Summary
Background and objectives
Residual kidney function in dialysis patients is associated with better survival, but there are no simple methods for its assessment. β-Trace protein is a novel endogenous filtration marker of kidney function that is not removed during hemodialysis and may serve as a marker for residual kidney function similar to serum creatinine in patients not on dialysis. The objective of this study was to determine the association of serum β-trace protein with mortality in incident hemodialysis patients.
Design, setting, participants, & measurements
Serum β-trace protein was measured in baseline samples from 503 participants of a national prospective cohort study of incident dialysis patients with enrollment during 1995–1998 and follow-up until 2004. Outcomes were all-cause and cardiovascular disease mortality analyzed using Cox regression adjusted for demographic, clinical, and treatment factors.
Results
Serum β-trace protein levels were higher in individuals with no urine output compared with individuals with urine output (9.0±3.5 versus 7.6±3.1 mg/L; P<0.001). There were 321 deaths (159 deaths from cardiovascular disease) during follow-up (median=3.3 years). Higher β-trace protein levels were associated with higher risk of mortality. The adjusted hazard ratio and 95% confidence interval for all-cause mortality per doubling of serum β-trace protein was 1.36 (1.09–1.69). The adjusted hazard ratios (95% confidence intervals) for all-cause mortality in the middle and highest tertiles compared with the lowest tertile were 0.95 (0.69–1.32) and 1.72 (1.25–2.37). Similar results were noted for cardiovascular disease mortality.
Conclusions
The serum level of β-trace protein is an independent predictor of death and cardiovascular disease mortality in incident hemodialysis patients.
Introduction
Optimizing care for dialysis patients remains a major challenge. Although the US costs for treatment of ESRD rose to $26.8 billion in 2008, accounting for 5.9% of the Medicare budget, the 5-year death rate for dialysis patients remains dismally high at 70% (1). Impressive improvements in morbidity and mortality have been documented in many chronic diseases over the last decade, but most of the recent randomized controlled trials of therapies in dialysis patients have yielded mostly negative results (2–4).
Native kidney function in dialysis patients, also referred to as residual kidney function (RKF), is strongly associated with better survival, with an 11%–48% lower risk of death per 1-ml/min per 1.73 m2 higher GFR. (5–11). RKF, even at the low GFR levels seen in dialysis patients, plays a crucial role in the clearance of uremic toxins that are not cleared by dialysis and also prevents volume overload and its sequelae, such as left ventricular hypertrophy and congestive heart failure. Although it is generally agreed that the contribution of RKF to clearance is important to quantify, the only method currently available for assessing RKF and residual Kt/Vurea is a 24- to 48-hour urine collection between dialysis days. This method is cumbersome; as a result, it is performed in fewer than 5% of all US incident hemodialysis patients (12) and is even difficult to perform in clinical trials (6). Thus, simpler methods for assessing kidney function similar to serum creatinine measurement in nondialysis patients, are needed.
β-Trace protein (BTP), a 23- to 29-kD serum protein, is a novel endogenous filtration marker. Unlike urea, creatinine, and cystatin C, it is not removed by hemodialysis because of its higher molecular weight. Serum levels of BTP are highly correlated with measured GFR (13–15) and have been shown to correlate with RKF (16), but their association with long-term outcomes in dialysis patients has not been studied. The aim of this study was to examine the association of serum BTP with mortality in incident hemodialysis participants of the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study.
Materials and Methods
Study Design
The CHOICE Study is a national prospective cohort study of incident dialysis patients (17). From October of 1995 to June of 1998, 1041 participants (767 on hemodialysis) from 19 US states were enrolled a median of 45 days after initiation of dialysis (95% within 3.5 months) and followed through December 31, 2004. Eligibility criteria were new onset of long-term dialysis therapy in the preceding 3 months, ability to provide informed consent, age>18 years, and ability to speak English or Spanish. A specimen bank was established among the Dialysis Clinics, Inc. participants of the CHOICE study. Nonfasting, predialysis serum specimens were centrifuged within 30–45 minutes of blood draw and sent overnight on ice to the Dialysis Clinics, Inc. Central Laboratory. Each blood draw was aliquoted into multiple vials and stored at −80°C. This study population includes 503 hemodialysis participants with banked sera. Included participants were younger (58 versus 62 years; P<0.001), less likely to be white (64% versus 76%; P=0.001) or have cardiovascular disease (56% versus 68%; P=0.001), and had lower estimated GFR at dialysis initiation (10.1 versus 11.3 ml/min per 1.73 m2; P=0.01) than those participants not included. Median time from dialysis initiation to blood draw was 4.8 months (25th to 75th percentiles=3.8–6.1 months). The Johns Hopkins Medicine Institutional Review Board (Baltimore, Maryland) and the clinical centers’ review boards approved the study, and participants provided written informed consent.
Data Collection
Exposure.
Serum BTP was measured by particle-enhanced immunonephelometric assays with a Siemens Dade Behring ProSpec analyzer (Siemens Healthcare Diagnostics, Newark, Delaware) at the University of Minnesota Medical Center, Fairview’s Collaborative Studies Clinical Laboratory. BTP is stable for 14 days on room temperature and multiple freeze–thaw cycles (18). The coefficient of variation (CV) for BTP was 4.0% at 1.8 mg/L and 5.7% at 0.6 mg/L. The reliability coefficient for BTP in 5% masked replicates assayed on different days was 0.885, and after the removal of two outliers, it was 0.968.
Outcomes.
The outcomes were all-cause and cardiovascular disease (CVD) mortality. Mortality information was independently adjudicated using information from clinic report, medical records, National Death Index, Centers for Medicare & Medicaid Services death notification forms, and Social Security records as previously described (19).
Other Variables.
Participants self-reported demographics, work history, medical history, and predialysis care. Body mass index (BMI; weight in kg/height in m2) was calculated based on the height and weight reported on the 2728 form. Late referral was defined as <4 months between first nephrologist evaluation and start of dialysis. The presence and severity of comorbid conditions were assessed using the Index of Coexistent Disease, a validated medical record-derived index that captures both presence and severity of comorbid conditions (20,21). Index of Coexistent Disease scores range from zero to three, with three as the highest severity level. Data on use of medications at baseline were abstracted from patient charts. Self-reported ability to make at least 1 full cup (250 ml) of urine daily was ascertained from study questionnaires at baseline and 1-year follow-up. Urine volume, measured as part of routine patient care, was available for a subsample (46%) of the participants at baseline. Data from routine patient care were available for BP, interdialytic weigh gain, serum calcium, phosphorus, potassium, blood urea nitrogen, and hemoglobin. High-sensitivity C-reactive protein (CRP) was measured using a colorimetric competitive enzyme-linked immunosorbent assay (CV=8.9%) as previously described (22). Serum albumin was measured in the same specimen as BTP at the University of Minnesota using bromocresol purple (CV=1.9%).
Statistical Analyses
Baseline characteristics of participants were compared by tertiles of serum BTP, and P values for linear trend were obtained using Pearson’s chi-squared test or linear regression as appropriate. Survival analysis techniques were used to analyze the risk of mortality, with serum BTP modeled categorically as tertiles and continuously as natural log of BTP. Individuals were censored at transplantation or the end of the study period (December 31, 2004). Missing data for variables were as follows: educational status (2.8%), smoking history (2.8%), BMI (5.6%), systolic and diastolic BP (3.8%). Missing data values were imputed with 10 data replicates using multiple imputation by the chained equations method implemented by the ice program and analyzed using the mim program in Stata 10.1 (Stata Corp.; www.stata.com). Cox proportional hazards regression was used to model the risk of death. Proportional hazards assumptions were checked graphically and by hypothesis-based tests (P=0.69). Hazard ratios (HRs) were calculated to assess the risk of death in unadjusted models and after adjustment for a priori-defined confounders, including demographic characteristics and clinical and treatment factors. Adjusted relative hazard of death was displayed with serum BTP modeled as a restricted cubic spline with knots at 10th, 50th, and 90th percentiles, with the 25th percentile of the lowest tertile as the reference point (23). In exploratory analyses, to assess the construct validity of serum BTP as a marker of RKF, we analyzed the association between BTP and self-described urine output, urine volume, and interdialytic weight gain among subgroups with available data. Statistical analyses were performed using Stata software, version 10.1 (Stata Corp.; www.stata.com). Statistical significance was defined as P<0.05 using two-tailed tests.
Results
Characteristics Associated with BTP
Baseline characteristics of the participants stratified by tertiles of serum BTP are listed in Table 1. The median time to specimen collection from start of dialysis was slightly longer among those individuals with higher BTP. Individuals with higher levels of BTP were less likely to self-report≥1 cup urine/d and had lower urine volumes. Higher levels of BTP were also associated with lower BMI, higher diastolic BP, lower estimated GFR at dialysis initiation, higher serum BUN, creatinine, potassium, phosphorus, and albumin. Importantly, there was no association between BTP and CRP, baseline Kt/V, or average duration of dialysis sessions.
Table 1.
Baseline characteristics of 503 incident hemodialysis participants of the Choices for Healthy Outcomes in Caring for ESRD Study by tertiles of serum β-trace protein
| N | Serum β-Trace Protein Tertiles (range, mg/L) | Linear Trend P Value | |||
|---|---|---|---|---|---|
| Lowest Tertile (1.8–6.1) | Middle Tertile (6.1–8.8) | Highest Tertile (8.8–21.1) | |||
| Number of participants | 503 | 168 | 168 | 167 | |
| Serum β-trace protein (mg/L)a | 503 | ||||
| mean (SD) | 4.6 (1.0) | 7.3 (0.8) | 11.4 (2.5) | ||
| median (25th to 75th percentiles) | 4.8 (3.8–5.5) | 7.3 (6.7–7.9) | 10.8 (9.8–12.2) | ||
| median time from dialysis initiation to specimen collection (months; 25th to 75th percentiles) | 4.5 (3.5–5.9) | 4.8 (3.8–6.0) | 5.1 (4.1–6.2) | 0.003 | |
| Demographic | |||||
| age | 503 | 59 (14) | 57 (15) | 58 (15) | 0.54 |
| sex (percent female) | 503 | 52 | 42 | 43 | 0.15 |
| race (percent white) | 503 | 64 | 68 | 59 | 0.26 |
| education (percent high school graduate) | 489 | 37 | 33 | 33 | 0.60 |
| employment (percent working) | 503 | 11 | 12 | 6 | 0.15 |
| marital status (percent married) | 503 | 52 | 49 | 47 | 0.65 |
| Clinical | |||||
| smoking status (percent ever smoker) | 489 | 56 | 59 | 61 | 0.64 |
| index of coexistent disease score (percent) | 502 | 0.33 | |||
| ≤1 | 33 | 25 | 31 | ||
| 2 | 38 | 42 | 44 | ||
| 3 | 29 | 33 | 25 | ||
| baseline comorbid conditions (%) | |||||
| diabetes | 502 | 59 | 60 | 52 | 0.27 |
| CVD | 502 | 56 | 55 | 55 | 0.97 |
| CHF | 502 | 50 | 49 | 48 | 0.91 |
| LVH | 502 | 29 | 23 | 29 | 0.35 |
| BMI median (kg/m2; 25th to 75th percentiles) | 475 | 27.2 (22.7–32.1) | 25.9 (22.8–31.0) | 25.0 (21.9–28.3) | 0.001 |
| systolic BP (mmHg) | 484 | 149 (19) | 153 (17) | 152 (18) | 0.10 |
| diastolic BP (mmHg) | 484 | 78 (10) | 81 (10) | 81 (10) | 0.03 |
| pulse pressure (mmHg) | 484 | 71 (15) | 73 (13) | 72 (14) | 0.59 |
| urine output≥1 cup/d | |||||
| status at baseline (percent yes) | 487 | 90 | 87 | 75 | 0.001 |
| status at year 1 | 425 | <0.001 | |||
| not at baseline or year 1 (%) | 11 | 15 | 28 | ||
| at baseline but not year 1 (loss of RKF; %) | 52 | 49 | 52 | ||
| at baseline and year 1 (preserved RKF; %) | 37 | 36 | 20 | ||
| urine volume (ml) | 231 | <0.001 | |||
| mean (SD) | 906 (584) | 815 (548) | 605 (381) | ||
| median (25th to 75th percentiles) | 738 (500–1150) | 600 (400–1100) | 525 (300–800) | ||
| ESRD-related | |||||
| eGFR at dialysis initiation (ml/min per 1.73 m2)b | 476 | 11 (4) | 10 (3) | 9 (4) | <0.001 |
| assigned primary cause of renal failure (%) | 503 | <0.001 | |||
| diabetes | 50 | 55 | 43 | ||
| hypertension | 18 | 11 | 23 | ||
| glomerulonephritis | 10 | 13 | 22 | ||
| late referral (%; <4 months) | 31 | 29 | 31 | 0.62 | |
| average dialysis Kt/Vc | 400 | 1.37 (0.28) | 1.31 (0.26) | 1.39 (0.27) | 0.42 |
| average dialysis duration in minutes | 434 | 216 (23) | 218 (23) | 219 (22) | 0.18 |
| Laboratory | |||||
| predialysis BUN (mg/dl) | 404 | 57 (14) | 59 (15) | 61 (15) | 0.02 |
| predialysis creatinine (mg/dl) | 501 | 6.6 (2.0) | 8.2 (2.4) | 9.8 (3.1) | <0.001 |
| potassium (mEq/L) | 500 | 4.6 (0.6) | 4.7 (0.6) | 4.9 (0.6) | <0.001 |
| albumin (g/dl) | 503 | 3.4 (0.6) | 3.5 (0.5) | 3.7 (0.6) | <0.001 |
| calcium (mg/dl) | 500 | 9.8 (0.7) | 9.8 (0.9) | 9.7 (0.8) | 0.56 |
| phosphorus (mg/dl) | 500 | 5.1 (1.3) | 5.8 (1.5) | 5.9 (1.7) | <0.001 |
| hemoglobin (g/dl) | 500 | 10.9 (1.1) | 11.2 (1.1) | 10.9 (1.1) | 0.48 |
| C-reactive protein (mg/dl; median [25th to 75th percentiles]) | 502 | 0.63 (0.30–1.39) | 0.58 (0.25–1.42) | 0.64 (0.22–1.70) | 0.77 |
| Baseline medications | |||||
| diuretics (%) | 503 | 30 | 29 | 24 | 0.38 |
| ACE inhibitors (%) | 503 | 28 | 30 | 29 | 0.89 |
| calcium channel blockers (%) | 503 | 60 | 57 | 68 | 0.07 |
| β-blockers (%) | 503 | 27 | 23 | 24 | 0.66 |
| phosphate binders (%) | 503 | 88 | 90 | 93 | 0.24 |
| EPO dose (units/wk)d | 338 | 42,184 (25,024) | 47,388 (24,157) | 48,956 (26,621) | 0.06 |
Numbers presented are mean (SD) or percent unless otherwise specified. Conversion factors for units: albumin in g/dl to g/L, ×10; calcium in mg/dl to mmol/L, ×0.2495; phosphorus in mg/dl to mmol/L, ×0.3229; hemoglobin in g/dl to g/L, ×10; BUN in mg/dl to urea in mmol/L, ×0.357; creatinine in mg/dl to umol/L, ×88.4. No conversion is necessary for potassium in mEq/L to mmol/L. CVD, cardiovascular disease; CHF, congestive heart failure; LVH, left ventricular hypertrophy; BMI, body mass index; RKF, residual kidney function; eGFR, estimated GFR; ACE, angiotensin converting enzyme; EPO, erythropoietin.
Normal reference range of serum BTP has not been determined. In the Atherosclerosis Risk in Communities Study, among those individuals in the highest eGFR group (>96.7 ml/min per 1.73 m2), serum β-trace protein (BTP) was 0.6±0.1 mg/L, and among those individuals in the lowest eGFR group (<60 ml/min per 1.73 m2), serum BTP was 1.1±0.9 mg/L (52).
eGFR at dialysis initiation is eGFR at the time of initiation of renal replacement therapy. It is calculated using the four-variable Modification of Diet in Renal Disease Study formula from serum creatinine reported on Form 2728 (53).
Dialysis dose (Kt/V) was calculated using the Daugirdas formula and was available for 327 (65%) participants at baseline (54).
EPO dose is average weekly erythropoietin dose requirements during the first 6 months after enrollment.
All-Cause Mortality
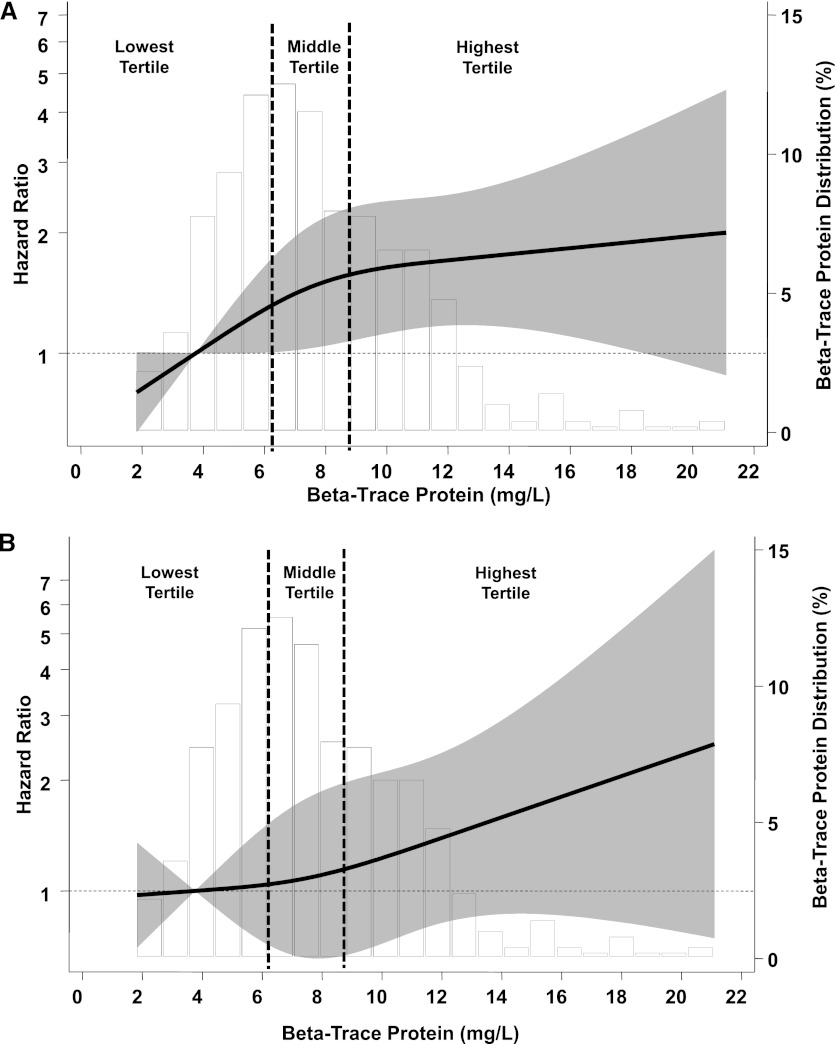
Of the 503 participants at baseline, 321 participants died during 1814 person-years of follow-up (median=3.3 years). Analyzed as a continuous variable, doubling of serum BTP was associated with 36% higher risk of death in the fully adjusted models (Table 2) (HR=1.36; 95% confidence interval [CI]=1.09–1.69; P=0.007). Compared with the lowest tertile of BTP, the highest tertile was associated with a 45% higher risk of death in unadjusted models (Table 3) (HR=1.45; 95% CI=1.09–1.92). After adjustment for demographic and clinical factors, compared with the lowest tertile, the highest tertile was associated with a 72% higher risk of death (HR=1.72; 95% CI=1.25–2.37). Figure 1A displays the relative hazard of death with serum BTP adjusted for mean values of confounders. With serum BTP of 3.8 mg/L as the reference point (25th percentile for tertile 1), the initial rise in hazard is steep followed by flattening in hazard at the highest levels.
Table 2.
Association between serum β-trace protein and mortality in 503 incident hemodialysis participants of the Choices for Healthy Outcomes in Caring for ESRD Study
| All-Cause Mortality | CVD Mortality | |||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Events | ||||
| number of deaths/participants | 321/503 | 159/503 | ||
| unadjusted incidence rate per 1000 person-years (95% CI) | 177 (159–198) | 88 (75–102) | ||
| Risk of deatha [HR (95% CI)] | ||||
| unadjusted | 1.21 (0.99–1.45) | 0.06 | 1.11 (0.84–1.47) | 0.45 |
| adjusted | ||||
| demographicb | 1.19 (0.97–1.47) | 0.09 | 1.08 (0.80–1.45) | 0.63 |
| plus clinical factorsc | 1.36 (1.09–1.69) | 0.007 | 1.20 (0.86–1.66) | 0.29 |
CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval.
Cox proportional hazards regression. Serum β-trace protein (BTP) is modeled as a natural log of BTP. HR presented represents hazard for each doubling of serum BTP.
Demographic characteristics: age, race (white or other), sex, educational status (completed high school or not), marital status (married or not), and employment status (employed or not employed).
Clinical and treatment factors in addition to demographic characteristics: smoking history (ever smoked), pulse pressure, body mass index, primary cause of kidney failure (diabetes, hypertension, glomerulonephritis, or other), Index of Coexistent Disease score (zero to three), CVD, congestive heart failure, left ventricular hypertrophy, diabetes, and serum albumin.
Table 3.
Association between serum β-trace protein tertiles and mortality in 503 incident hemodialysis participants of the Choices for Healthy Outcomes in Caring for ESRD Study
| Overall | Serum β-Trace Protein Tertiles (mg/L) | P for Linear Trend | |||
|---|---|---|---|---|---|
| Lowest Tertile (1.8–6.1) | Middle Tertile (6.1–8.8) | Highest Tertile (8.8–21.1) | |||
| All-cause mortality | |||||
| events | |||||
| number of deaths/participants | 321/503 | 103/168 | 100/168 | 118/167 | |
| unadjusted incidence rate per 1000 person-years (95% CI) | 177 (159–198) | 155 (128–189) | 166 (136–202) | 216 (180–258) | 0.03 |
| risk of death HR (95% CI)a | |||||
| unadjusted | Referent | 1.04 (0.77–1.40) | 1.45 (1.09–1.92) | 0.009 | |
| adjusted | |||||
| demographicb | Referent | 0.89 (0.65–1.21) | 1.39 (1.04–1.86) | 0.01 | |
| plus clinical factorsc | Referent | 0.95 (0.69–1.32) | 1.72 (1.25–2.37) | <0.001 | |
| CVD mortality | |||||
| events | |||||
| number of deaths/participants | 159/503 | 52/168 | 49/168 | 58/167 | |
| unadjusted incidence rate per 1000 person-years (95% CI) | 88 (75–102) | 78 (60–103) | 81 (61–108) | 106 (82–136) | 0.03 |
| risk of death HR (95% CI)a | |||||
| unadjusted | Referent | 0.95 (0.62–1.45) | 1.41 (0.94–2.10) | 0.08 | |
| adjusted | |||||
| demographicb | Referent | 0.73 (0.47–1.14) | 1.31 (0.87–1.98) | 0.13 | |
| plus clinical factorsc | Referent | 0.77 (0.47–1.24) | 1.63 (1.03–2.58) | 0.02 | |
CI, confidence interval; HR, hazard ratio; CVD, cardiovascular disease.
Cox proportional hazards regression.
Demographic characteristics: age, race (white or other), sex, educational status (completed high school or not), marital status (married or not), and employment status (employed or not employed).
Clinical and treatment factors in addition to demographic characteristics: smoking history (ever smoked), pulse pressure, body mass index, primary cause of kidney failure (diabetes, hypertension, glomerulonephritis, or other), Index of Coexistent Disease score (zero to three), CVD, congestive heart failure, left ventricular hypertrophy, diabetes, and serum albumin.
Figure 1.
Serum β-trace protein and adjusted risk of death. Adjusted relative hazard of all-cause mortality (A) and cardiovascular disease mortality (B) with serum β-trace protein (BTP) in 503 incident hemodialysis participants of the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Relative hazard predicted using Cox proportional hazards regression adjusted for demographic characteristics (age, race [white or other], sex, educational status [completed high school or not], marital status [married or not], and employment status [employed or not]) and clinical and treatment factors (smoking history [ever smoked], pulse pressure, body mass index, primary cause of kidney failure [diabetes, hypertension, glomerulonephritis, or other], Index of Coexistent Disease score [zero to three], cardiovascular disease, congestive heart failure, left ventricular hypertrophy, diabetes, and serum albumin). Bars present the distribution of serum BTP. Solid line represents the adjusted hazard ratio of serum BTP with mortality; BTP of 3.8 mg/L (25th percentile of the lowest tertile) is used as the reference point (hazard ratio=1). Gray bands surrounding the solid line represent the 95% confidence interval of the hazard ratios. Vertical dotted lines represent BTP tertiles: lowest tertile (<6.1 mg/L), middle tertile (6.1–8.8 mg/L), and highest tertile (>8.8 mg/L).
CVD Mortality
There were 159 CVD deaths over the follow-up period. The specific causes of death were coronary artery disease=120 (75.5%) stroke=23 (14.5%) peripheral arterial disease=10 (6.3%) and ischemic bowel=6 (3.8%) Analyzed as a continuous variable, doubling of serum BTP was associated with a trend to higher mortality, but it did not reach statistical significance (Table 2). Analyzed as tertiles, the highest BTP tertile was associated with a 63% higher risk of CVD mortality compared with the lowest BTP tertile after adjustment (Table 3) (HR=1.63; 95% CI=1.03–2.58). Figure 1B displays the relative hazard of CVD mortality with serum BTP adjusted for mean values of confounders.
Other Exploratory Analyses
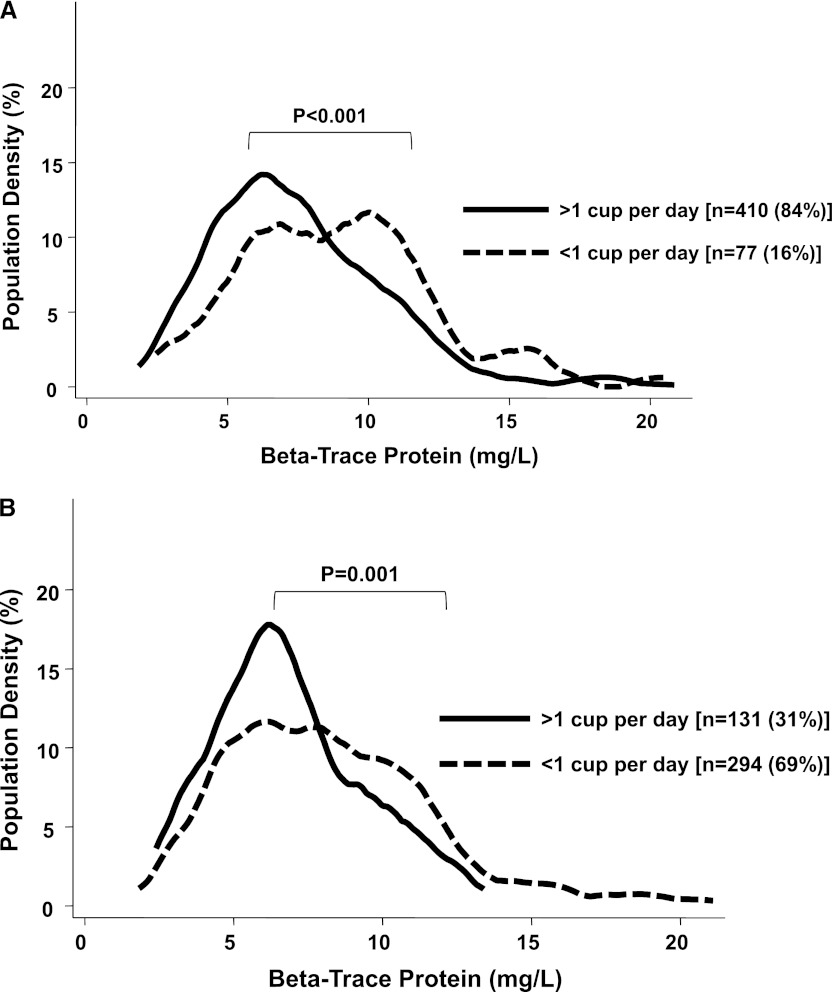
Urine volume (n=231; 46%) was the highest among those participants with the lowest tertile of BTP, intermediate among those participants in the middle tertile, and lowest among those participants in the highest tertile (Table 1). A similar trend was seen with self-reported urine output with the highest BTP levels in those participants without urine output at baseline or year 1. Figure 2 displays the distribution of serum BTP by self-reported urine output. Those participants with urine output at baseline (Figure 2A, solid black line) and those participants with urine output at baseline and year 1, indicating preserved RKF, (Figure 2B, solid black line) have lower BTP levels (to the left of the graph) and more people with lower levels (taller peak). Higher BTP levels were associated with greater interdialytic weight gain (Table 4). There was no significant effect modification by BMI on the association between serum BTP and mortality (P interaction=0.19 for all-cause mortality; P=0.17 for CVD mortality).
Figure 2.
Serum β-trace protein (BTP) and self-reported urine output. Serum BTP distribution by self-reported urine output at baseline (A) and year 1 (B) in incident hemodialysis participants of the CHOICE Study. Vertical axis (population density) represents the percent population at any given level of BTP. Solid line represents individuals with >1 cup/d self-reported urine output, and broken line represents individuals with <1 cup/d self-reported urine output.
Table 4.
Association between serum β-trace protein and interdialytic weight gain
| BTP | Interdialytic Weight Gain, kg | |||
|---|---|---|---|---|
| 0–3 Months (n=225) | 4–6 Months (n=450) | 7–9 Months (n=428) | 10–12 Months (n=403) | |
| Tertiles | ||||
| lowest tertile | 2.5 (1.0) | 2.6 (1.1) | 2.6 (1.1) | 2.8 (1.2) |
| middle tertile | 2.6 (1.0) | 2.7 (1.1) | 2.8 (1.1) | 2.8 (1.2) |
| highest tertile | 2.9 (1.0) | 2.9 (1.0) | 3.0 (1.0) | 3.0 (1.0) |
| P value for linear trend | 0.02 | 0.02 | 0.004 | 0.07 |
| Continuous | ||||
| per doubling of BTP | 0.33 (0.11, 0.55) | 0.18 (0.19, 0.35) | 0.24 (0.08, 0.40) | 0.18 (−0.002, 0.36) |
| P value | 0.004 | 0.03 | 0.004 | 0.05 |
Months represent time period after BTP measurement. BTP, β-trace protein.
Discussion
Mortality among hemodialysis patients remains dismally high, and there is a great need to identify new ways of improving hemodialysis care. In this report from a large, national prospective cohort study of incident hemodialysis patients in the United States, we show that serum BTP, a novel endogenous marker of kidney function, is associated with all-cause and CVD mortality, independent of a rigorously assessed list of covariates. Higher BTP was related to self-reported low urine output and measured volume, suggesting that it is acting (as hypothesized) as a measure of RKF. Thus, BTP provides a promising blood measure of RKF that could facilitate existing recommendations to integrate regular assessment of RKF, hitherto thwarted by the inconvenience and inaccuracy of 48-hour urine collection, into the care of hemodialysis patients.
RKF is increasingly being recognized as an important factor associated with survival among hemodialysis patients. Preserved RKF in hemodialysis patients was highly associated with better survival in a number of large representative populations (6,9,24). RKF in hemodialysis patients may prevent volume overload and its complications such as left ventricular hypertrophy and hypertension. Loss of RKF contributes to hyperkalemia and hyperphosphatemia (25), and it is also associated with accumulation of uremic toxins such as β-2-microglobulin and p-cresol (6,26). Higher BTP levels in our study, indicating lower RKF, were associated with higher diastolic BP, greater interdialytic weight gain, and higher serum potassium and phosphate, in addition to mortality.
Measurement and estimation of RKF in dialysis patients remains a challenge in clinical practice. Serum creatinine cannot be used for estimation of GFR in dialysis patients, and gold-standard measures of GFR, such as inulin, iothalamate, or iohexol clearance, can be cumbersome in routine clinical care. Estimation of RKF and calculation of renal Kt/Vurea are integral components of peritoneal dialysis (PD) prescription (27). RKF in PD patients is generally estimated from urea clearance in a 24-hour urine collection and then incorporated into the dialysis dose. Given the cumbersome nature of the urine collections, there has been increasing interest in filtration markers obtainable through a single blood draw that can obviate the need for urine collection. In PD patients, serum cystatin C is correlated with RKF (r2∼0.7), and a number of recent studies have explored its use for estimation of RKF (28–31). Cystatin C, a 13.2-kD protein, is about the same size as β-2 microglobulin (11.8 kD) and is removed effectively by high-flux hemodialysis, limiting its use as a serum marker of RKF in hemodialysis patients (17,32,33).
BTP is a 23- to 29-kD, 168-amino acid glycoprotein. Serum levels of BTP are highly correlated with measured GFR, and BTP seems comparable with serum creatinine in accuracy for estimation of GFR in nondialysis patients (13–15). Two major forms are recognized; brain-type BTP is a member of the lipocalin superfamily, and hematopoietic BTP is a member of the glutathione synthase class. Serum BTP assays measure only brain-type BTP (Mary Lou Gantzer, personal communication, 2010), which is produced by the epithelial cells of the choroid plexus in the central nervous system. From the cerebrospinal fluid, it diffuses into the systemic circulation. Serum BTP has a narrow range of distribution in healthy individuals, suggesting a constant rate of production (34,35). However, it is certainly possible that there may be non-GFR determinants of serum BTP, and large studies in populations with measured GFR may be able to address this important question. BTP glycoforms lacking N-acetylneuraminic acid residues are rapidly removed by liver, and the remaining are excreted in the urine (36). Similar to cystatin C and other low molecular weight proteins, BTP is freely filtered by the glomerulus, and it is completely reabsorbed and metabolized by the proximal tubule (37). Serum BTP levels increase as urine output declines in hemodialysis patients (16). BTP is not removed by low- or high-flux hemodialysis membranes during conventional hemodialysis (16). Hemodiafiltration, a specialized type of dialysis not performed for maintenance dialysis in the United States, can remove BTP (16,33). These characteristics suggest that serum BTP may serve as a useful biomarker for GFR estimation in hemodialysis patients. Estimation of renal Kt/Vurea by a simple blood test may allow for adjustment of hemodialysis frequency and delivered dose, especially in those individuals with significant RKF at start of hemodialysis and patients performing frequent hemodialysis, such as home hemodialysis patients. Although there is a growing body of literature on the use of BTP as an endogenous filtration marker, to our knowledge, no studies have analyzed the association between serum BTP levels and outcomes in dialysis patients.
In a recent small study (n=66), higher cystatin C levels, measured immediately before dialysis initiation, were associated with a higher risk of cardiovascular events in the follow-up period (38). In our study, higher serum BTP levels were independently associated with all-cause and CVD mortality. The effect was most pronounced comparing the lowest with the highest tertiles of serum BTP, and those individuals in the highest tertile (median level=10.8 mg/L) had 72% greater hazard of death (95% CI=25%–237% higher risk) compared with the lowest tertile of BTP (median level=4.8 mg/L) after adjustment for confounders. The middle tertile (median level=7.3 mg/L) did not have higher risk compared with the lowest tertile, likely reflecting the relatively narrow range of BTP in this group (6.1–8.8 mg/L) and similar incidence rates of mortality in the lowest and middle tertile. These findings are consistent with the role of BTP as a marker of RKF, with the early rise in BTP levels reflecting declining RKF and later values representing those individuals with no RKF. In our study, we did not measure cystatin C, and as a result, we cannot directly compare the risk of mortality in BTP versus cystatin C in hemodialysis patients.
We noted a trend toward higher albumin levels in patients in the highest BTP tertile. Higher BTP levels were also associated with lower BMI, despite higher albumin levels. No previous associations between BMI and BTP have been reported, and it is difficult to determine if this cross-sectional association is causal or incidental. We did not find any effect modification by BMI on the association between BTP and mortality. Importantly, there was no association between inflammation, measured using CRP, and serum BTP tertiles. Future studies in healthy populations and different disease states will be required to determine the non-GFR determinants of serum BTP.
BTP binds lipophilic substances in blood, such as retinoids, thyroid hormone, and bilirubin, and it may have a role in extracellular transport of these substances (39,40). Biologically, BTP increases conversion of prostaglandin H2 to prostaglandin D2. Prostaglandin D2 regulates body temperature, sleep–awake cycle, tactile pain response, and inflammation (41,42). In animal models, prostaglandin D2 can induce coronary vasoconstriction and reduce left ventricular contractility (43,44). BTP has been shown in human coronary artery atherosclerotic plaques, especially those plaques causing >75% luminal stenosis, and on immunohistochemistry, BTP seems to be localized to intima more than media (45). BTP has also been implicated in the progression of subclinical atherosclerosis (46–49). Thus, it is possible that, beyond marking lower RKF, accumulation of BTP in dialysis patients may have direct toxicity and contribute to the high prevalence of CVD, sudden cardiac death, and sleep apnea.
Our study has some limitations. First, assessment of RKF was based on self-report, and we did not have measurement of residual urea and creatinine clearance. However, this self-reported urine output itself is independently associated with all-cause mortality in hemodialysis patients (6,9,24). Serum BTP levels tended to be the highest in participants with lower urine volumes and lower self-reported urine output. Second, dialysis dose (Kt/V) was available for a limited number of participants. It is, however, unlikely that BTP levels would have differed by levels of Kt/V, because it is not cleared with conventional hemodialysis (16). Third, in the context of biomarker research, our study represents a second-phase evaluation (prospective validation) of a new biomarker (50,51). Although we had an adequate sample size and number of events in our cohort to support our findings, demonstration of the clinical use of BTP will need validation in future studies. Fourth, as with any cohort study, there is always the possibility of residual confounding as a result of unmeasured factors, and causal associations cannot be established. These limitations are balanced by the several strengths of our study, including prospective design with inclusion of only incident hemodialysis patients; detailed and precise information for demographic, clinical, and treatment factors; and systematic adjudication of baseline comorbid conditions as well as outcomes. These comprehensive data allowed us to extensively adjust for potential confounders in our analysis.
In summary, we found that serum BTP is independently associated with all-cause and CVD mortality in hemodialysis patients. Validation of these results in other studies may allow the use of BTP as a practical serum marker of RKF in hemodialysis patients to improve care and enhance prognostic ability.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the Cardiovascular Endpoint Committee: Bernard G. Jaar; Michael J. Choi; Josef Coresh; Joseph A. Eustace, MHS; Nancy E. Fink; Caroline Fox; Melanie H. Katzman, MHS; Michael J. Klag; Yongmei Liu; J. Craig Longenecker; Michal Melamed, MHS; Laura C. Plantinga; Neil R. Powe; Renuka Sothinathan, MHS; Richard M. Ugarte, MHS; and Gayanne Yenokian. We thank the patients, staff, laboratory, and physicians of Dialysis Clinic Inc. for their participation in the Choices for Healthy Outcomes in Caring for ESRD Study.
This work is in loving memory of Nancy Fink, whose dedication and commitment to research was instrumental in the design, implementation, and success of the Choices for Healthy Outcomes in Caring for ESRD Study.
Choices for Healthy Outcomes in Caring for ESRD Study was supported by Agency for Healthcare Quality and Research Grant R01-HS-008365 from July of 1994 to June of 1999; National Heart, Lung, and Blood Institute Grant R01-HL-62985 from September of 2000 to June of 2006; National Institute of Diabetes & Digestive & Kidney Diseases Grant R01-DK-059616 from September of 2000 to June of 2005; and National Institute of Diabetes & Digestive & Kidney Diseases Grant R01-DK-080123 from August of 2008 to June of 2013. T.S. was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant K23-DK-083514 and The National Kidney Foundation of Maryland Professional Development Award. R.S.P. was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant R01-DK-072367. N.R.P. was supported in part by National Institute of Diabetes & Digestive & Kidney Diseases Grants K24-DK-02643 and R01-DK-080123. J.C. was supported in part as an American Heart Association established investigator (01-4019-7N).
The reagents for β-trace protein assay were provided by Siemens to the University of Minnesota, where serum β-trace protein measurements were performed. Siemens had no role in the design, analysis, and interpretation of data or the preparation of this manuscript.
Parts of this work were presented at the 2011 Annual Meeting of the American Society of Nephrology, November 8–13, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02240312/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA, IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, Teehan B, Eknoyan G: Serum beta-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J Am Soc Nephrol 17: 546–555, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM: Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 33: 523–534, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d’Avolio G, Gelatti U: Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant 10: 2295–2305, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, NECOSAD Study Group : Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 15: 1061–1070, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT, NECOSAD Study Group : The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis 41: 1293–1302, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S, Mexican Nephrology Collaborative Study Group : Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE: Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 11: 556–564, 2000 [DOI] [PubMed] [Google Scholar]
- 13.White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA: Estimating GFR using serum beta trace protein: Accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76: 784–791, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Benlamri A, Nadarajah R, Yasin A, Lepage N, Sharma AP, Filler G: Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol 25: 485–490, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von Eckardstein A, Mild and Moderate Kidney Disease (MMKD) Study Group : Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem 56: 740–749, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gerhardt T, Pöge U, Stoffel-Wagner B, Klein B, Klehr HU, Sauerbruch T, Woitas RP: Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant 23: 309–314, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Powe NR, Klag MJ, Sadler JH.Anderson GF, Bass EB, Briggs WA, Fink NE, Levey AS, Levin NW, Meyer KB, Rubin HR, Wu AW: Choices for healthy outcomes in caring for end stage renal disease. Semin Dial 9: 9–11, 1996 [Google Scholar]
- 18.Sanders EL, Clark RJ, Katzmann JA: Cerebrospinal fluid leakage: Agarose gel electrophoresis detection of beta(2)-transferrin and nephelometric quantification of beta-trace protein. Clin Chem 50: 2401–2403, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Longenecker JC, Klag MJ, Marcovina SM, Liu YM, Jaar BG, Powe NR, Fink NE, Levey AS, Coresh J: High lipoprotein(a) levels and small apolipoprotein(a) size prospectively predict cardiovascular events in dialysis patients. J Am Soc Nephrol 16: 1794–1802, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Miskulin DC, Meyer KB, Athienites NV, Martin AA, Terrin N, Marsh JV, Fink NE, Coresh J, Powe NR, Klag MJ, Levey AS: Comorbidity and other factors associated with modality selection in incident dialysis patients: The CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis 39: 324–336, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Athienites NV, Miskulin DC, Fernandez G, Bunnapradist S, Simon G, Landa M, Schmid CH, Greenfield S, Levey AS, Meyer KB: Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial 13: 320–326, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis, New York, Springer, 2001 [Google Scholar]
- 24.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J: Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 56: 348–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penne EL, van der Weerd NC, Grooteman MP, Mazairac AH, van den Dorpel MA, Nubé MJ, Bots ML, Lévesque R, ter Wee PM, Blankestijn PJ, CONTRAST investigators : Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol 6: 281–289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, Meyer TW: Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol 6: 290–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peritoneal Dialysis Adequacy Work Group : Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 48[Suppl 1]: S98–S129, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ros S, Bajo A, del Peso G, Garcia de Miguel A, Santacruz S, Fernandez E, de Garcia R, Selgas R: Cystatin C as marker of residual renal function in patients on peritoneal dialysis: Relation with parameters of peritoneal function. J Nephrol 20: 468–473, 2007 [PubMed] [Google Scholar]
- 29.Delaney MP, Stevens PE, Al Hasani M, Stowe HJ, Judge C, Lamb EJ: Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: A cross-sectional study. Am J Kidney Dis 51: 278–284, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Sohn YB, Park SW, Jin DK, Paik KH: Serum cystatin C for estimation of residual renal function in children on peritoneal dialysis. Pediatr Nephrol 26: 433–440, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Filler G, Huang SH, Lindsay RM: Residual renal function assessment with cystatin C. Pediatr Nephrol 26: 333–335, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Al-Malki N, Heidenheim PA, Filler G, Yasin A, Lindsay RM: Cystatin C levels in functionally anephric patients undergoing dialysis: The effect of different methods and intensities. Clin J Am Soc Nephrol 4: 1606–1610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindström V, Grubb A, Alquist Hegbrant M, Christensson A: Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest 68: 685–691, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K: Beta-trace protein in serum: A new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem 45: 567–568, 1999 [PubMed] [Google Scholar]
- 35.Donadio C, Lucchesi A, Ardini M, Donadio E, Giordani R: Serum levels of beta-trace protein and glomerular filtration rate—preliminary results. J Pharm Biomed Anal 32: 1099–1104, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann A, Nimtz M, Conradt HS: Molecular characterization of beta-trace protein in human serum and urine: A potential diagnostic marker for renal diseases. Glycobiology 7: 499–506, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Nagata N, Fujimori K, Okazaki I, Oda H, Eguchi N, Uehara Y, Urade Y: De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, beta-trace, in the kidneys. FEBS J 276: 7146–7158, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Shin MJ, Song SH, Kwak IS, Lee SB, Lee DW, Seong EY, Kim IY, Rhee H, Lee N: Serum cystatin C as a predictor for cardiovascular events in end-stage renal disease patients at the initiation of dialysis. Clin Exp Nephrol, in press [DOI] [PubMed] [Google Scholar]
- 39.Tanaka T, Urade Y, Kimura H, Eguchi N, Nishikawa A, Hayaishi O: Lipocalin-type prostaglandin D synthase (beta-trace) is a newly recognized type of retinoid transporter. J Biol Chem 272: 15789–15795, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Beuckmann CT, Aoyagi M, Okazaki I, Hiroike T, Toh H, Hayaishi O, Urade Y: Binding of biliverdin, bilirubin, and thyroid hormones to lipocalin-type prostaglandin D synthase. Biochemistry 38: 8006–8013, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Shaw N, Li Y, Zhao Y, Zhang R, Liu ZJ: Structure-function analysis of human l-prostaglandin D synthase bound with fatty acid molecules. FASEB J 24: 4668–4677, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Saleem S, Shah ZA, Urade Y, Doré S: Lipocalin-prostaglandin D synthase is a critical beneficial factor in transient and permanent focal cerebral ischemia. Neuroscience 160: 248–254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hattori Y, Levi R: Effect of PGD2 on cardiac contractility: A negative inotropism secondary to coronary vasoconstriction conceals a primary positive inotropic action. J Pharmacol Exp Ther 237: 719–724, 1986 [PubMed] [Google Scholar]
- 44.Schrör K: Prostaglandin D2 (PGD2)—a potent coronary vasoconstrictor agent in the guinea pig isolated heart. Naunyn Schmiedebergs Arch Pharmacol 302: 61–62, 1978 [DOI] [PubMed] [Google Scholar]
- 45.Eguchi Y, Eguchi N, Oda H, Seiki K, Kijima Y, Matsu-ura Y, Urade Y, Hayaishi O: Expression of lipocalin-type prostaglandin D synthase (beta-trace) in human heart and its accumulation in the coronary circulation of angina patients. Proc Natl Acad Sci U S A 94: 14689–14694, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miwa Y, Oda H, Shiina Y, Shikata K, Tsushima M, Nakano S, Maruyama T, Kyotani S, Eguchi N, Urade Y, Takahashi-Yanaga F, Morimoto S, Sasaguri T: Association of serum lipocalin-type prostaglandin D synthase levels with subclinical atherosclerosis in untreated asymptomatic subjects. Hypertens Res 31: 1931–1939, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Inoue T, Eguchi Y, Matsumoto T, Kijima Y, Kato Y, Ozaki Y, Waseda K, Oda H, Seiki K, Node K, Urade Y: Lipocalin-type prostaglandin D synthase is a powerful biomarker for severity of stable coronary artery disease. Atherosclerosis 201: 385–391, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Miwa Y, Takiuchi S, Kamide K, Yoshii M, Horio T, Tanaka C, Banno M, Miyata T, Sasaguri T, Kawano Y: Identification of gene polymorphism in lipocalin-type prostaglandin D synthase and its association with carotid atherosclerosis in Japanese hypertensive patients. Biochem Biophys Res Commun 322: 428–433, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Cipollone F, Cicolini G, Bucci M: Cyclooxygenase and prostaglandin synthases in atherosclerosis: Recent insights and future perspectives. Pharmacol Ther 118: 161–180, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Siew ED, Ware LB, Ikizler TA: Biological markers of acute kidney injury. J Am Soc Nephrol 22: 810–820, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand S-LT, O’Donnell CJ, Smith SC, Jr, Wilson PWF, American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council : Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation 119: 2408–2416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J: Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 59: 653–662, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.