renal transplant patients versus the reference group. Except for higher trabecular thickness in the potassium citrate versus potassium chloride groups (P<0.001), all other baseline variables were comparable between treatment groups. Unlike with potassium citrate, a significant increase (P=0.02) in trabecular thickness was observed with potassium chloride (from 123±19 µm to 161±39 µm versus 175±27 µm to 165±28 µm, respectively). Moreover, the potassium citrate group showed a tendency for an elevation in cortical thickness from baseline to follow-up (P=0.06). Both findings correspond well with the µCT analysis. Histomorphometrically, the percentage in cortex porosity significantly increased with potassium citrate (P=0.03), but was unchanged with potassium chloride. In both treatment arms, no significant change in cortex/total bone ratio was observed. Finally, patients taking potassium citrate, unlike potassium chloride, showed significantly higher bone formation after 12 months of treatment as revealed by increased spacing between tetracycline bands from baseline to follow-up (11±3 µm to 13±2 µm; P=0.04).
Figure 5.
Bone characteristics assessed by histomorphometry of iliac crest samples from patients taking potassium citrate or potassium chloride at baseline and after 12 months of treatment, as well as of living organ donors (reference value). Results are given as absolute values for bone characteristics assessed by histomorphometry analysis. †Denotes the P value for significance between the reference population of healthy individuals and the combined renal transplant patients. Ref., reference; K-Cit, potassium citrate; K-Cl, potassium chloride.
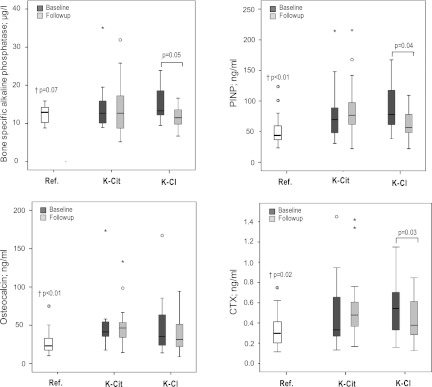
Influence of Treatment on Serum Bone Markers
Carboxyl-terminal crosslinked telopeptide of CTX were measured as an indicator of bone resorption, OC for general bone turnover, and both BAP and PINP for bone formation. Except for BAP (14.5±5.8 µg/L versus 12.1±2.2 µg/L; P=0.07), all biomarkers measured at baseline were significantly elevated in renal transplant patients versus controls, including OC (48.5±37.4 ng/ml versus 27.2±14.6 ng/ml; P<0.01), PINP (80.6±46.8 ng/ml versus 51.9±24.3 ng/ml; P<0.01), and CTX (0.8±1.9 ng/ml versus 0.3±0.2 ng/ml; P=0.02) (Figure 6). At baseline, no differences in these markers existed between treatment groups. No significant changes in all biomarkers occurred from baseline to follow-up with potassium citrate treatment. In contrast, significant decreases in PINP (P=0.04), CTX (P=0.03), and BAP (P=0.05) were observed with potassium chloride treatment.
Figure 6.
Serologic biomarkers of bone metabolism. Results are given as absolute values in boxplots, representing the median, 25th and 75th percentiles, and 2 SDs. †Denotes the P value for significance between the reference population of healthy individuals and the combined renal transplant patients. Ref., reference; K-Cit, potassium citrate; K-Cl, potassium chloride; PINP, N-terminal propeptide of precollagen I; CTX, C-terminal telopeptide/CrossLaps.
Discussion
To the best of our knowledge, this is the first study to examine the effects of potassium citrate on systemic acid/base homeostasis and associated changes in mineral bone metabolism and bone architecture in renal transplant patients with metabolic acidosis. Our data demonstrate that potassium citrate is safe and efficient to completely normalize metabolic acidosis in this population within 1 month of treatment. Moreover, assessment of bone by invasive and noninvasive tests suggests that administration of potassium citrate tends to ameliorate overall bone quality in acidotic renal transplant patients. Finally, a trend to better preservation of transplant GFR from potassium citrate can be observed.
Potassium citrate has previously been found to be effective in increasing serum bicarbonate in patients with distal renal tubular acidosis (24). In this trial, serum bicarbonate significantly increased within the first month, and thereafter remained within the normal range over 12 months of follow-up. Despite a significant increase in serum potassium over the first 6 months versus controls, no relevant episodes of hyperkalemia or other therapy-related side effects occurred. The decline in serum [K+] after month 6 may suggest lower treatment adherence with time. However, because correction of acid/base homeostasis was maintained over the whole study duration, it is more likely that hormonal and renal adaptations may account for the decreasing serum potassium on potassium citrate. Renal calcium and phosphate excretion were not significantly altered by citrate intake in this trial, unlike in other studies (24–26). If anything, we noticed a slight increase in calciuria and phosphaturia. In addition, no effects of potassium citrate on calcium and phosphate in serum were found. Similarly, vitamin D metabolites and iPTH also remained unaffected. This is in accordance with other human clinical trials, although some studies report alterations in these parameters from potassium citrate treatment (24,27,28).
The main rationale for treating metabolic acidosis in renal transplant patients in our trial was based on the hypothesis that restoring acid/base homeostasis may improve bone quality in this population. Several studies have documented clinically relevant bone disturbances in renal transplant patients such as osteopenia (6), osteomalacia (3), and bone turnover disorders (2). Similarly, alkali therapy has been shown to be beneficial for bone structure and bone metabolism in nontransplant populations (24,27,29). To our knowledge, ours is the first study to investigate the effect of alkali therapy on bone quality in renal transplant patients. Baseline characteristics of morphologic features did not reveal major differences in bone structure compared with our reference population of healthy nontransplant individuals. This is most likely due to the large variation in many of the examined variables, which are explained mainly by age and sex factors. Nevertheless, as expected, several parameters point to lower bone quality in renal transplant patients versus controls, such as significantly lower cortical thickness and cortex/bone ratio, as well as borderline significant reduction in cortical porosity. In addition, renal transplant patients had significant alterations in serological bone biomarkers, pointing to higher bone turnover compared with controls. Despite the difficulties in integrating a multitude of structural and biochemical characteristics of bone and bone metabolism, and translating them into meaningful clinical ratings, our overall findings suggest an improvement in bone quality in renal transplant patients treated with potassium citrate for 12 months. Specifically, bone surface, connectivity density, cortical thickness, and cortical porosity were better preserved in bone biopsies assessed both by µCT and classic histomorphometry versus renal transplant patients receiving potassium chloride as a control treatment. These architectural parameters have been shown to correlate with biochemical bone properties and thus are likely to be relevant for bone quality (30). In addition, serological biomarkers did not change in the potassium citrate group but decreased significantly in patients taking potassium chloride, indicating better maintenance of bone turnover by citrate administration. This conclusion is further supported by the findings of increased bone formation by tetracycline double labeling in participants taking potassium citrate. To note, bone density measurements by DXA did not reveal any meaningful differences between any groups, including normal controls, and any time point. Most certainly this can be explained by the fact that neither group had bone loss at baseline as judged by DXA criteria. Moreover, DXA may not be the adequate technique to assess bone structure in this population with renal osteopathy, which differs from classic osteoporosis for which the method has been validated.
Our findings are in line with other publications. For example, Jehle et al. also describe beneficial effects of potassium citrate versus potassium chloride therapy over 12 months on enhancement of areal BMD (29). In their study, they examined bone quality only by DXA and biomarkers of bone turnover. In contrast to our findings, DXA measurements improved significantly in patients receiving alkali treatment. However, their clinical trial was conducted in postmenopausal women devoid of any acid/base disorders and steroid treatment. In patients with medullary sponge kidney, T and Z scores of lumbar spine improved significantly after potassium citrate treatment, but duration of alkali therapy was of at least 12 months and up to 8 years, raising the question regarding the influence of treatment duration on alterations in BMD (27). A significant enhancement of areal BMD at both the total hip and the trochanter was observed in 10 patients with distal renal tubular acidosis after 12 months of potassium citrate treatment. Similar to our results, no significant improvements in BMD were found at the lumbar spine and the femoral neck (24).
From the conception of our trial, assessing the effect of alkali treatment on renal graft function was not a primary issue. However, during the course of this study, several publications appeared demonstrating improvement of GFR in patients with chronic renal insufficiency from endogenous kidney disease treated with sodium bicarbonate (31,32) or potassium citrate (33). All three studies demonstrated significant retardation in the rate of progression of CKD. In agreement with these publications, we found a trend of better preservation of renal function in transplant patients taking potassium citrate versus potassium chloride, although it was not statistically significant. Compared with the investigations in patients with native kidney disease, our study was much smaller and had a shorter follow-up (1 versus 2 years), and thus was underpowered to detect superiority of alkali treatment with regard to maintenance or improvement in eGFR.
Our study has several limitations. First and foremost, our trial population was rather small, with a total of 53 participants, of which 30 renal transplant patients underwent the intervention and were prospectively evaluated. The major obstacle in recruiting more patients was the repeat bone biopsy within 12 months. In addition, the study population was rather heterogeneous with regard to age, sex, and transplant vintage. In particular, treatment groups were not well balanced for every potential cofounder, with a predominance of patients taking steroids, with lower body weight, longer duration since transplantation, and of women in the potassium citrate group. If anything, however, this would mitigate the positive effects of citrate treatment on bone quality. Bone being a tissue affected heavily by all of these factors explains the high variability in DXA and morphologic findings. Moreover, repeat biopsies were not available from all patients, further limiting our analysis. Another factor that may have precluded more stringent results is the limited time course of 12 months of follow-up. Given the slow changes in bone architecture, some of the techniques applied, DXA in particular, may not have been sensitive enough to detect alterations within 1 year. Thus, our trial, which was not blinded, has rather to be considered as a proof of concept study, which does not allow final conclusions. Despite these restrictions, some trends clearly emerge, which are in accordance with the hypotheses generated based on experimental evidence from animal and clinical studies. Moreover, it is a major strength of our trial to have applied different approaches and techniques in assessing bone structure and metabolism, which resulted in findings that are compatible with each other. Finally, it is beyond the scope of our investigation to elucidate the mechanisms by which study treatment affects the findings described. Suffice it to say that both acidosis and its correction by alkali have many direct and indirect effects on bone and kidney as elaborated on in the introduction. In the study by Jehle et al., for example, changes in BMD assessed by DXA were highly correlated with changes in renal net acid excretion from alkali treatment with potassium citrate (29), supporting a direct link between acid/base homeostasis and bone biology.
What clinical recommendations can be made based on the results of our trial? Given the multitude of potential systemic adverse effects of metabolic acidosis and the available literature, including this study, suggesting a beneficial effect of both alkali treatment and restoration of acid/base balance, prescription of alkali to renal transplant patients with metabolic acidosis should be used generously. Potassium citrate, in our opinion, may be superior to sodium bicarbonate, because it lacks volume effects and obligatory calcium excretion associated with sodium. In this trial, administration of potassium citrate to acidotic transplant patients resulted in normalization of systemic acid-base status and was associated with improvements in bone quality.
Disclosures
None.
Acknowledgments
The authors thank all of the patients and volunteers who participated in this study. We are also grateful for the contributions of Dr. Patricia Wahl and Rebecca Winzeler, who assisted valuably as study coordinators. Finally, the authors thank Käthi Kämpf, Katalin Zlinszky, and Sabina Wunderlin for preparing the excellent histology slides.
This trial was sponsored by scientific grants from the Swiss National Science Foundation (3200B0-112299) and the Hermann Klaus Foundation (Zurich, Switzerland). All study medication was generously provided by Vifor (Fribourg, Switzerland).
Footnotes
A.S. and A.C. contributed equally to this work.
Present address: Dr. Daniel Uebelhart, Valmont, Private Rehabilitation Clinic, Glion-sur-Montreux, Switzerland.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chiu MY, Sprague SM, Bruce DS, Woodle ES, Thistlethwaite JR, Jr, Josephson MA: Analysis of fracture prevalence in kidney-pancreas allograft recipients. J Am Soc Nephrol 9: 677–683, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV: The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int 43: 436–442, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH: High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol 11: 1093–1099, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H: Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14: 2669–2676, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Movsowitz C, Schlosberg M, Epstein S, Ismail F, Fallon M, Thomas S: Combined treatment with cyclosporin A and cortisone acetate minimizes the adverse bone effects of either agent alone. J Orthop Res 8: 635–641, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler R, Kasperk C: Glucocorticoid-induced osteoporosis: Prevention and treatment. Steroids 63: 344–348, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD: Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 325: 544–550, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Yakupoglu HY, Corsenca A, Wahl P, Wüthrich RP, Ambühl PM: Posttransplant acidosis and associated disorders of mineral metabolism in patients with a renal graft. Transplantation 84: 1151–1157, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Murrills RJ, Stein LS, Dempster DW: Stimulation of bone resorption and osteoclast clear zone formation by low pH: A time-course study. J Cell Physiol 154: 511–518, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Shibutani T, Heersche JN: Effect of medium pH on osteoclast activity and osteoclast formation in cultures of dispersed rabbit osteoclasts. J Bone Miner Res 8: 331–336, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Geng W, Hill K, Zerwekh JE, Kohler T, Müller R, Moe OW: Inhibition of osteoclast formation and function by bicarbonate: Role of soluble adenylyl cyclase. J Cell Physiol 220: 332–340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraut JA, Mishler DR, Singer FR, Goodman WG: The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int 30: 694–700, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Goodman AD, Lemann J, Jr, Lennon EJ, Relman AS: Production, excretion, and net balance of fixed acid in patients with renal acidosis. J Clin Invest 44: 495–506, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemann J, Jr, Litzow JR, Lennon EJ: The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litzow JR, Lemann J, Jr, Lennon EJ: The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest 46: 280–286, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, Sirikulchayanonta V, Ongphiphadhanakul B, Radinahamed P, Karnsombut P, Kunkitti N, Ruang-raksa C, Rajatanavin R: Bone mineral density and histology in distal renal tubular acidosis. Kidney Int 59: 1086–1093, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Lemann J, Jr, Adams ND, Wilz DR, Brenes LG: Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int 58: 1267–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Coen G, Mazzaferro S, Ballanti P, Sardella D, Chicca S, Manni M, Bonucci E, Taggi F: Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: A cross-sectional study. Nephrol Dial Transplant 11: 813–819, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Bailey RR: Chronic acidosis with metabolic bone disease. N Z Med J 98: 483–484, 1985 [PubMed] [Google Scholar]
- 20.Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hory B, Drüeke TB: The parathyroid-bone axis in uremia: New insights into old questions. Curr Opin Nephrol Hypertens 6: 40–48, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Hernandez JD, Wesseling K, Pereira R, Gales B, Harrison R, Salusky IB: Technical approach to iliac crest biopsy. Clin J Am Soc Nephrol 3[Suppl 3]: S164–S169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leutenegger CM, von Rechenberg B, Huder JB, Zlinsky K, Mislin C, Akens MK, Auer J, Lutz H: Quantitative real-time PCR for equine cytokine mRNA in nondecalcified bone tissue embedded in methyl methacrylate. Calcif Tissue Int 65: 378–383, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, Stitchantrakul W, Leeprasert V, Ongphiphadhanakul B, Radinahamed P, Rajatanavin R: Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int 62: 2160–2166, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Gambaro G, van der Woude FJ: Glycosaminoglycans: Use in treatment of diabetic nephropathy. J Am Soc Nephrol 11: 359–368, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Sakhaee K, Maalouf NM, Abrams SA, Pak CY: Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab 90: 3528–3533, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Fabris A, Bernich P, Abaterusso C, Marchionna N, Canciani C, Nouvenne A, Zamboni M, Lupo A, Gambaro G: Bone disease in medullary sponge kidney and effect of potassium citrate treatment. Clin J Am Soc Nephrol 4: 1974–1979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karp HJ, Ketola ME, Lamberg-Allardt CJ: Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br J Nutr 102: 1341–1347, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R: Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol 17: 3213–3222, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D: Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol 23: 525–532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]