Summary
Background and objectives
Podocalyxin (PCX) is present on the apical cell membrane of podocytes and is shed in urine from injured podocytes. Urinary podocalyxin (u-PCX) is associated with severity of active glomerular injury in patients with glomerular diseases. This study examined the relationship between number of urinary podocytes, levels of u-PCX, and glomerular injury in adults with IgA nephropathy (IgAN).
Design, setting, participants, & measurements
Urine samples voided in the morning on the day of biopsy were obtained from 51 patients with IgAN (18 men and 33 women; mean age, 31 years). All renal biopsy specimens were analyzed histologically. Pathologic variables of IgAN were analyzed per Shigematsu classification, the Oxford classification of IgAN, and the Clinical Guidelines of IgAN in Japan. Levels of u-PCX were measured by sandwich ELISA.
Results
Histologic analysis based on Shigematsu classification revealed a significant correlation between levels of u-PCX and severity of acute extracapillary abnormalities (r=0.72; P<0.001), but levels of urinary protein excretion did not correlate with acute glomerular abnormalities. Levels of urinary protein excretion in patients with segmental sclerosis (n=19) were higher than in patients without (n=22) (0.49 [interquartile range (IQR), 0.20–0.88] g/g creatinine versus 0.20 [IQR, 0.10–0.33] g/g creatinine; P<0.01). The number of urinary podocytes in patients with segmental sclerosis was higher than in patients without (1.05 [IQR, 0.41–1.67] per mg creatinine versus 0.28 [IQR, 0.10–0.66] per mg creatinine; P<0.01).
Conclusions
Levels of u-PCX and the number of urinary podocytes are associated with histologic abnormalities in adults with IgAN.
Introduction
Glomerular podocytes, highly specialized epithelial cells, together with the glomerular basement membrane (GBM), play an important role in glomerular filtration (1). They form the final barrier to protein loss, which explains why podocyte injury is typically associated with marked proteinuria (2,3). Podocyte injury is accompanied by characteristic changes, including foot process effacement, cell body attenuation, pseudocyst formation, and detachment from the GBM (1). The ultimate podocyte lesion is detachment from the GBM, eventually followed by loss of the entire cell into the Bowman space (1). Denuded GBM, which results from detachment of podocytes, leads to the formation of a tuft adhesion to the Bowman capsule, the first lesion in the progression to glomerulosclerosis (1). Hara et al. suggested that prolonged or significant podocyte loss into urine during GN may play a causative role in the development of glomerulosclerosis (4). It has also been reported that urinary podocytes are associated with the severity of active glomerular injury in children with glomerular diseases (4–6).
Podocalyxin (PCX), a sialomucin most closely related to CD34 and endoglycan, is expressed by podocytes, hematopoietic progenitors, vascular endothelia, and a subset of neurons (7). The function of PCX is to maintain podocyte shape and distortion of the slit diaphragm (7). PCX is usually located on the apical cell membrane of podocytes and is shed into urine from injured podocytes (8). Human urinary PCX (u-PCX) originates not from podocyte exosomes but from tip vesiculation of glomerular podocyte microvilli (9). Habara et al. also showed that levels of u-PCX were significantly increased in patients with active GN compared with patients who have chronic GN in long-term remission (10).
IgAN is one of the most common types of primary GN in children and adults worldwide. It is characterized by mesangial deposition of IgA associated with mesangial cell proliferation and mesangial matrix expansion. In addition to these common histologic abnormalities, other glomerular abnormalities, such as segmental sclerosis, crescent formation, and adhesion to the Bowman capsules, are detected. Podocyte loss from the GBM in IgAN may cause or contribute to the progression of proteinuria, glomerulosclerosis, and filtration failure (11–14).
To more easily speculate about the current histologic findings in adult patients suspected of having IgAN, it is very important to find new noninvasive biomarkers. It is not known whether u-PCX and urinary podocytes are associated with histologic findings in adults with IgAN. In this study, we focused on u-PCX and urinary podocytes in adult patients with IgAN to understand the relationship between these urinary biomarkers and histologic findings.
Materials and Methods
Patients and Histologic Evaluation
From October 2007 to October 2011, urine samples voided on the morning of the day biopsy were obtained from 51 patients with IgAN. The clinical profile of patients with IgAN is shown in Table 1. Renal biopsies were performed on 49 patients in Juntendo University Hospital, Tokyo, Japan. The pathologic characteristics of the other two samples were also investigated at Juntendo University Hospital. Patients who were administrated angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, and corticosteroid treatment and who underwent tonsillectomy were excluded from this study.
Table 1.
Clinical profile of patients with IgA nephropathy
| Variable | Data |
|---|---|
| Patients (n) | 51 |
| Age (yr) | 31 (24–37) |
| Male:female (n) | 18:33 |
| Serum creatinine (mg/dl) | 0.70 (0.60–0.92) |
| Urinary protein (g/g creatinine) | 0.34 (0.17–0.55) |
| Estimated GFR (ml/min per 1.73 m2) | 83.6 (71.3–103.0) |
| Mean arterial pressure (mmHg) | 79 (73–84) |
| Shigematsu classification (n) | 35 |
| Oxford classification (n) | 41 |
| Clinical Guidelines of IgAN in Japan (n) | 51 |
Unless otherwise noted, the data are given as median (interquartile range). IgAN, IgA nephropathy.
Shigematsu classification and the Oxford classification were used to evaluate histologic findings of each case (15–17). To evaluate histologic findings in renal biopsy specimens of patients with IgAN, the sections were stained by three stains: hematoxylin-eosin, periodic acid-Schiff, and periodic acid methenamine silver-Masson trichrome (PAM-MT). The histologic evaluation of glomeruli for activity and chronicity was performed according to the method proposed by Shigematsu (15): extracapillary abnormality (acute and chronic) and endocapillary abnormality (acute and chronic). The extent of the active lesion was categorized into one of four grades (0, 1, 2, and 3), and extent of chronic lesion into four stages (0, 1, 2, and 3). Active lesions were commonly expressed segmentally; therefore, grade 1 indicated one segmental lesion, grade 2 indicated two segmental lesions, and grade 3 indicated three or more lesions in one glomerulus. Chronic lesions were categorized by the extent of PAM-positive collagenous matrix; therefore, stage 1 represented ≤30% in one glomerulus, stage 2 represented >31% to <59% in one glomerulus, and stage 3 represented ≥60% in one glomerulus. Regarding the glomerular lesions, this evaluation was applied to all the glomeruli in the biopsy specimens, and the average of the scores was taken. These semiquantitative evaluations were processed in statistical analyses. The minimal number of glomeruli evaluated per section was 10 according to Shigematsu classification.
The minimal number of glomeruli evaluated per section was eight according to the Oxford classification (16,17). We divided tubular atrophy and interstitial fibrosis, which ranged from 0% to 25% in the original score, into groups of 0%–4% and 5%–25% to analyze the interstitial changes in detail.
Moreover, patients were divided into four prognosis groups according to the Clinical Guidelines of IgAN in Japan (18); (1) good prognosis, (2) relatively good prognosis, (3) relatively poor prognosis, and (4) poor prognosis. In this study, we divided the patients into two groups as follows: group A (combined good and relatively good prognosis groups) and group B (combined relatively poor and poor prognosis groups).
Histologic findings of each slide were evaluated by two nephrologists who did not know the details of patients’ clinical data, including levels of u-PCX and the number of urinary podocytes.
This study was conducted according to the Declaration of Helsinki and was approved by Institutional Review Board of Juntendo University Hospital. Informed consent was obtained from all patients or from parents in the case of children younger than age 19 years.
Urine Sample Collection
Urine samples were collected in sterile plastic tubes and mixed with a protease inhibitor mixture (final concentration of 0.1% [w/v] NaN3, 3.3 μg/ml pepstatin A, 5 μg/ml aprotinin, 5 μg/ml leupeptin, and 5 mM benzamidine). The samples were frozen at −80°C and thawed once before analysis.
Preparation of PCX-Intra
A detailed procedure for construction of human PCX has been described elsewhere (19). A 228-bp cDNA fragment encoding the intracellular region of human PCX (Cys 453–Leu 528; PCX-Intra) was amplified by PCR from human template cDNA using the primers of 5′-TTTGGATCCTGCCACCAGCGCCTCTCCCAG-3′ (forward) and 5′-TTTGAATTCTTAGAGGTGCGTGTCTTCCTC-3′ (reverse). The PCR products were subcloned into the pGEX-6P-1 expression vector (GE Healthcare UK Ltd.) at its BamHI/EcoRI site.
Production of mAbs to PCX
cDNA encoding human PCX was obtained from a human kidney cDNA library using RT-PCR by the method of Kershaw et al. (19). The PC-46, a portion of extracellular, transmembrane, and intracellular domains of PCX (Gln 252–Leu 528), was obtained as glutathione-S-transferase fusion protein to produce mAbs (19). The epitopes on mAbs were characterized using PCX-Intra. The hybridomas were produced in immunized BALB/C mice as previously described by Tsutsumi et al. (20), and then selected and subcloned according to the immunofluorescence pattern that was assayed on human renal cortex or ELISA using plates coated with wheat germ agglutinin-binding as reported elsewhere (21). Among the positive clones, mAb no. 147 and mAb no. 5 were selected and characterized by Western blot and ELISA (8,21).
Measurement of Human u-PCX by ELISA
Two clones of antibody (no. 147 and no. 5) that recognize the intracellular peptide region were chosen for sandwich ELISA from protein A–bound fraction of ascitic fluid. No. 147 was fixed on the ELISA plate as a capture antibody and no. 5 was labeled with horseradish peroxidase and used as a tracer antibody. The PCX-Intra was used as a reference control for the calibration curve. The concentration was standardized to the reactivity of native PCX extracted from human glomeruli (8,22). A 1:1 mixture of urine and solution A (400 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid sodium–NaOH, 40 mM EDTA, 0.4% [v/v] Triton X-100, pH 7.0) were applied on the ELISA plate. The intra- and interassay coefficients of variation were 3.5% and 8.4%, respectively. The spiking recovery rate was 91.9%.
Measurement of Other Markers
Serum measures from the patients were determined in the clinical laboratory at Juntendo University Hospital. Levels of serum creatinine were measured using standard enzymatic methods. Levels of urinary protein excretion were measured by the pyrogallol red method using a reagent kit (Protein Assay Rapid Kit, Wako Pure Chemical Industries, Ltd.). Levels of urinary creatinine were measured by an automated machine (7170S; Hitachi) using a reagent kit (CRE-S; Denka Seiken Co., Ltd.). Levels of urinary protein excretion and u-PCX and the number of urinary podocytes were individually normalized to urinary creatinine levels. The following equation was used to calculate estimated glomerular filtration rate as described by Matsuo et al. (23):
 |
Quantification of Urinary Podocytes
Urinary podocytes were stained by immunofluorescence technique, and the number of urinary podocytes was counted as reported elsewhere (4,24).
Western Blot Analysis of u-PCX
Urine samples were filtered through a sieve with a 40-μm pore size and centrifuged at 44,000 rpm for 2 hours. Isolated human glomeruli were obtained as positive control on the basis of a previous report (8). Detection of protein in human glomerular lysate and urine sediment lysate was analyzed by Western blot according to the procedures described elsewhere (21).
Immunofluorescence
Immunofluorescence was performed as described elsewhere to detect the expression of PCX in the urine and glomeruli of patients with IgAN (9). To detect urinary podocytes, immunofluorescence was performed and Cytospin preparations were made. Freshly voided urine (10 ml) was centrifuged at 1800 rpm for 5 minutes. The sediments were prepared on poly-L-lysin–coated microscope slides at 1000 rpm for 5 minutes (Cytospin preparations), then air-dried for at least 30 minutes and fixed in acetone at −20°C for 5 minutes. After fixation in cold acetone, the slide glass was processed for the usual immunofluorescence staining. Similarly, frozen sections from renal biopsy specimens were stained by usual immunofluorescence procedure. The primary antibodies were antihuman PCX monoclonal antibody (mouse) (Denka Seiken Co., Ltd, Niigata, Japan) and antipodocin antibody (25). Nuclei were stained with 4′, 6-diamidine-2-phenylindole (DAPI).
Statistical Analyses
Statistical analyses were performed using Graph Pad PRISM software, version 4.0. Comparisons between groups were analyzed by the Mann-Whitney U test and Kruskall-Wallis tests. Differences at P<0.05 were considered significant. Because the three urinary measures and the scores of histologic examinations did not follow the normal distribution, Spearman rank correlation coefficients were used. The confidence intervals (CIs) were calculated using bootstrapping (26,27), a statistical method based on resampling that can be used to perform statistical inference, by SAS/BASIC, version 9 (SAS Institute, Cary, NC).
Results
Urinary PCX and Urinary Podocytes Detected in Patients with IgAN
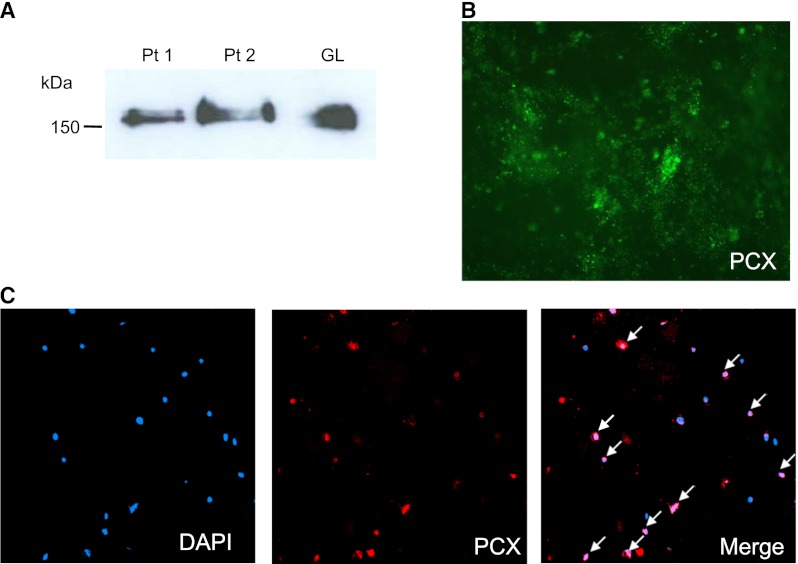
Urine samples were analyzed by Western blot to detect u-PCX in two randomly chosen patients with IgAN (Figure 1A). The 160- to 170-kD band in human glomerular lysate was used as a positive control. Positive bands on 160 to 170 kD were detected in urine samples from patients with IgAN (patients 1 and 2).
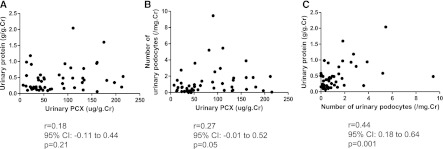
Figure 1.
Western blot analysis for urinary podocalyxin (u-PCX) and immunofluorescence for u-PCX and urinary podocytes. (A) Western blot analysis of urine from patients with IgA nephropathy (IgAN) and human glomerular lysate (GL) using monoclonal antibody against PCX. The 160- to 170-kD bands were seen in urine from patients with IgAN. Pt1 and Pt2, urinary sediments of two patients with IgAN. (B) Immunfluorescence of urine from a patient with IgAN. Urinary sediments were stained with anti-PCX monoclonal antibody. PCX staining showed a granular structure on urine from a patient with IgAN. Original magnification, ×400. (C) Immunfluorescence of urinary podocytes of a patient with IgAN showed double-staining for 4′, 6-diamidine-2-phenylindole (DAPI) (blue) and PCX (red). Some nucleated cells in urinary sediments showed positive PCX staining (arrows). Original magnification, ×200.
PCX-positive granular structures were frequently observed in the urine of several patients with IgAN who were randomly chosen (Figure 1B). Immunofluorescence of the urinary podocytes was performed in a patient with IgAN by double-staining for DAPI and PCX. The PCX-positive granular structures were located in the casts and sporadically around the casts. Some of the nucleated cells in the urinary sediments showed positive PCX staining (Figure 1C).
PCX was expressed in glomeruli of patients with IgAN (Supplemental Figure 1). PCX was co-localized with podocin, which is a podocyte marker (Supplemental Figure 2).
Correlation between Urinary PCX, Number of Urinary Podocytes, and Levels of Urinary Protein Excretion
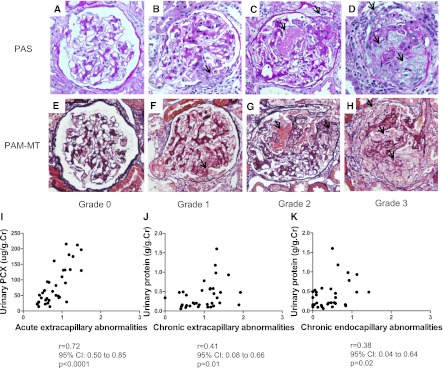
Levels of u-PCX did not correlate with levels of urinary protein excretion (Figure 2A). In addition, levels of u-PCX did not correlate with the number of urinary podocytes (Figure 2B). There was low correlation between the number of urinary podocytes and levels of urinary protein excretion (r=0.44; P=0.001) (Figure 2C).
Figure 2.
Relationship between levels of urinary podocalyxin (u-PCX), levels of urinary protein excretion, and number of urinary podocytes. (A) Relationship between levels of u-PCX and levels of urinary protein excretion. (B) Relationship between levels of u-PCX and number of urinary podocytes. (C) Relationship between number of urinary podocytes and levels of urinary protein excretion. There was low correlation between number of urinary podocytes and levels of urinary protein excretion.
Histologic Evaluation
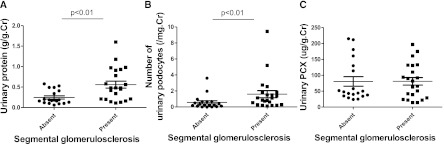
Urinary PCX Correlated with Acute Extracapillary Abnormalities (Shigematsu Classification)
We enrolled 35 patients to determine acute extracapillary abnormalities according to Shigematsu classification (15). The representative histologic findings of acute extracapillary abnormalities were analyzed by periodic acid-Schiff and PAM-MT stain (Figure 3, A–H). In grade 0, no acute extracapillary abnormality was observed (Figure 3, A and E). In grade 1, a small cellular crescent formation was observed (Figure 3, B and F). In grade 2, exudates that had escaped into the urinary space and a cellular crescent were observed (Figure 3, C and G). In grade 3, three cellular crescents were observed (Figure 3, D and H).
Figure 3.
Representative histologic findings of acute extracapillary abnormalities stained with periodic acid-Schiff and periodic acid methenamine silver-Masson trichrome. (A and E) In grade 0, no acute extracapillary abnormality was observed. (B and F) In grade 1, a small cellular crescent formation was observed (arrow). (C and G) In grade 2, exudates that have escaped into the urinary space and a cellular crescent were observed (arrows). (D and H) In grade 3, three cellular crescents were observed (arrows). Original magnification ×200. (I) Relationship between acute extracapillary abnormalities and levels of urinary podocalyxin. (J) Relationship between chronic extracapillary abnormalities and levels of urinary protein excretion. (K) Relationship between chronic endocapillary abnormalities and levels of urinary protein excretion. There was a positive correlation between the severity of acute extracapillary abnormalities and levels of urinary podocalyxin. There were low correlations between the severity of chronic glomerular abnormalities and levels of urinary protein excretion.
The relationships between levels of u-PCX, levels of urinary protein excretion, number of urinary podocytes, and pathologic score was analyzed using Shigematsu classification (15). Levels of u-PCX correlated positively with severity of acute extracapillary abnormalities (r=0.72; P<0.001) (Figure 3I). When the cutoff value for levels of u-PCX was defined as 110 μg/g creatinine and the cutoff value for the score of acute extracapillary abnormalities was defined as 1.0, the sensitivity and specificity were 0.77 and 0.96, respectively. Because the number of patients is not large, we tried to use the Efron bootstrap methods to prove the correlation between levels of u-PCX and the acute extracapillary abnormalities (26,27). The bootstrapping 95% CIs for the correlation between u-PCX and severity of acute extracapillary abnormalities are 0.50–0.85. Conversely, levels of urinary protein excretion did not correlate with severity of acute extracapillary abnormalities (Table 2). There was low correlation between levels of urinary protein excretion and chronic glomerular abnormalities (Figure 3, J and K). Other relationships between urinary markers and Shigematsu classification are shown in Table 2.
Table 2.
Relationship between urinary markers and each measure of Shigematsu classification
| Variable | r (95% Confidence Interval) | P Value |
|---|---|---|
| Acute endocapillary abnormalities | ||
| u-PCX | 0.33 (−0.01 to 0.60) | 0.05 |
| urinary protein | −0.03 (−0.37 to 0.32) | 0.88 |
| number of urinary podocytes | −0.01 (−0.35 to 0.34) | 0.97 |
| Acute extracapillary abnormalities | ||
| urinary protein | 0.19 (−0.16 to 0.50) | 0.28 |
| number of urinary podocytes | 0.07 (−0.28 to 0.40) | 0.69 |
| Chronic endocapillary abnormalities | ||
| u-PCX | 0.03 (−0.32 to 0.36) | 0.89 |
| number of urinary podocytes | 0.23 (−0.12 to 0.53) | 0.18 |
| Chronic extracapillary abnormalities | ||
| u-PCX | 0.02 (−0.33 to 0.36) | 0.92 |
| number of urinary podocytes | −0.02 (−0.36 to 0.32) | 0.91 |
u-PCX, urinary podocalyxin.
Levels of Urinary Protein Excretion and Number of Urinary Podocytes Were Higher in IgAN Patients with Glomerulosclerosis Than Those without (Oxford Classification)
We enrolled 41 patients to analyze their samples using Oxford classification. Levels of urinary protein excretion in patients with segmental sclerosis (n=19) were higher than those in patients without (n=22) (0.49 [interquartile range (IQR), 0.20–0.88] g/g creatinine versus 0.20 [IQR, 0.10–0.33] g/g creatinine; P<0.01; Figure 4A). Similarly, the number of urinary podocytes in patients with segmental sclerosis was higher than that in patients without (1.05 [IQR, 0.41–1.67]/mg creatinine versus 0.28 [IQR, 0.10–0.66]/mg creatinine; P<0.01; Figure 4B). When the cutoff value for levels of urinary protein excretion was defined as 0.3 g/g creatinine, the sensitivity and specificity were 0.70 and 0.68, respectively. When the cutoff value for number of podocytes was defined as 1.0 per mg creatinine, the sensitivity and specificity were 0.52 and 0.90, respectively. However, there was no difference between levels of u-PCX in IgAN patients with and those without segmental sclerosis (P=0.65) (Figure 4C). The relationship between the severity of mesangial hypercellularity and each urinary biomarkers is as follows: levels of urinary protein excretion, r=0.14 (95% CI, −0.18 to 0.43; P=0.38), levels of u-PCX, r=0.03 (95% CI, −0.29 to 0.34; P=0.38), and number of urinary podocytes, r=−0.12 (95% CI, −0.42 to 0.20; P=0.44). Levels of urinary protein excretion in patients with 5%–25% tubular atrophy/interstitial fibrosis were higher than those in patients with 0%–4% (0.39 [IQR, 0.17–0.59] g/g creatinine versus 0.18 [IQR, 0.12–0.37] g/g creatinine; P=0.04). There was no difference between levels of u-PCX, number of urinary podocytes, and severity of tubular atrophy/interstitial fibrosis (P>0.05 for all comparisons).
Figure 4.
Relationship of segmental sclerosis to each urinary biomarker. (A) Levels of urinary protein excretion. (B) Number of urinary podocytes. (C) Levels of urinary podocalyxin (PCX). Levels of urinary protein excretion and number of urinary podocytes in patients with IgA nephropathy with segmental sclerosis were higher than those in patients without (P<0.01, P<0.01, respectively). Error bars represent mean ± SEM.
Clinical Guidelines of IgAN in Japan
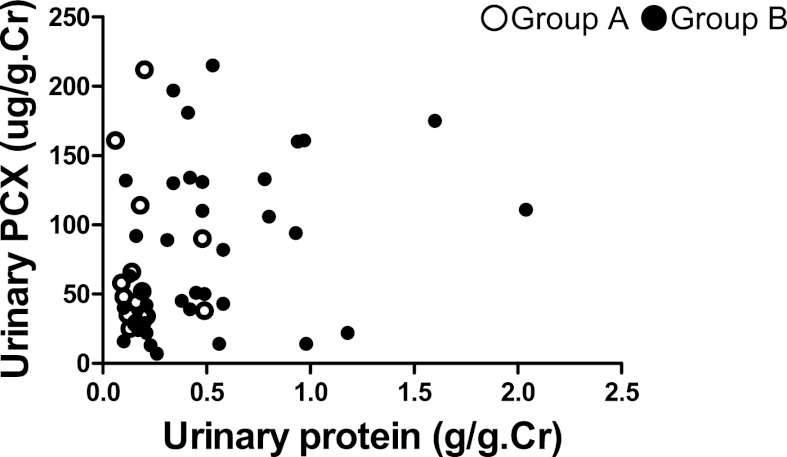
The clinical profile of 51 patients with IgAN categorized by Clinical Guidelines of IgAN in Japan is shown in Table 3. There was no significant difference in levels of u-PCX or the number of urinary podocytes between group A and group B (Table 3). Levels of urinary protein excretion in group B were significantly higher than those in group A (P<0.001) (Table 3). Some poor-prognosis patients had lower levels of urinary protein excretion and higher levels of u-PCX (Figure 5).
Table 3.
Clinical profile of patients evaluated by the Clinical Guidelines of IgAN in Japan
| Variable | Group A (n=16)a | Group B (n=35)b | P Value |
|---|---|---|---|
| Age (yr) | 26 (22–32) | 33 (25–39) | 0.15 |
| Male:female (n) | 6:10 | 12:23 | |
| Serum creatinine (mg/dl) | 0.69 (0.59–0.81) | 0.70 (0.61–0.93) | 0.48 |
| Estimated GFR (ml/min per 1.73 m2) | 91.9 (76.2–105.1) | 80.8 (69.9–98.4) | 0.28 |
| Mean arterial pressure (mmHg) | 74 (72–79) | 81 (76–85) | 0.09 |
| Urinary protein (g/g creatinine) | 0.17 (0.13–0.19) | 0.42 (0.22–0.68) | <0.001 |
| u-PCX (μg/g creatinine) | 50.05 (35.17–72.23) | 81.59 (34.02–131.63) | 0.70 |
| Urinary podocytes (n/mg creatinine) | 0.35 (0.25–1.53) | 0.82 (0.24–1.64) | 0.24 |
Unless otherwise noted, the data are given as median (interquartile range). IgAN, IgA nephropathy; u-PCX, urinary podocalyxin.
Good prognosis and relatively good prognosis groups.
Relatively poor prognosis and poor prognosis groups.
Figure 5.
Relationship between the Clinical Guidelines of IgAN in Japan and urinary biomarkers. Group A, good prognosis and relatively good prognosis groups; group B, relatively poor prognosis and poor prognosis groups. Some patients with relatively poor or poor prognosis had lower levels of urinary protein excretion and higher levels of urinary podocalyxin.
Discussion
PCX is excreted in the urine because of shedding of tip vesiculation from podocyte microvilli, not because of passive cellular destruction during or after detachment of podocytes from GBM (8,9). Achenbach et al. reported that PCX was detected on the Bowman capsule and was produced by the parietal epithelial surface (28). Conversely, we found that PCX was detected in the glomerulus, and only a little on the Bowman capsule, in patients with IgAN. Two possible reasons may explain difference between those earlier results and ours. The first reason is the difference in type of kidney diseases. Achenbach et al. enrolled patients with focal segmental glomerulosclerosis, membranous nephropathy, and membranoproliferative GN. In contrast, we enrolled patients with IgAN. The second reason is the difference in the antibody used against PCX for immunostaining.
Kanno et al. measured levels of u-PCX in children with glomerular diseases such as IgAN and concluded that u-PCX was a useful biomarker for estimating the severity of active glomerular injury and urinary index of acute extracapillary abnormalities in children (21). Habara et al. reported that levels of u-PCX in adults with various forms of active GN were significantly higher than those in patients with chronic GN in long-term remission (10). However, few published studies report the correlation between u-PCX and the histologic findings of adults with IgAN. In this study, we found a positive correlation between levels of u-PCX and acute extracapillary abnormalities in adults with IgAN. Conversely, levels of urinary protein excretion did not correlate with acute glomerular abnormalities. Acute extracapillary abnormalities could be followed by detachment of podocytes and GBM denudation, the initial steps in the development of glomerulosclerosis (29). It is possible that u-PCX reflects acute extracapillary abnormalities and is an index of ongoing podocyte injury leading to glomerulosclerosis.
Recently, IgAN patients with FSGS have been reported to have a relatively poor renal outcome than those without FSGS (30,31). Preventing the development of glomerulosclerosis as soon as possible is extremely important, particularly if the renal outcome can be improved by some treatments. Several studies have shown that corticosteroid treatment could decrease levels of urinary protein excretion and improve renal outcome in patients with IgAN (32–35). Shoji et al. reported that early corticosteroid treatment for active and proliferative IgAN in adults was effective in reducing renal injury (36). Immunosuppressive treatment, including corticosteroids, is recommended for crescentic IgAN with active glomerular inflammation (35). In future studies, we aim to clarify whether u-PCX is a useful biomarker to determine the effectiveness of corticosteroid treatment. We also need to perform a longitudinal study to examine such factors as changes in levels of u-PCX before and after corticosteroid treatment, as well as renal outcome.
Development of glomerulosclerosis in several human and experimental diseases is associated with podocyte loss (4,5,37,38). In this study, we showed that the number of urinary podocytes in IgAN patients with segmental sclerosis was significantly higher than that in patients without segmental sclerosis. Lemley et al. reported that the degree of podocyte loss was related to the extent of glomerulosclerosis in patients with IgAN (12). These results suggest that the number of urinary podocytes indicates the existence of glomerulosclerosis in adults with IgAN.
The number of glomeruli obtained by renal biopsy was sometimes not enough to evaluate the severity of glomerular changes. However, because u-PCX and urinary podocytes mainly originated from all glomeruli in the kidney, the urinary biomarkers seem to reflect the histologic findings of all glomeruli. The urinary biomarkers might help us to speculate on the histologic findings with renal biopsy. In this cohort study, we found the relationship between urinary biomarkers and renal histologic findings in adults with IgAN. The biomarkers have a potential role as an adjunct to the diagnosis of renal histology in patients with IgAN.
The limitations of this study were the low sensitivity in the relationship between u-PCX and acute extracapillary abnormalities and in the relationship between number of urinary podocytes and segmental sclerosis. These results suggested that even if the levels of u-PCX or number of urinary podocytes were low or small, some patients had active glomerular abnormalities or segmental sclerosis, respectively. We had to consider the risk of underestimating the pathologic findings based on only the levels of u-PCX or number of urinary podocytes. Moreover, this study was performed only in Juntendo University Hospital, and the number of patients was small. Future studies should involve more samples.
In conclusion, u-PCX is associated with the activity of IgAN in the acute phase, and urinary protein and urinary podocytes are also associated with the chronicity of IgAN.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Professor Kevin V. Lemley for helpful discussion and advice about this manuscript. We thank the Laboratory of Molecular and Biochemical Research, Research Support Center, and Laboratory of Biomedical Imaging Research, Biomedical Research Center, Juntendo University Graduate School of Medicine, for technical assistance.
This work was supported by research grants from the Takeda Science Foundation, Kanae Foundation for the Promotion of Medical Science and Kowa Life Science Foundation to K.A., by a Grant for Challenging Exploratory Research (21659217) to K.A., by a Grant-in-Aid for Scientific Research (C) (23591201) to K.A., by a Grant-in-Aid for Young Scientists (B) (24790858) to F.K., and by a Grant-in-Aid for Young Scientists (B) (24790856) to M.A.-T.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08110811/-/DCSupplemental.
References
- 1.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y: The role of podocytes in proteinuria. Nephrology (Carlton) 12[Suppl 3]: S15–S20, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hara M, Yanagihara T, Kihara I: Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein-Henoch purpura nephritis. Clin J Am Soc Nephrol 2: 231–238, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Yanagihara T, Takada T, Itoh M, Matsuno M, Yamamoto T, Kihara I: Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol 18: 35–41, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J: Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16: 1733–1741, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen JS, McNagny KM: The role of podocalyxin in health and disease. J Am Soc Nephrol 20: 1669–1676, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T: Apical cell membranes are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. J Am Soc Nephrol 16: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Kihara I: Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol 41: 1265–1275, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Habara P, Marecková H, Sopková Z, Malícková K, Zivorová D, Zima T, Tesar V: A novel method for the estimation of podocyte injury: Podocalyxin-positive elements in urine. Folia Biol (Praha) 54: 162–167, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Tomino Y: Pathogenesis of IgA nephropathy. Contrib Nephrol 157: 1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Yang HC, Hao CM, Lin ST, Gu Y, Ma J: Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol 23: 1241–1250, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Hishiki T, Shirato I, Takahashi Y, Funabiki K, Horikoshi S, Tomino Y: Podocyte injury predicts prognosis in patients with iga nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press Res 24: 99–104, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu H: Histological grading and staging of IgA nephropathy. Pathol Int 47: 194–202, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Tomino Y, Sakai H, Special Study Group (IgA Nephropathy) on Progressive Glomerular Disease : Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol 7: 93–97, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC: Molecular cloning and characterization of human podocalyxin-like protein. Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem 272: 15708–15714, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi H, Flanagan TD, Ogra PL: Monoclonal antibodies to the large glycoproteins of respiratory syncytial virus: Possible evidence for several functional antigenic sites. J Gen Virol 68: 2161–2167, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kanno K, Kawachi H, Uchida Y, Hara M, Shimizu F, Uchiyama M: Urinary sediment podocalyxin in children with glomerular diseases. Nephron Clin Pract 95: c91–c99, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kerjaschki D, Poczewski H, Dekan G, Horvat R, Balzar E, Kraft N, Atkins RC: Identification of a major sialoprotein in the glycocalyx of human visceral glomerular epithelial cells. J Clin Invest 78: 1142–1149, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hara M, Yanagihara T, Kihara I: Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lydia A, Asanuma K, Nonaka K, Takagi M, Jeong KH, Kodama F, Asao R, Asanuma E, Prodjosudjadi W, Tomino Y: Effects of 22-oxa-calcitriol on podocyte injury in adriamycin-induced nephrosis. Am J Nephrol 35: 58–68, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Efron B: The Jackknife, the Bootstrap, and Other Resampling Plans CBMS-NSF Monographs, 38. Philadelphia, PA, Society of Industrial and Applied Mathematics, 1982 [Google Scholar]
- 27.Efron B, Tibshirani RJ: An Introduction to the Bootstrap, Boca Raton, FL, Chapman & Hall, 1993 [Google Scholar]
- 28.Achenbach J, Mengel M, Tossidou I, Peters I, Park JK, Haubitz M, Ehrich JH, Haller H, Schiffer M: Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant 23: 3138–3145, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MM, Lewis EJ: Focal segmental glomerular sclerosis: The cellular lesion. Kidney Int 28: 968–974, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Hill GS, Karoui KE, Karras A, Mandet C, Duong Van Huyen JP, Nochy D, Bruneval P: Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. I. Immunohistochemical studies. Kidney Int 79: 635–642, 2011 [DOI] [PubMed] [Google Scholar]
- 31.El Karoui K, Hill GS, Karras A, Moulonguet L, Caudwell V, Loupy A, Bruneval P, Jacquot C, Nochy D: Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int 79: 643–654, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Samuels JA, Strippoli GF, Craig JC, Schena FP, Molony DA: Immunosuppressive treatments for immunoglobulin A nephropathy: A meta-analysis of randomized controlled trials. Nephrology (Carlton) 9: 177–185, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Barratt J, Feehally J: Treatment of IgA nephropathy. Kidney Int 69: 1934–1938, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, Imai E, Hori M, Tsubakihara Y: Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Asanuma K, Akiba-Takagi M, Kodama F, Asao R, Nagai Y, Lydia A, Fukuda H, Tanaka E, Shibata T, Takahara H, Hidaka T, Asanuma E, Kominami E, Ueno T, Tomino Y: Dendrin location in podocytes is associated with disease progression in animal and human glomerulopathy. Am J Nephrol 33: 537–549, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.