Summary
Background and objectives
Prescription patterns for hemodialysis patients with secondary hyperparathyroidism have varied widely since market introduction of cinacalcet. This study examined associations between prescription patterns and subsequent laboratory values.
Design, setting, participants, & measurements
Using a Mineral and Bone Disorder Outcomes Study for Japanese CKD Stage 5D Patients subcohort, 1716 prevalent hemodialysis patients (4048 sets for repeated measures between January 2008 and July 2009) with an intact parathyroid hormone (iPTH) level >180 pg/ml who used intravenous vitamin D receptor activator (VDRA) without cinacalcet were selected. Prescription patterns were defined based on cinacalcet administration (starting or not) and VDRA dosage change (decreased [<−25%], stable [−25% to 25%], or increased [>25%]). Proportion differences (PDs) were determined for decreasing iPTH levels by at least one category (<180, 180–299, 300–499, and ≥500 pg/ml) and for achieving target phosphorus (3.5–6.0 mg/dl) and calcium (8.4–10.0 mg/dl) levels, adjusting for potential confounders.
Results
The starting cinacalcet and increased VDRA patterns were associated with decreasing iPTH levels (PD, 0.25 and 0.13; 95% confidence intervals [95% CIs], 0.19–0.31 and 0.09–0.17, respectively); combination use had an additive association (PD, 0.34; 95% CI, 0.20–0.42). The starting cinacalcet and decreased VDRA combination was associated with simultaneously achieving target phosphorus (PD, 0.12; 95% CI: 0.04–0.20) and calcium (PD, 0.09; 95% CI, 0.01–0.17) levels.
Conclusions
Certain combinations of cinacalcet and VDRA were associated with decreasing iPTH and achieving targets for phosphorus and calcium. Combinations may prove advantageous versus VDRA alone in managing secondary hyperparathyroidism.
Introduction
Prescription patterns for hemodialysis patients with secondary hyperparathyroidism have begun to vary widely since cinacalcet introduction (1–3), and the effects of these patterns on laboratory values (1–9) and clinical outcomes (10,11) have attracted attention. Before cinacalcet introduction, the only treatment option available for elevated parathyroid hormone (PTH) levels was vitamin D receptor activator (VDRA) (12). However, since cinacalcet introduction, the number of treatment options has increased, and physicians can now choose from several suitable prescription patterns, according to the status of patients’ laboratory values and the therapeutic purposes. For example, physicians may be better able to administer an adequate VDRA dosage with concomitant therapy with cinacalcet. Although several studies have examined the effect of cinacalcet on subsequent laboratory values in patients (1,6–8), little is known about the additive effect of cinacalcet administration and VDRA dosage change (4,13).
Secondary hyperparathyroidism is characterized by elevated levels of PTH, comprising a major component of CKD mineral and bone disorder (CKD-MBD) (14). CKD-MBD is a common complication in hemodialysis patients and is associated with not only bone disease (15–17) but also mortality and cardiovascular disease (17–20). Clinical guidelines therefore recommend maintaining target levels of intact PTH (iPTH), phosphorus, and calcium (21–23). Specifically, the Japanese Society for Dialysis Therapy guidelines (released in 2006) recommend target levels of iPTH, phosphorus, and calcium at 60–180 pg/ml, 3.5–6.0 mg/dl, and 8.4–10.0 mg/dl, respectively. The target level of iPTH in Japan is lower than that in other countries (21–23), accounting for the characteristics of Japanese hemodialysis patients that differ from those in other countries, such as comparatively longer time on hemodialysis therapy (24). Generally, physicians assign patients MBD-related prescriptions according to the drugs’ therapeutic purposes, such as decreasing PTH levels or achieving target levels of phosphorus and calcium.
Here, we attempted to clarify the associations between prescription patterns and subsequent levels of iPTH, phosphorus, and calcium in the cinacalcet era. We defined prescription patterns by two factors—cinacalcet administration and VDRA dosage change—and examined the separate and additive associations of these factors using data from the Mineral and Bone Disorder Outcomes Study for Japanese CKD Stage 5D Patients (MBD-5D) (25).
Materials and Methods
Study Design
The MBD-5D study, which started just when cinacalcet became available in Japan (January 2008), was a 3-year prospective observational study involving prevalent hemodialysis patients with secondary hyperparathyroidism and comprises a whole cohort of all patients enrolled (8229 patients) and a subcohort of patients randomly selected (3276 patients) from the whole cohort. The study involved relatively large dialysis facilities (>100 patients) in Japan, and patients were informed of an opt-out option before the study started. The study protocol was approved by the ethics committee. Precise design details of the MBD-5D study were previously reported (25).
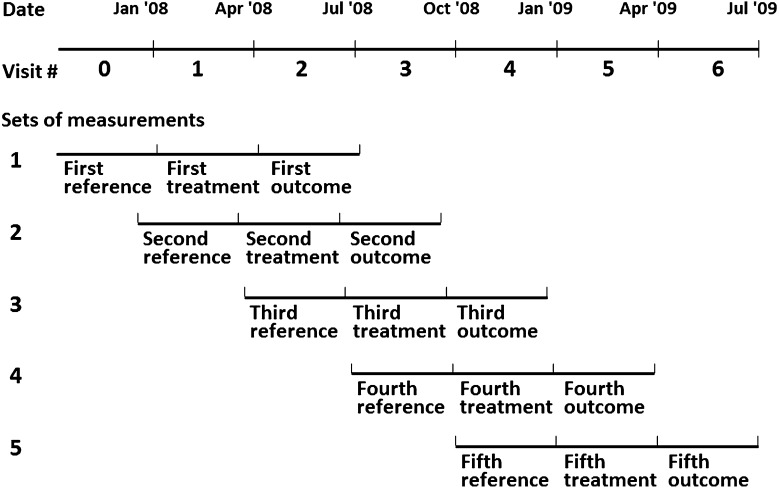
In this study, we used subcohort data obtained during the first 1.5 years of the MBD-5D study. Data were collected at enrollment (visit 0) and every 3 months subsequently from January 2008 through June 2009 (visit 1–6). We defined five imbricated sets of measures from seven visits (visit 0–6), and each set comprised three consecutive visits: reference, treatment, and outcome (Figure 1). We conducted set-level analyses to take full advantage of repeated-measures data. To note, although 3276 patients were initially allocated to the subcohort, this number decreased over time due to death (n=208), stopping hemodialysis (n=1), parathyroidectomy (n=56), and loss to follow-up (n=57) during the 1.5 years of follow-up.
Figure 1.
Set-level analyses of three consecutive visits: reference, treatment, and outcome. We defined five imbricated sets of measures from seven visits (visit 0–6) between January 2008 and July 2009 and conducted set-level analyses accounting for correlation between repeated measures within patients. In generalized estimating equation models, we included patient characteristics measured at study enrollment, laboratory data measured at reference visits, and treatment data measured at treatment visits. Outcome data were measured at outcome visits.
All patients enrolled in this study had iPTH levels of at least 180 pg/ml and received intravenous VDRA without cinacalcet at reference visits.
Exposure, Outcomes, and Covariates
Prescription patterns were defined based on cinacalcet and VDRA dosage. Cinacalcet patterns were defined as starting or not starting, based on the presence or absence of cinacalcet administration at treatment visits, respectively. Patterns of VDRA dosage were defined as decreased, stable, or increased, based on the proportion of dosage change between reference visits and treatment visits (decreased, <-25%; stable, −25% to 25%; and increased, >25%, respectively). Prescription patterns in patients were thus categorized into one of six groups, combining the patterns of cinacalcet administration (two groups) and VDRA dosage change (three groups). These six prescription patterns are as follows: decreased VDRA, stable VDRA, increased VDRA, starting cinacalcet and decreased VDRA, starting cinacalcet and stable VDRA, and starting cinacalcet and increased VDRA. VDRA dosages are shown as calcitriol dosage equivalents, with 10 μg of maxacalcitol equivalent to 1.5 μg of calcitriol (26).
The main outcome measure was proportion difference (PD) for decreasing iPTH levels (at least one category when categorized as <180, 180–299, 300–499, and ≥500 pg/ml). The secondary outcome measures were PD for achieving target levels of phosphorus (3.5–6.0 mg/dl) and calcium (8.4–10.0 mg/dl), which were defined based on Japanese clinical guidelines (23). Whole PTH levels measured using a third-generation PTH assay were converted to iPTH levels using the following equation: iPTH (pg/ml) = whole PTH (pg/ml) × 1.7 (23,27). We used values of calcium that were corrected for albumin concentration (28).
We also collected covariate demographic data (age, sex, and dialysis duration) and baseline laboratory data (levels of iPTH, phosphorus, and calcium) at the reference visit, and covariate data on dialysis (single-pool Kt/V and dialysate calcium level) and other medications (calcium-based phosphate binder, non-calcium–based phosphate binder, and oral VDRAs) at the treatment visit.
Statistical Analyses
To take full advantage of repeated-measures data, we used set-level data for all analyses. Figure 1 shows the framework of set-level data.
Set-level characteristics were described at baseline (reference or treatment visit). Continuous variables were expressed as medians and interquartile ranges, whereas categorical variables were expressed as proportions. The P values were calculated from generalized estimating equations with robust variance estimation, often used to analyze correlated data (29–31), to determine the difference between two groups based on cinacalcet patterns at the treatment visit.
We used generalized estimating equations with robust variance estimation to estimate PDs and 95% confidence intervals (95% CIs) for the association between prescription patterns and decreased iPTH levels, with the stable VDRA group established as the reference group. We adjusted for potential confounders such as age, sex, dialysis duration, presence of calcium- or non-calcium–based phosphate binders, dialysate calcium, single-pool Kt/V, and levels of iPTH, phosphorus, and calcium.
In sensitivity analyses to examine the robustness of the association, we examined models including these factors along with VDRA dosage change and levels of iPTH, phosphorus, and calcium at the visit directly before the reference visit. We also examined associations among sets with iPTH levels ≥300 pg/ml (2209 sets) and ≥500 pg/ml (853 sets) according to the cut-off points established in the guidelines of Kidney Disease Outcomes Quality Initiative and Kidney Disease Improving Global Outcomes (21,22).
To examine the additive association of starting cinacalcet and increased VDRA, we divided sets into four groups on the basis of cinacalcet administration (starting or not) and VDRA dosage (increased [>25%] or not [<25%]). We estimated adjusted PDs for decreasing iPTH levels and assessed the presence or absence of an additive association when patients received the starting cinacalcet and increased VDRA treatment simultaneously. If an additive association was noted, the sum of the PDs for separate associations of starting cinacalcet and increased VDRA do not depart from the PD for combination of starting cinacalcet and increased VDRA. This theory is based on the concept of biologic interaction (32,33).
In addition to the above investigation regarding decreasing iPTH levels, we also estimated PDs and 95% CIs for the association between prescription patterns and achieving target levels of phosphorus (3.5–6.0 mg/dl) or calcium (8.4–10.0 mg/dl) after adjusting for potential confounders. Sets with missing data on levels of phosphorus (n=20) or calcium (n=19) at outcome visits were excluded from analysis. As above, we used generalized estimating equations with robust variance estimation. In addition, to examine the association between prescription patterns and new achievement of target levels of phosphorus or calcium, we also analyzed those sets for which phosphorus or calcium levels exceeded the target range at baseline (>6.0 mg/dl for phosphorus levels or >10.0 mg/dl for calcium levels). All analyses were carried out with SAS 9.2 (SAS Institute, Cary, NC) and STATA 11.0 software (STATA, College Station, TX).
Results
A total of 4048 sets of 1716 patients (median 2 sets per patient; range, 1–5 sets per patient) were analyzed in this study. During 1.5 years of follow-up, 579 patients (33.7%) started receiving cinacalcet, with a mean dosage of 25.1 mg/d (SD 11.8) at start and 29.0 mg/d (SD 14.3) 3 months later. To note, these starting and titration dosages are lower than those in a previous study in the United States (1).
Table 1 shows set-level characteristics overall and by cinacalcet patterns. With regard to patient characteristics, the median age was 61 years, the median iPTH level was 319 pg/ml, the median phosphorus level was 5.7 mg/dl, the median calcium level was 9.7 mg/dl, and 4.1% of participants used oral VDRA in addition to intravenous VDRA. Patients who started receiving cinacalcet tended to be younger, tended to have longer dialysis duration, higher levels of iPTH, calcium, phosphorus, higher Kt/V, and were more likely to receive phosphate binders than those who did not start cinacalcet.
Table 1.
Set-level characteristics (overall and by cinacalcet patterns)
| Characteristic | Overall (4048 Sets) | Cinacalcet Patterna | ||
|---|---|---|---|---|
| Not Starting (3469 Sets) | Starting (579 Sets) | P Value | ||
| Age (yr) | 61 (53–70) | 62 (53–70) | 59 (52–66) | <0.001 |
| Male | 59.1 | 59.5 | 57.2 | 0.31 |
| Dialysis duration (yr) | 9.9 (5.6–16.0) | 9.5 (5.1–15.6) | 12.3 (8.3–17.7) | <0.001 |
| Intact parathyroid hormone (pg/ml) | 319 (239–466) | 304 (232–441) | 415 (303–603) | <0.001 |
| Calcium (mg/dl) | 9.7 (9.1–10.2) | 9.6 (9.1–10.1) | 10.1 (9.7–10.4) | <0.001 |
| Phosphorus (mg/dl) | 5.7 (4.9–6.5) | 5.6 (4.9–6.5) | 5.8 (5.1–6.7) | 0.001 |
| Kt/V | 1.4 (1.3–1.6) | 1.4 (1.3–1.6) | 1.4 (1.3–1.6) | 0.003 |
| Dialysate calcium (≥3.0 mEq/L) | 46.2 | 45.1 | 52.4 | 0.002 |
| Phosphate binder use | 89.6 | 89.0 | 93.6 | <0.001 |
| calcium based | 63.8 | 65.4 | 54.4 | <0.001 |
| non-calcium based | 54.4 | 51.7 | 70.0 | <0.001 |
| Oral VDRA useb | 4.1 | 4.2 | 3.8 | 0.67 |
Data are the median (interquartile range) or percentage. Characteristics measured at baseline (visits of reference or treatment). VDRA, vitamin D receptor activator.
Overall sets were divided into two groups on the basis of cinacalcet patterns at treatment visits.
Sets with oral VDRA in addition to intravenous VDRA.
Association between Prescription Patterns and iPTH levels
Table 2 shows unadjusted and multivariable-adjusted associations between prescription patterns and decreasing iPTH levels. After adjusting for potential confounders, the starting cinacalcet and increased VDRA patterns were both associated with decreased iPTH levels (PD, 0.25; 95% CI, 0.19–0.31; and PD, 0.13; 95% CI, 0.09–0.17, respectively). In addition, starting cinacalcet was associated with decreasing iPTH levels in any VDRA category. An additive association with decreasing iPTH levels was noted with the starting cinacalcet and increased VDRA combination (PD, 0.34; 95% CI, 0.25–0.42).
Table 2.
Association between prescription patterns and decrease in iPTH
| Prescription Pattern | iPTH | Unadjusted Model(4048 Sets) | Adjusted Modelc(4029 Sets) | |||||
|---|---|---|---|---|---|---|---|---|
| Cinacalcet | VDRA | Sets (n) | ACa (pg/ml) | Proportionb (%) | PD | 95% CI | PD | 95% CI |
| Not | Decreased | 561 | −67.8 | 45.8 | 0.00 | −0.05 to 0.05 | 0.01 | −0.03 to 0.06 |
| Not | Stable | 2153 | −56.7 | 45.3 | Reference | Reference | Reference | Reference |
| Not | Increased | 755 | −127.6 | 60.3 | 0.14 | 0.09–0.18 | 0.13 | 0.09–0.17 |
| Starting | Decreased | 131 | −141.8 | 61.8 | 0.15 | 0.07–0.24 | 0.19 | 0.10–0.28 |
| Starting | Stable | 307 | −205.1 | 67.4 | 0.22 | 0.16–0.27 | 0.25 | 0.19–0.31 |
| Starting | Increased | 141 | −266.8 | 80.9 | 0.34 | 0.27–0.40 | 0.34 | 0.25–0.42 |
iPTH, intact parathyroid hormone; VDRA, vitamin D receptor activator; AC, absolute change; PD, proportion difference; 95% CI, 95% confidence interval.
Means of AC in iPTH between reference and outcome visits.
Proportions of patients with decrease in iPTH.
Adjusted for age, sex, dialysis duration, iPTH levels, phosphorus levels, calcium levels, use of calcium- or non-calcium–based phosphate binders, single-pool Kt/V, and dialysate calcium.
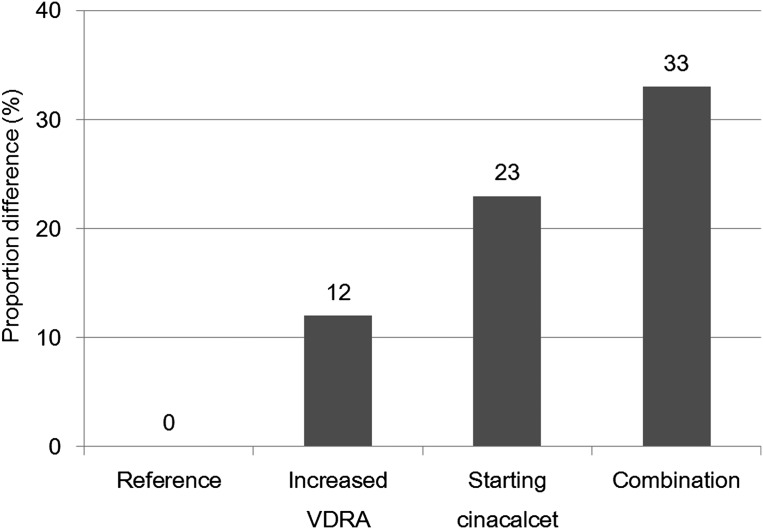
Figure 2 shows separate and combined associations of increased VDRA and starting cinacalcet, according to adjusted PD for decreasing iPTH levels. The combined association (33%) was nearly equal to the sum of the associations of the two factors (12% and 23%).
Figure 2.
Adjusted PD for decrease in iPTH levels according to increased VDRA, starting cinacalcet, and their combination. Reference, increased VDRA, starting cinacalcet, and combination were defined by patterns of not starting cinacalcet and not increased VDRA, not starting cinacalcet and increased VDRA, starting cinacalcet and not increased VDRA, and starting cinacalcet and increased VDRA, respectively. We estimated adjusted PDs for decrease in iPTH compared with the reference group, using generalized estimating equation models. PD, proportion difference; iPTH, intact parathyroid hormone; VDRA, vitamin D receptor activator.
We noted a similar association between the combination pattern of starting cinacalcet and increased VDRA and decreasing iPTH levels even after adjusting for changes in VDRA dosage (PD, 0.33; 95% CI, 0.23–0.44), and levels of iPTH, phosphorus, and calcium (PD, 0.32; 95% CI, 0.22–0.42) at one visit before the reference visit. In addition, we found consistent results among sets with iPTH levels ≥300 pg/ml (PD, 0.32; 95% CI, 0.25–0.40) and ≥500 pg/ml (PD, 0.35; 95% CI, 0.22–0.48) at baseline.
Association between Prescription Patterns and Levels of Phosphorus and Calcium
Table 3 shows adjusted associations between prescription patterns and achieving target levels of phosphorus and calcium. We included 4026 sets for phosphorus and 4027 for calcium, regardless of whether target ranges for levels had been achieved at reference visits. The combination pattern of starting cinacalcet and decreased VDRA was associated with achieving target levels of both phosphorus (PD, 0.12; 95% CI, 0.04–0.20) and calcium (PD, 0.09; 95% CI, 0.01–0.17). Furthermore, patterns involving starting cinacalcet in general were also associated with achieving target levels of calcium (PD, 0.08; 95% CI, 0.02–0.14).
Table 3.
Association between prescription patterns and achievement in target levels of phosphorus and calcium
| Prescription Pattern | ACa (pg/ml) | Proportionb (%) | PDc | 95% CI | ||
|---|---|---|---|---|---|---|
| Cinacalcet | VDRA | |||||
| Phosphorus (4026 sets) | ||||||
| Not | Decreased | −0.29 | 64.0 | 0.03 | −0.02 to 0.07 | |
| Not | Stable | −0.12 | 60.9 | Reference | ||
| Not | Increased | −0.01 | 61.6 | 0.00 | −0.03 to 0.04 | |
| Starting | Decreased | −0.82 | 72.5 | 0.12 | 0.04–0.20 | |
| Starting | Stable | −0.49 | 61.2 | 0.03 | −0.02 to 0.09 | |
| Starting | Increased | −0.35 | 67.4 | 0.07 | 0.00–0.15 | |
| Calcium (4027 sets) | ||||||
| Not | Decreased | −0.14 | 62.6 | 0.01 | −0.03 to 0.05 | |
| Not | Stable | 0.13 | 65.5 | Reference | ||
| Not | Increased | 0.32 | 65.4 | −0.02 | −0.06 to 0.01 | |
| Starting | Decreased | −0.69 | 65.7 | 0.09 | 0.01–0.17 | |
| Starting | Stable | −0.35 | 69.3 | 0.08 | 0.02–0.14 | |
| Starting | Increased | −0.16 | 60.3 | 0.03 | −0.05 to 0.11 | |
VDRA, vitamin D receptor activator; AC, absolute change; PD, proportion difference; 95% CI, 95% confidence interval; iPTH, intact parathyrpid hormone.
Means of AC between reference and outcome visits.
Proportions of patients with achievement of target levels.
Adjusted for age, sex, dialysis duration, intact PTH levels, phosphorus levels, calcium levels, use of calcium- or non–calcium-based phosphate binders, single-pool Kt/V, and dialysate calcium.
Table 4 shows adjusted associations between prescription patterns and improvements in hyperphosphatemia and hypercalcemia toward respective target ranges. We analyzed sets with elevated levels of phosphorus (>6.0 mg/dl; 1528 sets) or calcium (>10.0 mg/dl; 1263 sets) at reference visits and found that the combination pattern of starting cinacalcet and decreased VDRA was associated with improvement in both hyperphosphatemia (PD, 0.23; 95% CI, 0.10–0.36) and hypercalcemia (PD, 0.17; 95% CI, 0.07–0.28). Furthermore, the starting cinacalcet pattern was also associated with improvement in hypercalcemia (PD, 0.22; 95% CI, 0.14–0.31).
Table 4.
Association between prescription patterns and improving hyperphosphatemia and hypercalcemia toward the target range
| Prescription Pattern | ACa (pg/ml) | Proportionb (%) | PD | 95% CI | ||
|---|---|---|---|---|---|---|
| Cinacalcet | VDRA | |||||
| Hyperphosphatemia (1528 sets)c | ||||||
| Not | Decreased | −1.06 | 51.9 | 0.05 | −0.02 to 0.13 | |
| Not | Stable | −0.83 | 44.2 | Reference | ||
| Not | Increased | −0.80 | 42.0 | −0.03 | −0.10 to 0.04 | |
| Starting | Decreased | −1.58 | 69.0 | 0.23 | 0.10–0.36 | |
| Starting | Stable | −1.06 | 46.2 | 0.03 | −0.06 to 0.11 | |
| Starting | Increased | −0.82 | 46.2 | 0.02 | −0.12 to 0.16 | |
| Hypercalcemia (1263 sets)d | ||||||
| Not | Decreased | −0.53 | 42.0 | 0.05 | −0.03 to 0.12 | |
| Not | Stable | −0.22 | 35.6 | Reference | ||
| Not | Increased | −0.25 | 39.4 | 0.00 | −0.08 to 0.09 | |
| Starting | Decreased | −0.89 | 57.7 | 0.17 | 0.07–0.28 | |
| Starting | Stable | −0.61 | 64.1 | 0.22 | 0.14–0.31 | |
| Starting | Increased | −0.31 | 48.0 | 0.10 | −0.02 to 0.22 | |
VDRA, vitamin D receptor activator; AC, absolute change; PD, proportion difference; 95% CI, 95% confidence interval; iPTH, intact parathyrpid hormone.
Means of AC between reference and outcome visits.
Proportions of patients with achievement of target levels.
Adjusted for age, sex, dialysis duration, intact PTH levels, calcium levels, use of calcium- or non–calcium-based phosphate binders, single-pool Kt/V, and dialysate calcium.
Adjusted for age, sex, dialysis duration, intact PTH levels, phosphorus levels, use of calcium- or non–calcium-based phosphate binders, single-pool Kt/V, and dialysate calcium.
Discussion
This study showed that prescription patterns based on cinacalcet and VDRA were associated with subsequent levels of iPTH, phosphorus, and calcium, after adjusting for potential confounders. In particular, patterns involving either starting cinacalcet or increased VDRA were associated with decreasing iPTH levels, and their combination produced an additive association. We also found that the combination of starting cinacalcet and decreased VDRA was associated with achieving target levels of both phosphorus and calcium. These findings may indicate clinically effective prescription patterns appropriate for various therapeutic purposes.
With the introduction of cinacalcet, physicians can now choose effective combination patterns comprising cinacalcet and VDRA to help achieve target levels of iPTH, phosphorus, and calcium (1). One-third of patients in the study cohort started on cinacalcet during 1.5 years of combination therapy with VDRA. Because this study started just when cinacalcet was introduced in Japan, we were able to observe how cinacalcet prescription spread in daily practice and to examine the association between cinacalcet administration and subsequent laboratory values. Time trends of MBD-related medications in the MBD-5D study were previously reported (3). In this study, we noted that changes in prescription patterns of VDRA dosage varied widely, with some patients receiving decreased dosages and others increased dosages.
Several studies have reported the effect of cinacalcet on decreasing iPTH levels (1,4,5), with associations between cinacalcet administration and decreased iPTH levels in this study found to be consistent with those in randomized control studies (4,5) and a previous observational study (1). However, little is known about the effect of concomitant therapy with cinacalcet and VDRA (15), particularly with regard to the effect of VDRA dosage change in concomitant therapy. We therefore established six exposure categories for prescription patterns based on cinacalcet administration and VDRA dosage change. Results of observation revealed an additive association of starting cinacalcet and increased VDRA with decreased iPTH levels. Furthermore, their combined association nearly equaled the additive associations of the individual associations of the two factors (Figure 2). These observed additive associations can be explained by a biologically plausible mechanism given that cinacalcet and VDRA have separate target receptors (34): Cinacalcet is a positive allosteric modulator of calcium-sensing receptor, whereas VDRA, as its name suggests, activates vitamin D receptor (35). The combination of starting cinacalcet and increased VDRA may prove beneficial in effectively decreasing iPTH levels. However, no health benefits of decreasing iPTH with cinacalcet were examined in this study, and clarifying optimal regimens for reducing iPTH with cinacalcet will be the subject of future studies.
Although a number of previous studies have reported that cinacalcet administration reduced levels of phosphorus and calcium (1,4,5,7–9), few have examined the effects of concomitant therapy with VDRA. We found that a combination pattern involving starting cinacalcet and decreased VDRA was effective in achieving target levels of phosphorus and calcium. Furthermore, this combination was effective in achieving those targets even when administered to patients with either or both hyperphosphatemia and hypercalcemia. Thus, combination therapy with starting cinacalcet and decreased VDRA may be useful in controlling levels of phosphorus and calcium simultaneously.
The major strength of this study was that we defined prescription patterns based on two factors of cinacalcet administration and VDRA dosage change, allowing us to examine the separate and combined associations of these compounds on target patients. Because such concomitant therapy is common in daily practice, the results from this study should prove useful for physicians prescribing these compounds. The second strength was that we conducted sensitivity analyses to examine the robustness of the association, the results of which supported robustness. The third strength was that we conducted set-level analyses to take full advantage of repeated-measures data from the MBD-5D study (3). Finally, this cohort included data from daily practice, in which prescription patterns are not limited by the study protocol, thereby enabling examination of the associations of actual prescription patterns with laboratory values in the cinacalcet era.
Several obvious limitations to this study warrant mention. First, we cannot rule out confounding by indication. Physicians decide prescription patterns according to patients’ demographics and laboratory values, among other parameters. We attempted to compensate for this by adjusting for available covariates data from the reference and treatment visits and adjusting for covariates from the visit before reference visits in sensitivity analyses and found consistent results. However, residual confounding from unmeasured factors may have persisted. Second, patients in this study may not be representative of hemodialysis patients with high iPTH levels in other countries, particularly given that target levels of iPTH are lower in Japan than in other countries. However, we found similar associations in patients with iPTH levels ≥300 and ≥500 pg/ml according to the target range in other countries, and therefore our results may not be limited to only Japanese hemodialysis patients (21,22). Third, although we considered VDRA dosage in determining prescription patterns, we did not consider cinacalcet dosage. However, >85% of starting dosages were <25 mg/d, and dosage differences between patients were small. Fourth, data on methods of serum albumin measurement (bromcresol purple or bromcresol green) (36), which may affect corrected calcium values, were unavailable in the dataset. Fifth, iPTH levels in a number of patients (n=86) were calculated based on whole PTH levels (27). However, given that we found consistent results even after excluding these patients, we believe this was a negligible limitation. Sixth, data on patient adherence to medications was also unavailable in the dataset. Seventh, it is unclear if these findings apply to incident hemodialysis patients, because we did not included prevalent hemodialysis patients in this study. Taking into account these limitations, interpreting and generalizing these results should be carried out with caution.
Allowing for these methodological issues, we found that prescription patterns of cinacalcet administration and VDRA dosage change were associated with subsequent levels of iPTH, phosphorus, and calcium. In particular, the combination of starting cinacalcet and increased VDRA was effective in decreasing iPTH levels, whereas the combination of starting cinacalcet and decreased VDRA was more useful in achieving target levels of phosphorus and calcium. These findings may be useful in determining clinically effective prescription patterns for different therapeutic purposes in the cinacalcet era. Combination patterns may prove advantageous in managing secondary hyperparathyroidism compared with VDRA alone. A randomized trial is needed to examine the health benefits of these findings.
Disclosures
M.F. has acted as a consultant for Kyowa Hakko Kirin, has received honoraria from Kyowa Hakko Kirin, and has received grants (research support) from Kyowa Hakko Kirin. T.A. has acted as a consultant for Kyowa Hakko Kirin, has received grants (research support) from Kyowa Hakko Kirin, and is a member of the speakers’ bureau of Kyowa Hakko Kirin. S. Fukuhara has acted as a scientific advisor for Kyowa Hakko Kirin and has received grants (research support) from Kyowa Hakko Kirin.
Acknowledgments
We thank the following MBD-5D study advisory investigators: Masashi Suzuki (Shinrakuen Hospital), Yoshindo Kawaguchi (Shiomidai Hospital), Akira Saito (Yokohama Daiichi Hospital), Yoshiki Nishizawa (Osaka City University Graduate School of Medicine), Yusuke Tsukamoto (Shuwa General Hospital), Satoshi Kurihara (Tsukinomori Clinic), Takashi Akiba (Tokyo Women’s Medical University), Eriko Kinugasa (Showa University Northern Yokohama Hospital), Yuzo Watanabe (Kasugai Municipal Hospital), Yoshihiro Tominaga (Nagoya Daini Red Cross Hospital), Takashi Shigematsu (Wakayama Medical University), Masaaki Inaba (Osaka City University Graduate School of Medicine), Jun Minakuchi (Kawashima Hospital), Hideki Hirakata (Fukuoka Red Cross Hospital), Keitaro Yokoyama (Jikei University School of Medicine), Naoki Kimata (Tokyo Women’s Medical University), Fumihiko Koiwa (Showa University Fujigaoka Hospital), Ryoichi Ando (Musashino Red Cross Hospital), Junichiro J. Kazama (Niigata University), Takatoshi Kakuta (Tokai University School of Medicine), Hirotaka Komaba (Tokai University School of Medicine), Daijo Inaguma (Nagoya Daini Red Cross Hospital), Eiji Ishimura (Osaka City University Graduate School of Medicine), Hideki Tahara (Osaka City University Graduate School of Medicine), Kazuhiko Tsuruya (Kyushu University), and Akira Fujimori (Konan Hospital).
Funding for the MBD-5D study was provided by Kyowa Hakko Kirin (manufacturer of intravenous calcitriol, cinacalcet hydrochloride, and sevelamer hydrochloride).
Footnotes
M.F. and S. Fukuma contributed equally to this study.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA: Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 4: 354–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenas MD, Alvarez-Ude F, Gil MT, Moledous A, Malek T, Nuñez C, Devesa R, Carretón MA, Soriano A: Implementation of ‘K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease’ after the introduction of cinacalcet in a population of patients on chronic haemodialysis. Nephrol Dial Transplant 22: 1639–1644, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Akizawa T, Kido R, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, Fukuhara S, Kurokawa K: Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: Associations with changing practice patterns. Clin J Am Soc Nephrol 6: 2280–2288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messa P, Macário F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Wilkie M, Zani VJ, Molemans B, Carter D, Locatelli F: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3: 36–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishbane S, Shapiro WB, Corry DB, Vicks SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S, Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: The ACHIEVE study results. Clin J Am Soc Nephrol 3: 1718–1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, Drüeke TB, Cunningham J, Sherrard DJ, McCary LC, Olson KA, Turner SA, Martin KJ: Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67: 760–771, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, Koshikawa S, KRN1493 study group : Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant 23: 328–335, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, Liu J, St Peter WL: Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 78: 578–589, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM: Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 68: 1793–1800, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Evenepoel P: Calcimimetics in chronic kidney disease: Evidence, opportunities and challenges. Kidney Int 74: 265–275, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Wilkie M, Pontoriero G, Macário F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Zani VJ, Carter D, Messa P: Impact of vitamin D dose on biochemical parameters in patients with secondary hyperparathyroidism receiving cinacalcet. Nephron Clin Pract 112: c41–c50, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa M, Nakanishi S, Kazama JJ: Basic and clinical aspects of parathyroid hyperplasia in chronic kidney disease. Kidney Int Suppl 102: S3–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Nakai S, Akiba T, Kazama J, Yokoyama K, Fukagawa M, Tominaga Y, Iseki K, Tsubakihara Y, Patient Registration Committee of the Japanese Society for Dialysis Therapy, Tokyo, Japan : Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial 12: 49–54, 2008 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral bone disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Guideline Working Group, Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial 12: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 24.McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D: International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int 78: 215–223, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Fukuhara S, Akizawa T, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, Kurokawa K: Mineral and bone disorders outcomes study for Japanese chronic kidney disease stage 5D patients: Rationale and study design. Ther Apher Dial 15: 169–175, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Fukagawa M, Komaba H, Onishi Y, Fukuhara S, Akizawa T, Kurokawa K, MBD-5D Study Group : Mineral metabolism management in hemodialysis patients with secondary hyperparathyroidism in Japan: Baseline data from the MBD-5D. Am J Nephrol 33: 427–437, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Reichel H, Esser A, Roth HJ, Schmidt-Gayk H: Influence of PTH assay methodology on differential diagnosis of renal bone disease. Nephrol Dial Transplant 18: 759–768, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton P, Gurrin L, Sly P: Extending the simple linear regression model to account for correlated responses: An introduction to generalized estimating equations and multi-level mixed modelling. Stat Med 17: 1261–1291, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, Negassa A, Edwardes MD, Forrester JE: Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol 157: 364–375, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Zeger SL, Liang KY: An overview of methods for the analysis of longitudinal data. Stat Med 11: 1825–1839, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ: Epidemiology: An Introduction, New York, Oxford University Press, 2002 [Google Scholar]
- 33.de Mutsert R, Jager KJ, Zoccali C, Dekker FW: The effect of joint exposures: Examining the presence of interaction. Kidney Int 75: 677–681, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Drüeke TB, Ritz E: Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin J Am Soc Nephrol 4: 234–241, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kato A, Takita T, Furuhashi M, Fujimoto T, Suzuki H, Hakamada M, Maruyama Y: Influence of the assay for measuring serum albumin on corrected total calcium in chronic hemodialysis patients. Ther Apher Dial 15: 540–546, 2011 [DOI] [PubMed] [Google Scholar]