Summary
Background and objectives
Forkhead box P3 regulatory T cells control inflammatory responses, but it remains unclear whether they inhibit brain death-initiated inflammation and tissue injury in deceased kidney donors.
Design, setting, participants, & measurement
To study the actions of regulatory T cells at various stages of the donation and transplantation procedure, forkhead box P3, regulatory and inflammatory cytokine expression, and tissue injury markers were determined in time 0 kidney biopsies from deceased and living donors. Additionally, the interaction between forkhead box P3+ T cells and kidney injury molecule-1 by activated primary tubular epithelial cells was studied.
Results
After cold storage, the deceased donor kidneys expressed the higher mRNA levels of kidney injury molecule-1 and CD3ε. In these samples, the inflammatory cytokines IL-8 and IFN-γ and markers associated with regulation (forkhead box P3, TGF-β, and IL-10) were highly expressed compared with living donor kidneys. Correlations were found between mRNA expression levels of forkhead box P3 and kidney injury molecule-1 and forkhead box P3 and IFN-γ. Immunohistochemical analysis confirmed the presence of forkhead box P3+ T cells in donor kidneys. Renal function (analyzed by serum creatinine levels) at the first week posttransplantation correlated with kidney injury molecule-1 and forkhead box P3 mRNA levels. In vitro studies showed that kidney injury molecule-1 expression by primary tubular epithelial cells was 63% (mean) lower when cocultured with regulatory T cells compared with control T cells.
Conclusions
These results show that donor forkhead box P3+ T cells infiltrate the deceased donor kidney, where they may control inflammatory and injury responses.
Introduction
In transplantation, ischemic graft injury is an unavoidable process that occurs at key stages during the donation and transplantation procedure. Of note, tissue injury is induced by the pathophysiologic events in brain death donors even before organ retrieval. Brain death is associated with a storm of inflammatory cytokines, and infiltrates can be found in the peripheral tissues, which together with other cardiovascular instability, results in organ damage (1–4). This brain death-induced donor kidney damage is associated with upregulated kidney injury molecule-1 (KIM-1) expression in the kidney (5). Furthermore, in donor organs, ischemia/reperfusion injury induces additional IFN-γ and IL-8 upregulation in grafted parenchymal cells followed by recruitment of inflammatory cells of both the innate and adaptive immune system. This aggressive immune response is considered as an important cause of tissue injury in the first phase after transplantation (6,7). However, tissue injury itself perturbs immune homeostasis by inducing compensatory anti-inflammatory responses (8). For instance it was shown that CD4+CD25+forkhead box P3 (FoxP3) +IL-10+ regulatory T cells (Tregs) control inflammatory responses after burn injury (9). Evidence that Tregs participate in tissue injury comes from experimental AKI models. Depletion of Tregs increased renal tubular damage, whereas infusion of these T cells reduced IFN-γ production and improved tissue repair (10). The finding of a counterinflammatory mechanism in AKI prompted us to study whether Tregs play a role in controlling inflammatory responses that are present in deceased donor kidneys. These Tregs share a complex relationship with IL-17–producing cells, major players in induction of inflammation, because differentiation into IL-17 CD4 T cells and Tregs is directed by TGF-β. When naive CD4 T cells are exposed to TGF-β and antigen, they differentiate into Tregs, whereas in the presence of the proinflammatory cytokines IL-6 and IL-23, they differentiate into Th17 cells (11).
Here, we analyzed whether tissue damage characteristic for deceased donor kidneys initiates a compensatory reaction by FoxP3+ Tregs. For that purpose we studied time 0 biopsies of kidneys from deceased donors with brain death, warm ischemia, and prolonged cold ischemia times and living donors. Biopsies were taken at the end of cold storage and after reperfusion. In these samples, IL-8, IFN-γ, IL-17, FoxP3, and Treg-associated molecules and tissue injury markers were measured. Additionally, the inhibitory potential of FoxP3+ T cells on KIM-1 expression by activated primary tubular epithelial cells (PTECs) was studied (5,12).
Materials and Methods
Donor and Patient Characteristics
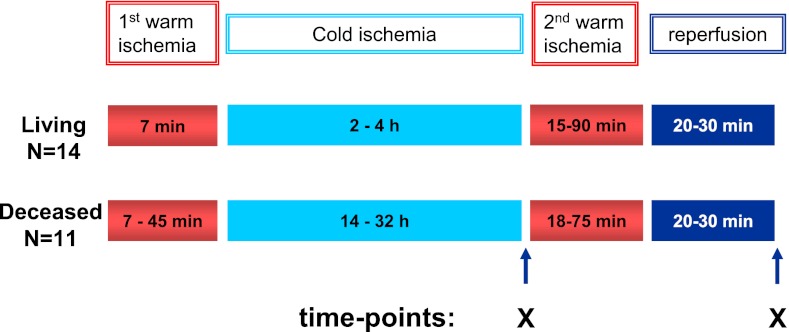
A total of 50 kidney biopsies were obtained for analysis from 11 deceased heart-beating donors (mean age=44 years; range=28–57 years) and 14 living donors (mean age=44 years; range=26–66 years). Biopsies were studied of 25 donors who enrolled in the study over a period of 1 year. Donor characteristics are shown in Table 1. Biopsies were taken at the end of cold storage and 20–30 minutes after reperfusion (Figure 1). Of the deceased donors, six were female; cause of brain death was cerebrovascular in six cases and trauma/other in the other five donors. Nine donors were treated with vasoactive drugs. Preoperative warm ischemia time was comparable among living and deceased donors (range=15–90 minutes). In living donor kidneys, the time of cold ischemia was 3.0 hours (median; range=2.1–4.2 hours), and for the deceased donor kidneys, the time of cold ischemia was 19.0 hours (median; range=14.4–32.5 hours; P<0.001). The mean ages were 43 years (range=25–55 years) and 43 years (range=23–60 years) for recipients of kidneys from living and deceased donors, respectively. Patients (n=25) received maintenance immunosuppressive therapy consisting of tacrolimus, mycophenolate mofetil, and prednisone. Morning recipient serum creatinine levels were used to assess graft function. The study was approved by the Erasmus Medical Center review board (no. 196.927/2000/235), and informed consent of the patients was obtained.
Table 1.
Donor and patient characteristics
| Living Donor Kidneys (n=14) | Deceased Donor Kidneys (n=11) | P Value | |
|---|---|---|---|
| Donor | |||
| mean age in years (range) | 44 (26–66) | 44 (28–57) | 0.62 |
| sex (male/female) | 8/6 | 5/6 | |
| mean cold ischemia time in hours (range) | 3.0 (2.1–4.2) | 19.0 (14.4–32.5) | <0.001 |
| Recipient | |||
| mean age in years (range) | 43 (25–55) | 43 (23–60) | 0.47 |
| sex (male/female) | 10/4 | 7/4 | 1.00 |
| HLA-A mismatch (mean ± SD) | 1.0 ± 0.7 | 0.81 ± 0.9 | 0.56 |
| HLA-B mismatch (mean ± SD) | 1.4 ± 0.7 | 0.7 ± 0.6 | 0.04 |
| HLA-DR mismatch (mean ± SD) | 1.0 ± 0.7 | 0.6 ± 0.5 | 0.17 |
| delayed graft function | 2/14 | 4/11 | 0.35 |
| acute rejection | 4/14 | 3/11 | 1.00 |
| graft failure within 1 year | 1/14 | 2/11 | 0.56 |
Figure 1.
Schematic overview of biopsy sampling. Per donor kidney, two biopsies were obtained. The first biopsy was taken after cold ischemia of the kidney, and the second sample was taken after 20–30 minutes of reperfusion. Sampling times points are marked by X.
Quantitative Real-Time PCR
mRNA extraction from the biopsies, cDNA transcription, and amplification were performed as described before (13). Measurements were performed using Assays on Demand (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used in each sample to control sample-to-sample variations in mRNA concentrations. The amount of target molecule was quantified by measuring cycle threshold (Ct), which was transformed to the number of cDNA copies [2(40 − Ct)]. The relative concentrations were normalized to the relative concentration of the housekeeping gene GAPDH present in each sample and multiplied by 106 because of the lower concentration of the target gene compared with the concentration of GAPDH.
Immunohistochemistry
Protein expression of FoxP3 and CD3 was studied by immunohistochemistry. Negative control experiments for both antigens were performed by omitting the incubation with the primary antibody. In brief, paraffin-embedded sections were deparaffinized, rehydrated, and incubated in H2O2 diluted in PBS to block the endogenous peroxidase activity. Antigen retrieval was performed by microwave treatment in citrate buffer. Sections were washed with PBS and incubated for 30 minutes in 10% normal human serum. Hereafter, sections were incubated with the primary antibody CD3 (DAKO, Glostrup, Denmark) or FoxP3 (eBiosciences, San Diego, CA) overnight at 4°C. Biotinylated donkey anti-rat or goat anti-rabbit secondary antibody combined with the Vectastain ABC Elite Kit was used to visualize the immunoreactivity; 3,3′-diaminobenzidine was used as chromogen, and sections were counterstained with hematoxylin.
PTECs Treg Interaction Studies
PTECs were obtained from nephrectomy specimens operated for various reasons and cultured from fragments of histologic normal kidney tissue. Outgrowth of PTEC was confirmed by morphologic appearance CD13+ CD26+ staining. Cells were used between passages 3–5 of culture. PTECs were plated overnight in 96-well plates and induced with recombinant IFN-γ (50 ng/ml) and TNF-α (20 ng/ml). After 24 hours, the PTECs were cocultured with 5×104 FACS-sorted T cell populations (CD4+CD45RO+CD25high+ CD127−/lo Treg cells and CD4 CD25−CD45RO− control T cells) in RPMI1640 supplemented with 10% heat inactivated human serum. The Treg cells and control T cells were isolated by FACSAria (BD Biosciences) and stimulated with soluble αCD3 (0.3 µg/ml) and αCD28 (0.4 µg/ml) antibodies for 3 days in the presence of 2000 IU recombinant IL-2.The sorted T cells were obtained from peripheral blood of blood bank donors that was stained with monoclonal antibodies for CD3-Amcyan, CD4-pacific blue, CD25 PE-Cy7, CD45RO-APC, and CD127-PE (BD Biosciences). Intracellular staining with the FoxP3 (e-Bioscience) antibody showed that CD4+CD45RO+CD25high+ CD127−/lo Treg cells expressed FoxP3 (>95%), which was not detectable in the CD25−CD45RO− control T cells. Activated Treg and control T cells were cocultured with activated PTECs for 3 days. To measure gene expression by the PTEC and T cell subsets, the T cells were separated from the PTEC layer by trypsinization procedures followed by Ficoll gradient centrifugation. KIM-1 and IL-10 mRNA expression by the PTECs, Tregs, and control T cells and the PTEC cocultures were determined by quantitative PCR as described above.
Statistical Analyses
A log transformation was performed on the mRNA levels of the analyzed markers to reduce the positive skew of the distribution. For determination of levels of statistical significance, two-sided probability values were used according to the t test if the data had a normal distribution; otherwise, groups were compared using Mann–Whitney U test for continuous variables and Fisher exact test for dichotomous variables. The relationships between variables were assessed by Spearman correlation and univariate linear regression analysis. A value of P<0.05 was considered significant. Statistical analysis was performed using Prism software.
Results
Clinical Data
Delayed graft function requiring dialysis occurred in 4 of 11 deceased donor kidneys and 2 living donor kidneys; 3 of 11 patients who received a deceased donor and 4 of 14 patients transplanted with a kidney from a living donor experienced an acute rejection episode within the first 3 months after transplantation, and 3 of 25 kidneys were rejected within 1 year after transplantation (2 were deceased donor kidneys and one was a living donor kidney) (Table 1).
KIM-1, Cytokine, Retinoid-Related Orphan Receptor-γc, and FoxP3 Expression in Deceased and Living Donor Kidneys
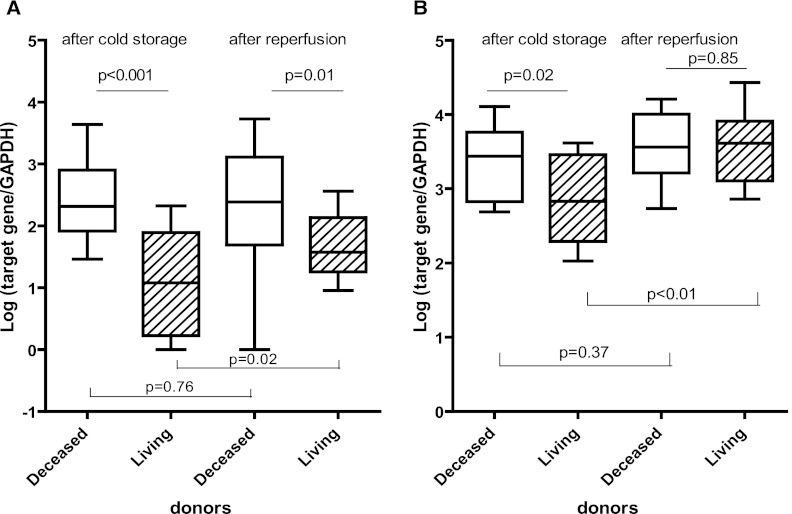
To examine the mRNA expression levels in deceased and living donor kidneys, biopsies were analyzed that were taken after donation and cold storage (Figure 1). In biopsies taken after both cold storage and reperfusion, the mRNA expression levels of KIM-1 were higher in deceased kidney donors than in biopsies of living donor kidneys (P<0.001 and P=0.01, respectively) (Figure 2A). In contrast to the deceased donor kidneys, reperfusion affected the KIM-1 expression in living donor kidneys. After reperfusion, significantly higher KIM-1 mRNA levels were found compared with living donor kidneys after cold storage (P=0.02). The proinflammatory cytokine IL-8 was expressed at higher levels by the deceased donor kidneys after cold storage compared with the living donors (P=0.02) (Figure 2B). This finding was not observed in specimens taken after reperfusion (P=0.85). Reperfusion affected the mRNA expression levels of IL-8 in living but not diseased donor kidneys. In living donor kidneys, a 30% higher mRNA expression level was measured after reperfusion compared with the samples taken after cold storage (P<0.01).
Figure 2.
Kidney injury molecule-1 (KIM-1) and IL-8 mRNA expression levels in donor kidney biopsies. Box plots show the minimum to maximum and 25th, 50th (median), and 75th percentiles for levels of mRNA for KIM-1 (A) and IL-8 (B) in biopsies from living (n=14) and deceased donor kidneys (n=11) after cold ischemia and reperfusion. In the biopsies taken after both cold storage and reperfusion, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) -normalized, log-transformed mRNA levels of KIM-1 and IL-8 were significantly higher in the deceased than living donor kidneys. The mRNA expression levels of KIM-1 and IL-8 did not significantly change after reperfusion in the deceased donor kidneys in contrast to the living donor kidneys (P=0.02 and P<0.01, respectively). Because of shortage of material, the mRNA levels of KIM-1 could not be tested in four biopsies.
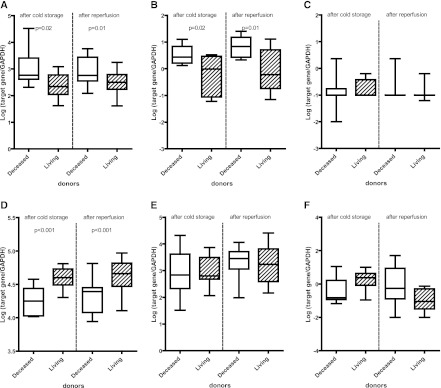
Next, we studied the contribution of CD3 T cells in brain death and renal ischemia reperfusion injury and the involvement of Tregs in the modulation of this response. In the deceased donor kidneys, the highest mRNA level of CD3ε was measured (Figure 3A) (after cold storage, P=0.02; after reperfusion, P=0.01). In samples from deceased donor kidneys, high levels of IFN-γ mRNA were also found (Figure 3B). mRNA levels of IL-17A were hardly detectable. After cold storage in 27% (3/11) of the deceased donor kidneys and 21% (3/14) of the living donor kidneys, IL-17A gene expression was found, which was not influenced by reperfusion (Figure 3C). To explain the absence of IL-17–producing cells in kidney from deceased and living donor kidneys, we measured the mRNA expression levels of the transcription factor retinoid-related orphan receptor-γc (RORγc) important for the differentiation into Th17 cells (14). Both in the cold storage and after reperfusion specimens, RORγc mRNA was expressed at lower levels in deceased than living donor kidneys (Figure 3D) (P<0.001). However, these low RORγc expression levels were not the result of shortage of the Th17 priming cytokines, because ample mRNA for IL-6 and IL-23 was detectable (Figure 3, E and F).
Figure 3.
mRNA expression levels of CD3ε, cytokines, and transcription factors in donor kidney biopsies. Box plots show the minimum to maximum and 25th, 50th (median), and 75th percentiles for GAPDH-normalized, log-transformed mRNA levels of CD3ε (A), IFN-γ (B), IL-17A (C), Retinoid-related orphan receptor-γc (RORγc) (D), IL-6 (E), and IL-23 (F) in biopsies from living (n=14) and deceased (n=11) donor kidneys after cold ischemia and reperfusion. The mRNA levels of CD3ε and IFN-γ were expressed significantly higher in deceased than living donor kidneys, whereas the highest mRNA levels for RORγc were measured in the living donor kidneys. The difference between deceased and living donor kidneys was significant in the specimens taken after cold storage as well as specimens taken after reperfusion. Because of shortage of material, the mRNA levels of IFN-γ could not be tested in six biopsies.
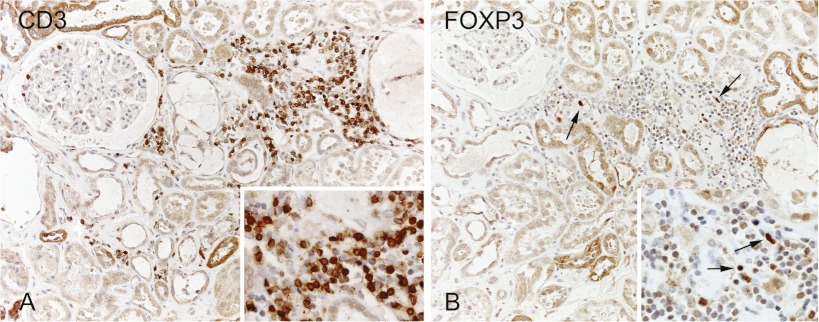
In the deceased donor kidneys, FoxP3 mRNA was present at high levels, suggesting that FoxP3+ T cells are present in donor kidneys after cold storage and before transplantation (P=0.01) (Figure 4A). Also, IL-10, a key cytokine for Treg function, and TGF-β mRNA were expressed at higher levels by deceased than living donor kidneys (P=0.02 and P=0.004, respectively) (Figure 4, B and C). Moreover, FoxP3+-expressing infiltrating cells were detected in specimens of donor kidneys after reperfusion (Figure 5).
Figure 4.
mRNA expression levels of forkhead box P3 (FoxP3), IL-10, and TGF-β in donor kidney biopsies. Box plots show the minimum to maximum and 25th, 50th (median), and 75th percentiles for levels of GAPDH-normalized, log-transformed mRNA levels of FoxP3 (A), IL-10 (B), and TGF-β (C) in biopsies from living (n=14) and deceased (n=11) donor kidneys after cold ischemia and reperfusion. The mRNA levels of FoxP3, IL-10, and TGF-β were expressed significantly higher in deceased than living donor kidneys. The difference between deceased and living donor kidneys was significant in the specimens taken after both cold storage and reperfusion.
Figure 5.
Immunohistochemical staining of CD3 and FoxP3. Example of infiltrating CD3 and FoxP3-positive cells in the kidney of a deceased donor (A). Marked expression of FoxP3 (arrows in B) was found in the cellular infiltrate in the kidney of a deceased donor (B). Sections are counterstained with hematoxylin. Original magnification, 200×; 400× in Insets.
To determine if the FoxP3 mRNA levels were associated with tissue damage and inflammation, we studied the correlation with KIM-1 and IFN-γ. Correlations were found between mRNA expression levels for FoxP3 and KIM-1 (rs=0.58, P=0.006) and FoxP3 and IFN-γ (rs=0.48, P=0.04). The FoxP3 mRNA expression levels inversely correlated with RORγc mRNA expression levels, suggesting that FoxP3+ T cells control the response of inflammatory IL-17 T cells by repressing RORγc (rs=−0.46, P=0.02).
The analyzed mRNA expression levels were not associated with the cause of brain death, treatment with vasoactive drugs, or occurrence of acute rejection or graft failure (all P>0.05).
Graft Function and mRNA Intragraft Expression
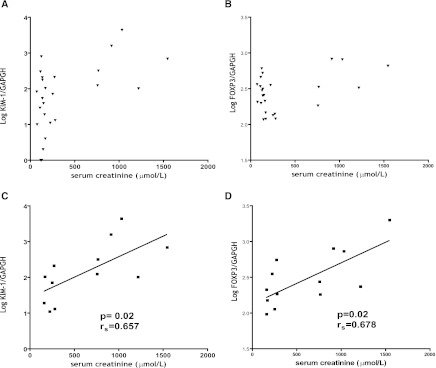
We determined whether kidney function shortly after transplantation correlated with intragraft mRNA expression levels. For the patient group studied, the median serum creatinine levels at day 7 after transplantation were 161 µmol/L (range=73–1546 µmol/L). No correlation between serum creatinine levels and mRNA expression levels was found for all samples analyzed (Figure 6, A and B). However, when we focused on the samples of patients with impaired kidney function at day 7 (>161 µmol/L, median of the study), a correlation between KIM-1, RORγc, and FoxP3 was found (rs=0.66, P=0.02; rs=0.68, P=0.02, respectively) (Figure 6, C and D).
Figure 6.
Correlation between KIM-1 and FoxP3 mRNA expression levels and kidney function. (A and B) No correlation between mRNA expression levels of KIM-1 and FoxP3 in all studied kidney biopsies from deceased and living donor kidneys obtained after cold ischemia was found. (C and D) Kidney KIM-1 and FoxP3 mRNA expression levels correlated with impaired kidney function at day 7 (>161 µmol/L, median of the study, n=12).
FoxP3+ T Cells and PTECs
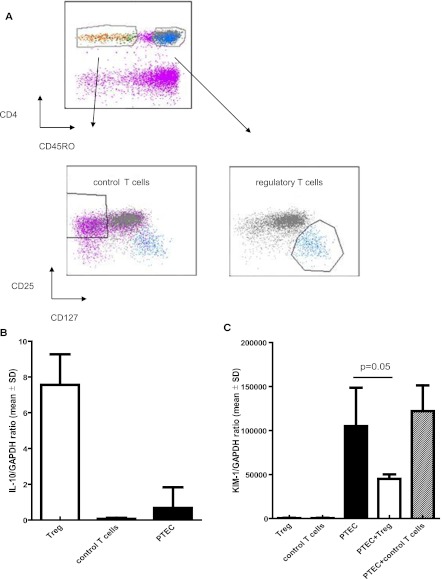
To investigate whether FoxP3+ T cells do have the potential to suppress the KIM-1 expression by PTEC, FACS sorted αCD3/αCD28/IL-2–activated FoxP3+Treg cells and control FoxP3− control T cells were cocultured for 3 days with TNF-α/IFN-γ–activated PTEC. Figure 7A shows the gating strategy of the sorted T cell populations. Tregs highly expressed IL-10 message and were negative for KIM-1, whereas PTECs expressed IL-10 at low levels and were strongly positive for KIM-1 (Figure 7, B and C). In three sets of experiments, we consistently found that KIM-1 expression by activated PTECs was 63% (mean) lower when cocultured with Tregs compared with control T cells (P=0.05) (Figure 7C).
Figure 7.
CD4+CD45RO+CD25high+CD127−/loFoxP3+ T cells but not CD4+CD25−CD45RO− T cells inhibit KIM-1 expression by proximal tubulus epithelial cells (PTECs). A shows the gating strategy of the sorted T cell populations. B depicts IL-10 mRNA expression levels of activated PTECs, activated CD4+CD45RO+CD25high+CD127−/loFOXP3+ T regulatory (Treg) cells, and CD4+CD25−CD45RO− T (control) cells. C shows the results of the coculture experiments. T cells were activated by αCD3/αCD28 and IL-2 and were cocultured with activated PTECs for 3 days. To measure gene expression by the PTEC and T cell subsets, the T cells were separated from the PTEC layer by standard trypsinization procedures followed by Ficoll gradient centrifugation. KIM-1 expression by PTEC was inhibited by 63% (mean) when cocultured with Treg cells compared with control T cells.
Discussion
We investigated regulatory and T-helper 17 cell-associated markers in the donor kidneys from deceased and living donors. Our study shows a marked overexpression of the kidney injury markers KIM-1, CD3ε, IFN-γ, IL-8, IL-10, TGF-β, and FoxP3 in deceased donor kidneys. In addition, hardly any IL-17A mRNA was detectable, whereas RORγc, the transcription factor for Th17 cells, was highly expressed in living donor kidneys.
The role of Th17 in tissue injury caused by the events of the donation and transplantation procedures is unknown. It has been speculated that, because of its chemoattractant function for neutrophils, IL-17 would be important in ischemia reperfusion injury (15–18). In samples taken after brain death and warm ischemia followed by a median cold ischemia time of 19 hours and 20–30 minutes reperfusion, we did not find evidence for the involvement of Th17 in kidney injury. This finding could be the result of sample timing. The work by Loong et al. (18) showed that IL-17–infiltrating cells are present in allografts at day 5 posttransplantation. The role of Th17 cells in human kidney injury is restricted to studies in renal autoimmune diseases and rejection after kidney transplantation (19,20). These studies show that Th17 cells can infiltrate the kidney and that these cells contribute to injury. In our study, the IL-17 transcription factor RORγc inversely correlated with the FoxP3. We hypothesize that FoxP3+ T cells inhibit IL-17 production or prevent the differentiation of activated T cells into Th17 cells. In mouse cells, FoxP3 suppresses transcriptional activity of RORγt by direct interaction, thereby inhibiting Th17 differentiation (21).
Here, we found that, during inflammation and tissue injury reflected by respectively high IFN-γ and KIM-1 mRNA levels, FoxP3 T cells infiltrate the kidney and that this finding mainly occurs in the donor as a result of brain death and warm ischemia-related events. Importantly, a biologic mechanism was found for FoxP3+ T cells, because they inhibited KIM-1 mRNA expression by PTECs in contrast to control FoxP3− T cells. Studies analyzing inflammatory responses after brain damage caused by cerebral ischemia reported an increased presence of FoxP3+ Tregs in blood and spleen (22). It is known that enhanced regulatory T cell activity is an element of the host response to tissue injury (9,23). These Tregs control the inflammatory immune response characteristic for burn injury (9). In our study, the positive correlation between FoxP3 and KIM-1 mRNA levels suggests a responsive reaction by Tregs. These Tregs may accumulate in the kidney subsequent to inflammation and injury. Data in the work by Veronese et al. (24) suggest an interaction between Tregs and PTECs and support a role for FoxP3+ T cells in tissue injury. Immunohistochemical analysis showed that, during allograft rejection, the proportion of CD4+ cells in tubules expressing FoxP3 is significantly higher than in the interstitium. To show the biologic activities of FoxP3+ IL-10–producing T cells directly on parenchymal cell function, we preformed coculture experiments and found that the expression of molecular marker for injury KIM-1 by PTECs was inhibited. Thus, FoxP3+ T cells have the potential to modulate KIM-1 through an IL-10–mediated mechanism. In line with this finding, it is tempting to speculate that Tregs have beneficial activities in repair processes in immunosuppressed patients. It has been shown that immunosuppressive agents like mTOR inhibitors and rabbit antithymocyte globulins help to expand or augment the activity of Tregs, whereas at the same time, these agents target effector T cells (25,26). Therefore, treatment of the organ donor, the harvested organ, and/or the recipient with these agents may beneficially contribute to the recovery of the organ. We speculate that this finding will lead to less tissue injury and improved kidney function after transplantation. The outcome of KIM-1 and FoxP3 measurements in urine samples of recipients of deceased donor kidneys who are treated with rabbit antithymocyte globulins induction therapy may support this hypothesis.
Proof that Treg function is important in tissue repair processes comes from Treg depletion studies. In mouse models of ischemic AKI, depletion of FoxP3 T cells potentiated kidney damage (27). In addition, it was shown that FoxP3+ T cells infiltrate the ischemic reperfused transplanted kidney during the healing process and promote repair by inhibiting the T cell production of IFN-γ and TNF-α (10).
In conclusion, our results show that donor FoxP3+ T cells infiltrate the deceased donor kidney, where they may control inflammatory and injury responses.
Disclosure
None.
Acknowledgment
The authors thank D. Lindenbergh-Kortleve for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, Faist E: Influence of brain death on cytokine release in organ donors and renal transplants. Transplant Proc 33: 1284–1285, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lopau K, Mark J, Schramm L, Heidbreder E, Wanner C: Hormonal changes in brain death and immune activation in the donor. Transpl Int 13[Suppl 1]: S282–S285, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Barklin A: Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand 53: 425–435, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Novitzky D, Horak A, Cooper DK, Rose AG: Electrocardiographic and histopathologic changes developing during experimental brain death in the baboon. Transplant Proc 21: 2567–2569, 1989 [PubMed] [Google Scholar]
- 5.Pratschke J, Neuhaus P, Tullius SG: What can be learned from brain-death models? Transpl Int 18: 15–21, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lemay S, Rabb H, Postler G, Singh AK: Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation 69: 959–963, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H: Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int 75: 526–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC: Postinjury multiple organ failure: A bimodal phenomenon. J Trauma 40: 501–510, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA: CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol 174: 2957–2963, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV: Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 73: 608–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemos FBC, Ijzermans JNM, Zondervan PE, Peeters AMA, van den Engel S, Mol WM, Weimar W, Baan CC: Differential expression of heme oxygenase-1 and vascular endothelial growth factor in cadaveric and living donor kidneys after ischemia-reperfusion. J Am Soc Nephrol 14: 3278–3287, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT: Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Wood KJ: Interleukin-23 and TH17 cells in transplantation immunity: Does 23+17 equal rejection? Transplantation 84: 1071–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS: Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant 6: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gorbacheva V, Fan R, Li X, Valujskikh A: Interleukin-17 promotes early allograft inflammation. Am J Pathol 177: 1265–1273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY: Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol 197: 322–332, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Van Kooten C, Boonstra JG, Paape ME, Fossiez F, Banchereau J, Lebecque S, Bruijn JA, De Fijter JW, Van Es LA, Daha MR: Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol 9: 1526–1534, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, Caligiuri G, Graff-Dubois S, Morelon E, Thaunat O: Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol 184: 5344–5351, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR: TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453: 236–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD: Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol 176: 6523–6531, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA: Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol 176: 225–236, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Veronese F, Rotman S, Smith RN, Pelle TD, Farrell ML, Kawai T, Benedict Cosimi A, Colvin RB: Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant 7: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177: 8338–8347, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sewgobind VD, van der Laan LJ, Kho MM, Kraaijeveld R, Korevaar SS, Mol W, Weimar W, Baan CC: The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins. Clin Exp Immunol 161: 364–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]