Summary
Background and objectives
The contribution of urolithiasis, if any, to the development of ESRD is unclear.
Design, setting, participants, & measurements
All stone formers in Olmsted County, Minnesota, first diagnosed between 1984 and 2008 were identified by diagnostic codes with up to four controls matched on age and sex. Charts were reviewed to validate symptomatic stone formers in a random subset. Incident ESRD events were identified by the US Renal Data System.
Results
Altogether, 51 stone formers and 75 controls developed ESRD among 6926 stone formers and 24,620 matched controls followed for a mean of 9 years. Stone formers had an increased risk of ESRD after adjusting for diabetes, hypertension, dyslipidemia, gout, and CKD (hazard ratio: 2.09; 95% confidence interval: 1.45–3.01). This increased risk of ESRD remained in the subset of 2457 validated symptomatic stone formers (hazard ratio: 1.95; 95% confidence interval: 1.09–3.49). The attributable risk of ESRD from symptomatic urolithiasis was 5.1% based on a prevalence of 5.4% for stone formers. For stone formers versus controls who developed ESRD, there was an increased likelihood of past hydronephrosis (44% versus 4%), recurrent urinary tract infections (26% versus 4%), acquired single kidney (15% versus 3%), neurogenic bladder (12% versus 1%), and ileal conduit (9% versus 0%), but not diabetes (32% versus 49%) or hypertension (44% versus 52%).
Conclusions
Symptomatic stone formers are at increased risk for ESRD independent of several cardiovascular risk factors. Other urological disease is relatively common among stone formers who develop ESRD.
Introduction
Symptomatic urolithiasis is commonly encountered in clinical practice and occasionally causes AKI secondary to urinary tract obstruction. Several population-based studies have also shown an increased risk of CKD among stone formers (1–4). This association with CKD may have been biased by inadequate validation of stone formers or by differential evaluation and follow-up for CKD between stone formers and controls (5). ESRD represents significant clinical morbidity that is less prone to detection bias than earlier stages of CKD. The risk of ESRD in stone formers is less clear (1,6–8), but urolithiasis is not usually considered to be an important contributor to the burden of ESRD in the population. Among patients on hemodialysis, ESRD attributed to urolithiasis occurs in only 2%–3% (9,10) and the US Renal Data System (USRDS) reports urolithiasis as the primary cause of ESRD in only 0.2% of cases (11). Prior studies assessing the risk of ESRD in stone formers lack adequate validation. In particular, patients who develop ESRD may be more likely to be miscoded with urolithiasis or undergo kidney imaging that detects asymptomatic stones (present in 10% of potential kidney donors by computed tomography) (12). We previously reported that there was no statistically significant increased risk of ESRD in stone formers (1); however, on subsequent chart review, we found that the clinical databases used to detect ESRD were not very accurate and ESRD events outside of Olmsted County were missed. To address these concerns, we performed a population-based cohort study to assess the risk of ESRD in stone formers with up to 25 years of follow-up. We manually reviewed charts to validate symptomatic stone formers and used the USRDS database to identify ESRD endpoints. The risk of ESRD in stone formers independent of several cardiovascular risk factors was assessed. To gain insight into potential disease pathways, comorbidities were also compared between stone formers and controls who developed ESRD. Finally, we estimated attributable risk (AR) in order to quantify the relevance of urolithiasis to the burden of ESRD in the population.
Materials and Methods
Study Population
Population-based research is feasible in Olmsted County because medical care is largely self-contained within the community. Over 95% of the population has at least one clinic visit with a local health care provider every 2–3 years, allowing essentially complete enumeration of the local population (13). The residency status of each person ever seen by an Olmsted County provider since 1966 was compiled by the Rochester Epidemiology Project to provide a sampling framework for the entire county population (14). Moreover, diagnostic codes (coded from the final diagnoses in clinical notes) dating back to 1935 are indexed and linked among virtually all Olmsted County providers through the Rochester Epidemiology Project (13). All residents with their first documented kidney or bladder stone in Olmsted County between 1984 and 2008 were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes 592, 594, and 274.11. Up to four controls with no prior history of stones according to these codes were selected for each stone former from among all Olmsted County residents. Controls were matched on index date (first coded stone episode for stone formers and county residency on that same date for controls) ± 1 year, age (at index date) ± 1 year, and sex. Controls who developed a stone after their index date were censored at that point and subsequently included in the incident stone former cohort with their own matched controls.
Validation of Outcome and Exposure
ESRD events were identified by matching the historical cohort of Olmsted County stone formers and controls with the USRDS database (11). Charts were manually reviewed to validate the ESRD events in all stone formers and controls, to determine the perceived primary cause of ESRD, and to identify cardiovascular and urological comorbidities present before the development of ESRD. If the primary cause of ESRD could not be determined from chart review, the primary cause listed in the USRDS database based on the ESRD medical evidence form (Centers for Medicare and Medicaid Services Form 2728) was used. A random subset of stone former charts from 1984 to 2003 was manually reviewed (budgetary constraints limited the number of charts that could be reviewed and an early subset was chosen to maximize follow-up time for ESRD events). This manual review excluded anyone who was miscoded or misdiagnosed for urolithiasis or had only incidentally discovered asymptomatic radiographic stones. Stone formers were considered validated if they had characteristic symptoms (abdominal pain and/or gross hematuria) that were attributed by physicians at the end of the episode of care as being due to kidney, ureter, or bladder stones. Radiographic confirmation was not required because imaging studies were not always performed.
Comorbidities
Comorbidities known to be associated with urolithiasis and ESRD were identified from ICD-9 codes, including hypertension, diabetes mellitus, obesity, dyslipidemia, gout, and CKD (see Appendix for codes). Comorbidities were considered prevalent (baseline) if they occurred before the index date.
Statistical Analyses
Persons with prevalent ESRD (before the index date) were excluded from all analyses. Follow-up was terminated at the earliest occurrence of ESRD, last clinic visit, death, or December 31, 2008. To confirm the robustness of our conclusions, analyses were performed using four groups of stone formers (group 1: all coded stone formers from 1984 to 2008; group 2: the subset from 1984 to 2003; group 3: a random subset of group 2 from 1984 to 2003 whose charts were reviewed; and group 4: the subset of group 3 who were validated as symptomatic stone formers). Each subset was compared with its respective controls. The association of urolithiasis with ESRD was assessed using hazard ratios (HRs) from a stratified (to account for matching) Cox proportional hazards models with and without adjustments for baseline comorbidities. Separate analyses excluded persons with baseline CKD (by diagnostic codes). Additional models assessed for statistical interactions between stones and each comorbidity, as well as demographic variables, and calendar year on the risk of ESRD. The cumulative incidence of ESRD was estimated using Kaplan–Meier plots, censoring deaths without prior ESRD. Rates of ESRD, using mortality as a competing risk, were lower by only 0.1% at 15 years in both stone formers and controls.
To calculate the 15-year AR of ESRD from urolithiasis, we first calculated the point prevalence of urolithiasis by identifying all Olmsted County residents as of January 1, 2009, who had a prior diagnostic code for urolithiasis and estimated the validated symptomatic fraction. AR was then calculated as follows: AR = Prevalencestones × (RR − 1)/[1 + Prevalencestones × (RR − 1)], where relative risk (RR) was the 15-year relative cumulative incidence of ESRD in stone formers compared with controls (15). All analyses used SAS v9.1 software (SAS Institute, Cary, NC).
Results
Characteristics of the Cohort
Altogether, 6926 stone formers and 24,620 matched controls were identified from the Olmsted County population between 1984 and 2008. As a consequence of the matching, stone formers and controls had the same mean age (45 years) and sex distribution (58% men). Duration of medical record documentation before the index date (mean 22.4 versus 23.1 years) and length of follow-up from index date to ESRD, last clinic visit, or death (mean 7.8 versus 7.9 years) were also similar. Consistent with the racial composition of the county, 93% of stone formers and 92% of controls were white. The prevalence of baseline comorbidities was higher in the stone formers than the controls (Table 1). There were 4910 stone formers identified between 1984 and 2003, 2995 stone formers in the random subset that was chart-reviewed, and 2457 validated symptomatic stone formers. The 538 stone formers who were excluded during chart validation were either miscoded (n=215), had asymptomatic stones only (n=253), were not county residents (n=22), or lacked an authorization to review their medical records for research (n=48).
Table 1.
Baseline comorbidities in stone formers identified by ICD-9 codes from 1984 to 2008 (group 1) and control participants in Olmsted County, Minnesota
| Comorbidity | Stone Formers (n=6926), n (%) | Controls (n=24,620), n (%) | P |
|---|---|---|---|
| CKD | 383 (5.5) | 811 (3.3) | <0.001 |
| Hypertension | 1501 (21.7) | 4848 (19.7) | <0.001 |
| Diabetes mellitus | 766 (11.1) | 2048 (8.3) | <0.001 |
| Obesity | 1655 (23.9) | 5044 (20.5) | <0.001 |
| Dyslipidemia | 1647 (23.8) | 5389 (21.9) | <0.001 |
| Gout | 219 (3.2) | 648 (2.6) | 0.02 |
ICD-9, International Classification of Diseases, Ninth Revision.
Risk of ESRD
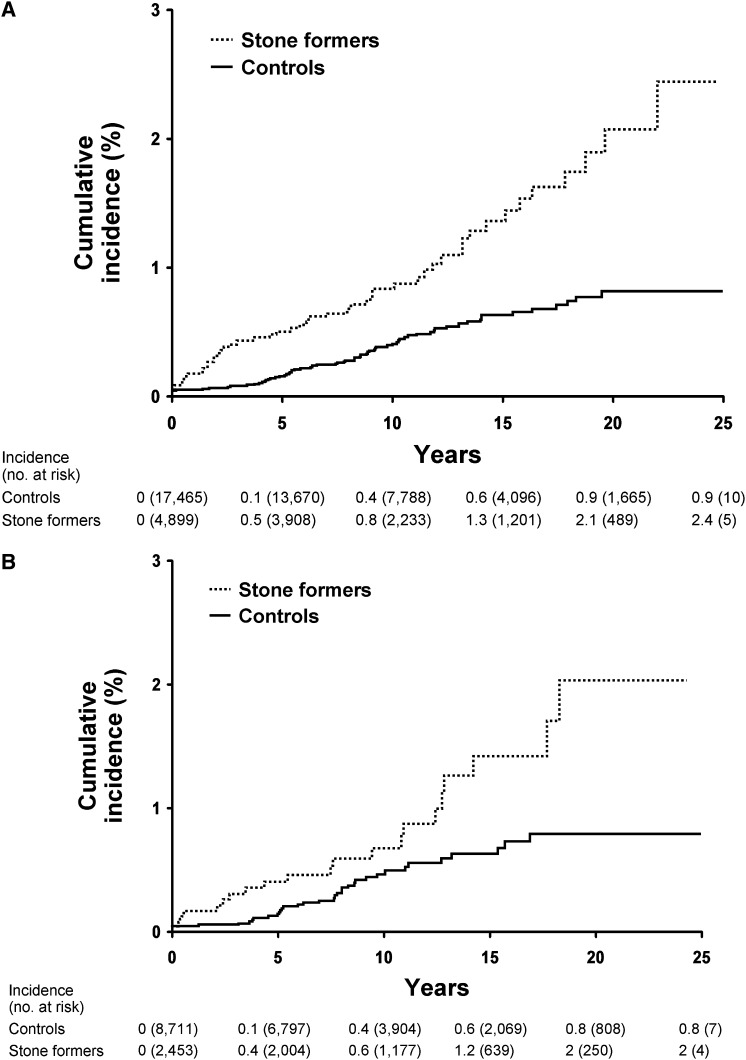
Altogether, the USRDS database showed that 68 (1.0%) stone formers and 122 (0.5%) controls had ESRD. There were 51 stone formers and 75 controls who developed ESRD after the index date (17 stone formers and 47 controls with ESRD before the index date were excluded). Through December 31, 2008, there were 709 controls who later became stone formers; one developed ESRD during follow-up as a control and four developed ESRD during follow-up as a stone former. Table 2 summarizes the risk of ESRD in stone formers in the full sample and within each of the subgroups analyzed separately. Using diagnostic codes to identify stone formers in the years 1984–2008, the ESRD HR among stone formers was 2.36 (95% confidence interval: 1.65–3.37). After chart validation, the risk of ESRD remained statistically significant (HR: 1.98; 95% confidence interval: 1.13–3.45), although slightly attenuated. Figure 1 shows the cumulative incidence of ESRD in stone formers compared with controls for analysis groups 2 and 4.
Table 2.
Risk of ESRD among stone formers compared with control participants in Olmsted County, Minnesota, by stone former group
| Group of Stone Formers Identified | Number of Stone Formers (Events) | Number of Controls (Events) | HR (95% CI) | Cumulative 15-Yr Incidence of ESRD (%) | |
|---|---|---|---|---|---|
| Stone Formers | Controls | ||||
| Group 1: ICD-9 codes (1984–2008) | 6911 (51) | 24,577 (75) | 2.36 (1.65, 3.37) | 1.3 | 0.7 |
| Group 2: ICD-9 codes (1984–2003) | 4899 (46) | 17,465 (66) | 2.42 (1.66, 3.53) | 1.3 | 0.6 |
| Group 3: Random subset of group 2 that underwent chart review | 2985 (29) | 10, 627 (42) | 2.35 (1.46, 3.78) | 1.4 | 0.7 |
| Group 4: Those in group 3 with a validated symptomatic stone | 2453 (20) | 8711 (33) | 1.98 (1.13, 3.45) | 1.2 | 0.6 |
Table excludes participants with prevalent ESRD. HR, hazard ratio; 95% CI, 95% confidence interval; ICD-9, International Classification of Diseases, Ninth Revision.
Figure 1.
Risk of ESRD is in stone formers versus matched controls. Cumulative incidence (Kaplan–Meier method) of ESRD is significantly higher among the ICD-9–coded stone formers versus matched controls in (A) analysis group 2 (P=0.001), and also among the validated symptomatic stone formers versus matched controls who comprised (B) group 4 (P=0.01) in Olmsted County, Minnesota, from 1984 to 2003. ICD-9, International Classification of Diseases, Ninth Revision.
Table 3 shows that the risk of ESRD in stone formers remained even after adjustment for comorbidities. Moreover, excluding persons with baseline CKD did not substantially change the magnitude of ESRD risk, although it was of borderline statistical significance in the validated symptomatic stone former subgroup (P=0.06). There was no detectable interaction between the risk of ESRD with urolithiasis and age, sex, or any comorbidity in the full cohort or the validated subset (P≥0.18 for each interaction). There was also no evident interaction between the risk of ESRD with urolithiasis and calendar year (P=0.81 for validated subset).
Table 3.
Risk of ESRD among stone formers compared with control participants in Olmsted County, Minnesota, adjusting for comorbidity status
| Adjusting Factor | Hazard Ratio (95% Confidence Interval) | |
|---|---|---|
| Coded Stone Formers (Group 1) | Random Subset of Validated Symptomatic Stone Formers (Group 4) | |
| None | 2.36 (1.65, 3.37) | 1.98 (1.13, 3.45) |
| Diabetes | 2.24 (1.57, 3.20) | 1.91 (1.09, 3.35) |
| Obesity | 2.30 (1.61, 3.29) | 1.96 (1.12, 3.42) |
| Hypertension | 2.38 (1.67, 3.40) | 2.09 (1.19, 3.65) |
| Dyslipidemia | 2.36 (1.65, 3.37) | 1.99 (1.14, 3.47) |
| Gout | 2.31 (1.62, 3.31) | 1.95 (1.12, 3.41) |
| CKD | 2.07 (1.44, 2.98) | 1.76 (0.99, 3.11) |
| Diabetes, obesity, hypertension, dyslipidemia, gout, and CKD | 2.09 (1.45, 3.01) | 1.95 (1.09, 3.49) |
| Excluding persons with baseline CKD | 2.25 (1.42, 3.56) | 1.96 (0.98 3.90) |
Characteristics of ESRD in Stone Formers
On manual chart review, 34 of the 51 stone formers with incident ESRD were valid symptomatic stone formers, whereas all of the 75 controls with incident ESRD had no prior evidence of symptomatic stone disease. Therefore, diagnostic codes had a high negative predictive value for validated symptomatic stone diseases. As shown in Table 4, symptomatic stone formers were more likely than controls to have other urological disease identified as the primary cause of ESRD, and they were more likely to have urological comorbidities (recurrent urinary tract infections, acquired single kidney, ileal conduit, neurogenic bladder, and hydronephrosis). Ileal conduit was the only form of bladder replacement surgery performed among cohort patients with ESRD. Of the 34 stone formers who developed ESRD, 4 had bladder stones and 4 had an episode of AKI attributed to urolithiasis. The stone composition was unknown in 17, uric acid in 9, calcium oxalate in 6, calcium phosphate in 4, and struvite in 1; none had a diagnosed rare hereditary stone disease (e.g., cystinuria, primary hyperoxaluria, dihydroxyadeninuria, or Dent disease).
Table 4.
Characteristics of incident ESRD among validated symptomatic stone formers compared with ESRD among control participants in Olmsted County, Minnesota
| Characteristic | Stone Formers Who Developed ESRD (n=34), n (%) | Controls Who Developed ESRD (n=75), n (%) | P Value (Fisher’s Exact Test) |
|---|---|---|---|
| Primary cause of ESRD | |||
| diabetes mellitus | 6 (18) | 22 (29) | 0.24 |
| hypertension | 6 (18) | 13 (17) | 1.0 |
| GN | 4 (12) | 10 (13) | 1.0 |
| cystic kidney disease | 2 (6) | 3 (4) | 0.64 |
| urolithiasis | 4 (12) | 0 (0) | 0.008 |
| other urologic disease | 6 (18) | 3 (4) | 0.03 |
| other known cause | 4 (12) | 19 (25) | 0.13 |
| unknown cause | 2 (6) | 5 (7) | 1.0 |
| Comorbidities present before onset of ESRD | |||
| diabetes mellitus | 11 (32) | 37 (49) | 0.14 |
| hypertension | 15 (44) | 39 (52) | 0.54 |
| recurrent urinary tract infections | 9 (26) | 3 (4) | 0.001 |
| acquired single kidney | 5 (15) | 2 (3) | 0.03 |
| ileal conduit | 3 (9) | 0 (0) | 0.03 |
| neurogenic bladder | 4 (12) | 1 (1) | 0.03 |
| hydronephrosis | 15 (44) | 3 (4) | <0.001 |
Attributable Risk of ESRD
The prevalence of stone formers by diagnostic code was 6.4% among a random 2% sample of the Olmsted County population on January 1, 2009 (n=2848; mean age 37 years). Taking into account the estimated 16% that are miscoded/misdiagnosed or asymptomatic based on this study, the prevalence of validated symptomatic stone formers was estimated at 5.4%. In addition, from the present analysis, the risk ratio of ESRD was 2.0 using the 15-year cumulative incidence of ESRD of 1.2% in stone formers and 0.6% in controls. Using these estimates, the calculated attributable risk of ESRD from symptomatic stone disease was calculated at 5.1% in the overall Olmsted County population.
Discussion
In this population-based cohort study, stone formers identified by ICD-9 codes were at 2.4-fold higher risk of developing ESRD. However, using diagnostic codes alone led to a differential bias, because 33% of coded stone formers who developed ESRD were miscoded or asymptomatic, whereas only 16% of those who did not develop ESRD were miscoded or asymptomatic. It is possible that this bias occurred because ESRD patients had other diseases initially miscoded for urolithiasis that contributed to ESRD (e.g., renal cell cancer). Furthermore, renal imaging is routinely performed in patients with CKD, and this would increase the detection of asymptomatic stones. To address these concerns, we manually validated charts to exclude the subset of stone formers who were miscoded or asymptomatic. Although this attenuated the risk, the two-fold higher risk of ESRD in validated symptomatic stone formers remained statistically significant and was independent of baseline cardiovascular risk factors and CKD. Stone formers who developed ESRD were more likely to have other urological diseases than controls who developed ESRD, but they were not more likely to have diabetes or hypertension. To the extent that the 5.1% AR for ESRD is causal, stone formers represent a small but relevant contributor to the overall burden of ESRD in the population.
Other studies have suggested an increased risk of ESRD among stone formers. A case-control study in African Americans established a prevalence of urolithiasis among ESRD patients that is much higher than among the general population (8% versus 3%) (8). Among primary care patients with up to 7 years of follow-up, a history of urolithiasis was an independent predictor of ESRD in women (HR: 2.07) but not in men (HR not reported) (7). In an integrated health care delivery system with 25-years of follow-up, a history of kidney or bladder stones predicted ESRD (HR: 1.91), but not after multivariable adjustment that included serum creatinine and dipstick proteinuria (6). For the purposes of assessing the risk of ESRD, adjusting for baseline CKD may be an overadjustment, particularly among stone formers with past stone events that preceded the onset of CKD. The reported prevalence of stone formers in these two cohorts was relatively low at 0.7% (7) and 2.5% (6), but these studies may have not detected all the stone formers. A survey of the US population (National Health and Nutrition Examination Survey 2007–2010, aged ≥20 years) reported a urolithiasis prevalence estimate of 8.8% (7.7% for symptomatic only) (16). When restricting our sample of the US population to ages ≥20 years, we found a similar code-based prevalence of urolithiasis of 8.6% (7.2% after excluding miscoded/misdiagnosed or asymptomatic only).
Urolithiasis is the reported primary cause of ESRD for only 0.2% of incident ESRD patients in the USRDS (11), but ESRD is often not due to a single cause. The AR of ESRD from urolithiasis (5.1%) may be 20-fold higher because the primary cause of ESRD is multifactorial and the contribution from urolithiasis may not be easily determined or accurately documented. It is difficult to determine the primary cause when stones occur in other urological diseases that lead to ESRD (in particular, recurrent urinary tract infections, ileal conduit, and neurogenic bladder). Among patients starting renal replacement therapy, other more active comorbidities (such as hypertension) may be perceived to be the primary cause of ESRD. The large decrease in urine calcium with more advanced CKD (17) leads to decreased stone growth and passage and this may further contribute to under-recognition of urolithiasis as a primary cause of ESRD.
The mechanisms by which urolithiasis might lead to ESRD are not clear. In this study, urological comorbidities were more common among stone formers than among controls who developed ESRD. Recurrent urinary tract infections, acquired single kidney, ileal conduit, neurogenic bladder, and hydronephrosis are plausible contributors to ESRD. Urolithiasis, particularly struvite stones, can be a nidus for recurrent urinary tract infections leading to chronic tubulointerstitial disease (18). In urology referral practices, approximately 40% of stone formers who develop ESRD have a solitary functioning kidney, most commonly from a staghorn or high stone burden, infection, or ureteral obstruction (10,19). An ileal conduit diversion after a radical cystectomy can lead to urolithiasis, urinary tract infections, and reduced kidney function (20). A neurogenic bladder can lead to vesicoureteral reflux, recurrent urinary tract infections, urolithiasis, and CKD (21). Hydronephrosis from an obstructing stone can cause ischemic injury to the kidney, some of which may be irreversible (5,22). Secular trends in the case mix of ESRD patients have suggested a decrease in stone-related ESRD over time, possibly due to less invasive urological therapies (10). However, that prior report was not based on ESRD incidence rates, and we found no evidence of an interaction between calendar year and risk of ESRD in stone formers in this study. Nevertheless, the effect of urological therapies on risk of ESRD in stone formers merits further investigation.
One might hypothesize that an increased prevalence of cardiovascular risk factors such as hypertension could explain the increased risk of ESRD among stone formers. Although we found that diabetes, obesity, hypertension, dyslipidemia, and gout were more prevalent among stone formers, adjustment for these cardiovascular risk factors did not substantively affect the risk of ESRD. Furthermore, among stone formers who developed ESRD, diabetes and hypertension trended toward being less common comorbidities than among controls who developed ESRD. Still, diabetes and hypertension may be the most potent predictors of ESRD among stone formers as they are in the general population (23). Our data only suggest that the contribution of urolithiasis to ESRD risk is not via these cardiovascular risk factors.
There are several strengths of this population-based cohort study that used matched controls and up to 25 years of follow-up. By virtue of our access to complete community medical records, we found evidence that a code-based identification of stone formers (groups 1, 2, and 3) inflated the risk of ESRD. By using the subset of validated symptomatic stone formers (group 4), we avoided this source of bias in our risk estimates of ESRD. There were also limitations to this study. Anyone who declined therapy for kidney failure would not have been detected by the USRDS as having ESRD. We had limited statistical power, particularly in the validated symptomatic stone formers. This limited the number of comorbidities we chose (a priori) for the adjusted analyses and prevented further subgroup analysis (e.g., comparing stone formers identified by imaging versus symptoms alone). Other urological diseases as determined by manual chart review were available in the small subset of participants who developed ESRD, but not in the remaining participants who did not develop ESRD; therefore, we cannot currently accurately calculate a HR for ESRD after removing those with recurrent urinary tract infections, acquired single kidney, neurogenic bladders, or ileal conduits. The findings are most generalizable to white populations similar to the Olmsted County population (14). The AR of ESRD from urolithiasis may differ in nonwhite populations in which stone formers are less common. Finally, in the multivariable analysis, we used diagnostic codes that lacked chart validation for comorbidities.
Although numerous studies have related urolithiasis to CKD, there have been significant limitations to prior reports. CKD is largely an asymptomatic condition and the clinical evaluation of symptomatic urolithiasis can lead to detection of previously unrecognized of CKD. Conversely the imaging evaluation of CKD can lead detection of previously unrecognized asymptomatic urolithiasis. To address these concerns, we used validated symptomatic stone formers based on chart review and the “hard” endpoint of ESRD. We found evidence that symptomatic stone formers are at increased risk for ESRD independent of hypertension, diabetes, obesity, dyslipidemia, and gout. Other urological diseases are relatively common in stone formers who develop ESRD. Further studies are needed to understand the interaction between urological disease and the increased risk of ESRD in stone formers.
Disclosures
None.
Acknowledgments
This project was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK 83007 and DK78229) and was made possible by the Rochester Epidemiology Project (AG034676) from the National Institutes of Health, US Public Health Service. Part of the data were provided by the USRDS. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. A.D.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The conclusions of the authors do not represent either the NIDDK or the USRDS.
This study was presented in part at the 43rd Annual Meeting of the American Society of Nephrology, November 18, 2010, Denver, Colorado.
Appendix
| Clinical Diagnosis | ICD-9 Codes |
|---|---|
| CKD | 250.4, 274.10, 274.19, 403, 582, 583, 585, 587, 791.0 |
| Hypertension | 401 |
| Diabetes mellitus | 250, 357.2, 362.01, 362.02, 366.41, 648.8, 790.2 |
| Obesity | 278.0, 278.1, 278.8 |
| Dyslipidemia | 272.0, 272.1, 272.2, 272.3, 272.4 |
| Gout | 274.0, 274.81, 274.82, 274.89, 274.9 |
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillen DL, Worcester EM, Coe FL: Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. Kidney Int 67: 685–690, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Vupputuri S, Soucie JM, McClellan W, Sandler DP: History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol 14: 222–228, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Wang W, Huang Y, Shen P, Pei D, Yu H, Shi H, Zhang Q, Xu J, Lv Y, Fan Q: Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant 24: 2117–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rule AD, Krambeck AE, Lieske JC: Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol 6: 2069–2075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 169: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hippisley-Cox J, Coupland C: Predicting the risk of chronic kidney disease in men and women in England and Wales: Prospective derivation and external validation of the QKidney Scores. BMC Fam Pract 11: 49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stankus N, Hammes M, Gillen D, Worcester E: African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res 35: 83–87, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Tosetto E, Graziotto R, Artifoni L, Nachtigal J, Cascone C, Conz P, Piva M, Dell’Aquila R, De Paoli Vitali E, Citron L, Nalesso F, Antonello A, Vertolli U, Zagatti R, Lupo A, D’Angelo A, Anglani F, Gambaro G: Dent’s disease and prevalence of renal stones in dialysis patients in Northeastern Italy. J Hum Genet 51: 25–30, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M: ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis 44: 799–805, 2004 [PubMed] [Google Scholar]
- 11.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 57[Suppl 1]: A8–, e1–e526., 2011 [DOI] [PubMed] [Google Scholar]
- 12.Lorenz EC, Lieske JC, Vrtiska TJ, Krambeck AE, Li X, Bergstralh EJ, Melton LJ, 3rd, Rule AD: Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant 26: 2695–2700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ, 3rd: History of the Rochester Epidemiology Project. Mayo Clin Proc 71: 266–274, 1996 [DOI] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA: Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester epidemiology project. Am J Epidemiol 173: 1059–1068, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jewell NP: Statistics for Epidemiology, Boca Raton, FL, Chapman & Hall/CRC, 2004 [Google Scholar]
- 16.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project: Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craver L, Marco MP, Martínez I, Rue M, Borràs MLM, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1-5—achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Murray T, Goldberg M: Chronic interstitial nephritis: Etiologic factors. Ann Intern Med 82: 453–459, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Worcester E, Parks JH, Josephson MA, Thisted RA, Coe FL: Causes and consequences of kidney loss in patients with nephrolithiasis. Kidney Int 64: 2204–2213, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Madersbacher S, Schmidt J, Eberle JM, Thoeny HC, Burkhard F, Hochreiter W, Studer UE: Long-term outcome of ileal conduit diversion. J Urol 169: 985–990, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gormley EA: Urologic complications of the neurogenic bladder. Urol Clin North Am 37: 601–607, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Bander SJ, Buerkert JE, Martin D, Klahr S: Long-term effects of 24-hr unilateral ureteral obstruction on renal function in the rat. Kidney Int 28: 614–620, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, Li X, Rule AD, Lieske JC: Risk factors for CKD in persons with kidney stones: A case-control study in Olmsted County, Minnesota. Am J Kidney Dis 55: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]