Summary
Background and objectives
Relatively little is known about the long-term outcomes of different histologic types of primary glomerulonephritis in Asian populations.
Design, setting, participants, & measurements
From 1993 to 2006, 987 patients undergoing renal biopsy were studied, and 580 patients (mean age=44.4 years, male=58.5%) with the four most common forms of glomerulonephritis (membranous nephropathy, focal and segmental glomerulosclerosis, IgA nephropathy, and minimal change disease) were selected for analysis. Median follow-up period was 5.9 (interquartile range=5.7) years.
Results
The focal and segmental glomerulosclerosis group displayed the highest incidence of ESRD (25.8%) and the fastest decline of estimated GFR (4.6 ml/min per 1.73 m2 per year). The IgA nephropathy group also had a higher rate of ESRD than the membranous nephropathy patients (19.2% versus 4.3%, P<0.001). In contrast, the membranous nephropathy group exhibited an overall death rate similar to the focal and segmental glomerulosclerosis group (17.2% versus 14.4%) but higher than the IgA nephropathy and minimal change disease patients (4.6% and 3.7%, respectively, P<0.001). The most powerful predictor for ESRD was focal and segmental glomerulosclerosis, whereas the strongest predictor for all-cause mortality was membranous nephropathy with higher proteinuria. Protectors against ESRD included male sex and higher hemoglobin.
Conclusions
Most predictors for ESRD and overall mortality found in this ethnic Chinese cohort were similar to other studies. However, some risk factors linked with distinct glomerular pathologies displayed differential clinical outcomes.
Introduction
The progressively increasing incidence and prevalence of ESRD not only threatens public health but also increases the financial burden of healthcare in many countries of the world (1,2). According to large renal registries (3), glomerulonephritis (GN) remains one of the most common etiologies of ESRD. Membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), and IgA nephropathy (IgAN) are the most frequently diagnosed forms of GN prone to renal progression in adult patients. Idiopathic MN and primary FSGS usually present with a nephrotic syndrome with or without renal insufficiency, whereas IgAN often exhibits persistent hematuria and proteinuria (4). Although many of these patients may benefit from specific immunosuppressive therapies, a significant proportion is resistant to treatment, and eventually, they develop ESRD (5–7). These three histologic types constitute the majority of primary GN cases reported in industrialized countries (6–10). Complementing the observations made in Western countries, primary GN is also one of the most common causes of ESRD in Asia (11–18), including Taiwan, where CKD is prevalent and the incidence of ESRD is the highest in the world (19,20).
There are many reports showing regional epidemiologic and clinicopathological features pertaining to various glomerulopathies in Western countries (4,9,21–23). By contrast, relatively little is known about the long-term outcomes of different histologic types of primary GN in Asian populations. In this study, we describe in detail the clinical outcomes and predictors for ESRD and mortality in an ethnic Chinese cohort of primary GN in Taiwan, and we explore the possible implications associated with these observations.
Materials and Methods
Study Population and Data Collection
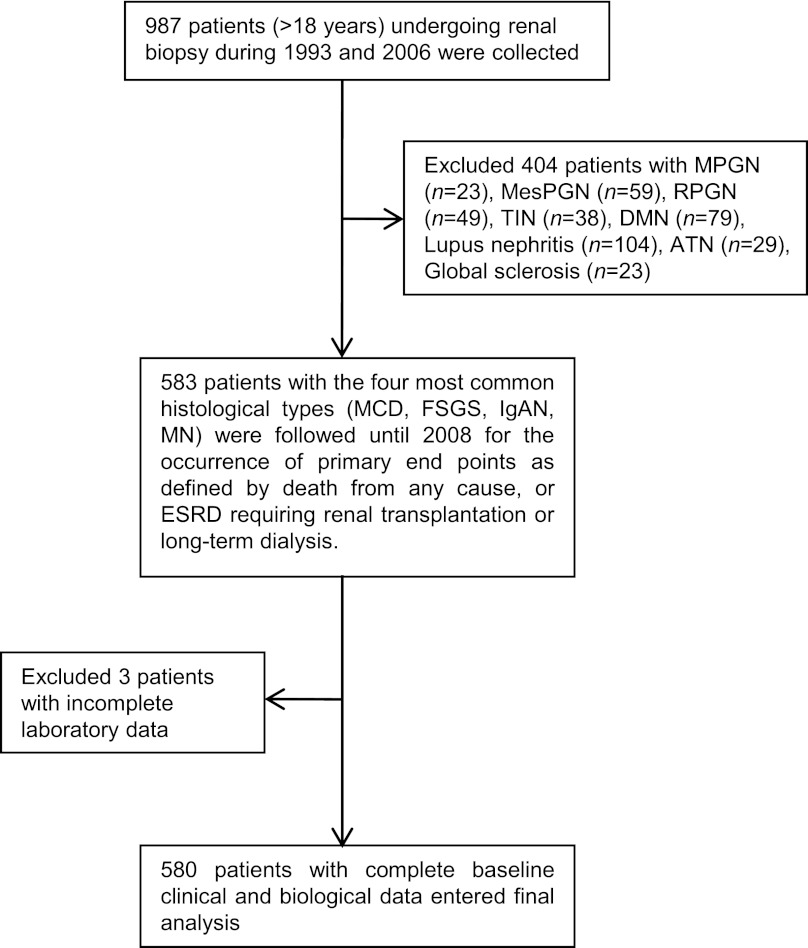
This retrospective observational study was approved by the Research Ethics Committee of National Taiwan University Hospital (No. 200810046R). The hospital is a major medical center located in northern Taiwan that serves as a referral center for more than 5 million residents of the metropolitan Taipei area. In addition, many patients all over Taiwan are referred to this hospital for evaluation of kidney diseases, including sonography-guided renal biopsy. From 1993 to 2006, a total of 987 patients older than 18 years underwent native kidney biopsy at the Renal Division of this hospital for nephrotic syndrome, unexplained renal failure, or less commonly, persistent urinary abnormalities such as subnephrotic proteinuria or hematuria. After screening, we excluded patients with membranoproliferative glomerulonephritis and mesangioproliferative glomerulonephritis because of the relatively fewer number of cases for analysis, and patients with secondary glomerulonephritis or other renal pathologies, such as diabetic nephropathy, lupus nephritis, rapid progress glomerulonephritis, acute tubular necrosis, and tubulointerstitial nephritis. We also excluded three patients from this study because of incomplete laboratory data. In total, 580 patients with the four most common glomerulopathies were chosen for analysis, which included minimal change disease (MCD), MN, FSGS, and IgAN. Figure 1 shows the enrollment process of this observational cohort.
Figure 1.
Flowchart of the participants in the cohort. ATN, acute tubular necrosis; DMN, diabetic nephropathy; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MesPGN, mesangioproliferative glomerulonephritis; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; RPGN, rapidly progressive glomerulonephritis; TIN, tubulointerstitial nephritis.
The demographic characteristics and laboratory data of these patients at presentation or before renal biopsy were recorded. These data included parameters such as age, sex, diabetes, hypertension, immunosuppressants treatment, BUN, serum creatinine, albumin, hemoglobin, total cholesterol, triglycerides, and urine protein. The magnitude of urinary protein excretion (either 24-hour urine quantitation or single-voided urine dipstick) was performed by the Department of Laboratory Medicine of this hospital. Patient survival was determined through the databank of the National Health Insurance Research Database, which contains healthcare data from >99% of the entire population of 23.74 million in Taiwan, and it covers all inpatient and outpatient medical benefit claims. We also crosslinked our study population with the nationwide Taiwan Society of Nephrology registry of 2008, which receives data reports of all dialysis patients every 3 months. All subjects were followed until 2008 for occurrence of primary endpoints, such as death from any cause or ESRD requiring renal transplantation or long-term dialysis. GFR was estimated using the simplified Modification of Diet in Renal Disease (MDRD) formula to calculate the rate of renal progression of the different cohorts (24). Decline of estimated GFR (eGFR) was computed by calculating the differences of eGFR normalized by intervals between the time at biopsy and the time at the last clinic visit before occurrence of primary endpoints or end of 2008.
Statistical Analyses
Statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC), and R version 2.9.2 (Free Software Foundation, Inc., Boston, MA) statistical software. In statistical testing, a two-sided P value ≤0.05 was considered statistically significant, and 0.05<P value ≤0.10 was considered borderline statistically significant. Continuous data were expressed as mean ± SD unless otherwise specified. Frequencies and percentages were calculated for categorical variables. The one-way ANOVA was used to compare the means of continuous data among groups, whereas the chi-squared or Fisher exact test was used to analyze categorical proportions. In addition to univariate analysis, Cox proportional hazards models were used to identify independent predictors for dialysis dependence and mortality. In multivariate analysis for mortality, we used time to dialysis, which was the time of dialysis to the time at which a death of any cause occurred. This time was computed for each subject and then included in the Cox proportional hazards model of mortality as a time-dependent covariate to clarify the relationship between dialysis and mortality. For patients who did not need dialysis at the time when death from any cause occurred, the time duration was set to zero. Finally, Kaplan–Meier estimates of survival curves with log-rank testing results were drawn to express the differences of patient survival and dialysis dependence between the MCD, MN, FSGS, and IgAN groups.
Our regression analyses proceeded in two steps. First, we conducted regression analysis within each GN subgroup to explore the potential heterogeneity in the covariates’ effects across those four GN subgroups. Next, we added the interaction terms between the covariates with potential heterogeneous effects and the three dummy variables representing the four GN subgroups into the regression analysis of the pulled data from the four GN subgroups to increase the testing power and estimation efficiency.
The goal of regression analysis was to find one or more parsimonious regression models that fitted the observed data well for outcome prediction or effect estimation. To ensure the quality of analysis results, basic model-fitting techniques for (1) variable selection, (2) goodness-of-fit (GOF) assessment, and (3) regression diagnostics were used in our regression analyses. Specifically, the stepwise variable selection procedure (with iterations between the forward and backward steps) was applied to obtain the final candidate regression model. All univariates, including the demographic characteristics and laboratory data, and significant and nonsignificant relevant covariates were considered, and the significance levels for entry and stay were set to 0.15 or larger. Then, the best final regression model was identified manually by reducing the significance levels to 0.05 corresponding to the chosen α-level. Any discrepancy between the results of univariate analysis and multivariate analysis was likely caused by the confounding effects of the uncontrolled covariates in the univariate analysis. Both the adjusted generalized R2 and the Grønnesby–Borgan GOF test were examined to assess the GOF of the fitted Cox proportional hazards model. However, the value of the adjusted generalized R2 for the Cox proportional hazards model is usually low. Larger P values of the Grønnesby–Borgan GOF test indicate better fits. Generalized additive models were applied to detect nonlinear effects of continuous covariates if needed, and statistical tools for regression diagnostics, such as verification of proportional hazards assumption, residual analysis, detection of influential cases, and check for multicollinearity, were used to discover model or data problems.
Results
Demographic Characteristics and Laboratory Data
There were 580 patients entered into the final analysis. Their mean age was 44.4 years, with 58.5% being male. By December of 2008, the median follow-up period was 5.9 (interquartile range=5.7) years. The most common GN was MN (n=209) followed by FSGS (n=132), IgAN (n=130), and MCD (n=109). As shown in Table 1, patients with MN were older, and together with patients with MCD, they were mostly male. Diabetes was most prevalent in patients with MN, and hypertension occurred more frequently in patients with FSGS.
Table 1.
Baseline demographic characteristics and laboratory data in patients with different types of primary glomerulonephritis
| Demography and Biochemistry | Total (n=580) | FSGS (n=132) | IgAN (n=130) | MCD (n=109) | MN (n=209) | P Valuea |
|---|---|---|---|---|---|---|
| Age (years) | 44.4±16.8 | 44.3±15.1 | 34.5±12.1 | 35.7±15.9 | 55.2±14.3 | <0.001 |
| Male (%) | 58.5 | 57.6 | 46.9 | 63.3 | 63.6 | 0.02 |
| Diabetes (%) | 7.9 | 8.3 | 2.3 | 4.6 | 13.9 | 0.002 |
| Hypertension (%) | 32.5 | 48.5 | 25.4 | 21.1 | 33.0 | <0.001 |
| Serum albumin (g/dl) | 3.0±1.0 | 3.2±1.0 | 3.9±0.7 | 2.7±1.2 | 2.5±0.7 | <0.001 |
| Hemoglobin (g/dl) | 13.0±2.2 | 12.8±2.1 | 12.5±2.3 | 14.2±1.9 | 12.7±2.1 | <0.001 |
| BUN (mg/dl) | 22.9±17.6 | 30.1±20.3 | 24.2±22.2 | 19.7±13.0 | 19.0±12.3 | <0.001 |
| Serum creatinine (mg/dl) | 1.5±1.3 | 1.8±1.3 | 1.8±1.7 | 1.2±0.9 | 1.3±1.2 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 70.4±33.8 | 56.8±33.7 | 63.4±32.7 | 87.3±34.0 | 74.6±29.6 | <0.001 |
| ≥90 (%) | 27.6 | 13.5 | 17.7 | 48.6 | 31.6 | <0.001 |
| 60–89 (%) | 34.1 | 25.6 | 35.4 | 33.1 | 39.7 | <0.001 |
| 30–59 (%) | 25.5 | 39.9 | 29.2 | 11.9 | 21.1 | <0.001 |
| 15–29 (%) | 8.8 | 16.5 | 8.5 | 4.6 | 6.2 | <0.001 |
| <15 (%) | 4.0 | 4.5 | 9.2 | 1.8 | 1.4 | <0.001 |
| Serum cholesterol (mg/dl) | 311±151 | 288±159 | 217±78 | 414±198 | 328±109 | <0.001 |
| Serum triglyceride (mg/dl) | 223±161 | 245±172 | 133±72 | 246±152 | 252±178 | <0.001 |
| Proteinuria | ||||||
| mild (1+ or 2+; %) | 28.7 | 31.1 | 56.3 | 25.9 | 11.6 | <0.001 |
| severe (>3.5g/d or ≥3+; %) | 71.3 | 68.9 | 43.7 | 74.1 | 88.4 | <0.001 |
| Steroid treatment alone (%) | 42.0 | 19.7 | 30.0 | 64.2 | 52.2 | <0.001 |
| Other cytotoxic agents alone (%) | 1 | 1 | 0 | 0 | 1 | |
| Combined treatment of steroid and cytotoxic agents (%) | 18.1 | 30.0 | 6.2 | 8.3 | 23.0 | <0.001 |
| Intervals between onset of disease and date of biopsy (days) | 12.7 | 10.3 | 10.7 | 12.8 | 15.4 | <0.001 |
FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy; eGFR, estimated GFR as calculated by the simplified Modification of Diet in Renal Disease formula: 186×(creatinine [mg/dl])−1.154×(age [years])−0.203×0.742 (if female).
All P values were obtained from one-way ANOVA for testing the equality in population means among the FSGS, IgAN, MCD, and MN groups of patients.
MN patients had the highest mean urine protein and lowest mean serum albumin. MCD patients had the highest mean hemoglobin and total cholesterol. BUN and serum creatinine were higher in FSGS and IgAN groups. Serum triglyceride of IgAN patients was lower compared with other groups.
Patient Outcomes
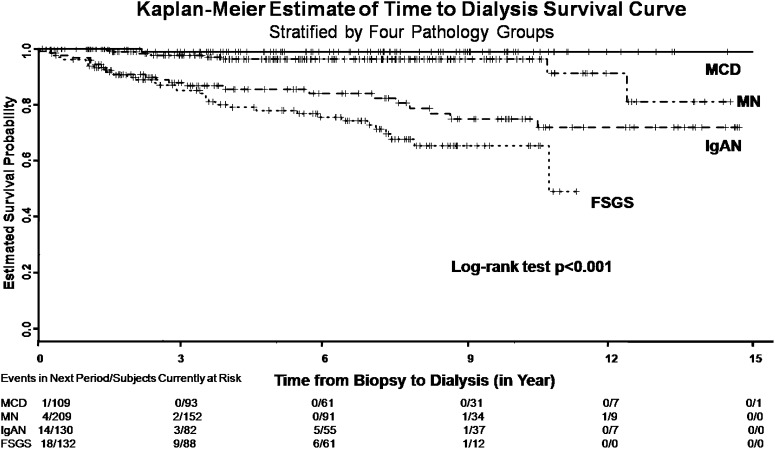
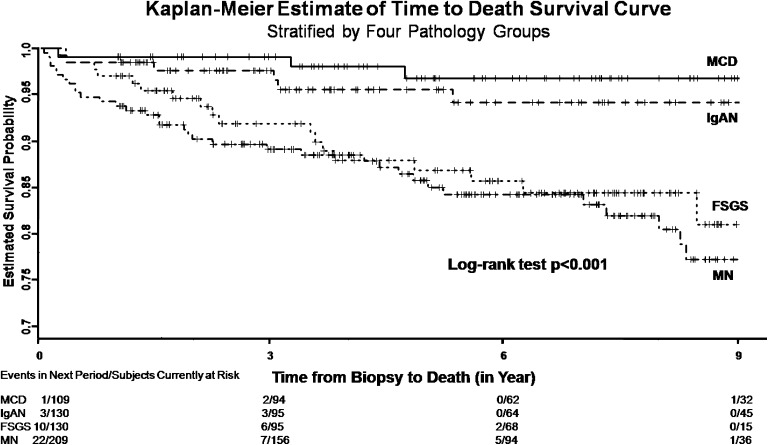
There were significant differences in the use of corticosteroids (Table 1). Patients with MN and MCD received corticosteroid treatment more frequently than the other two groups that had less severe proteinuria and higher serum creatinine. At the end of follow-up period, 69 (11.9%) patients developed ESRD, and 65 (11.2%) patients died from any cause (Table 2). By Kaplan–Meier survival analysis, we found that patients with FSGS developed ESRD more rapidly than the other three groups (Figure 2). The proportion of ESRD was significantly higher in the FSGS (25.8%) and IgAN (19.2%) groups (P<0.001) (Table 2). By contrast, patients with MN reached death most rapidly (Figure 3), and the proportion of overall death was significantly higher in the MN (17.2%) and FSGS (14.4%) groups (P<0.001) (Table 2). To know the renal progression of the different cohorts over the observation period, we were able to calculate the decline rates of eGFR in 417 (72%) patients who were followed up in this center after the biopsy (Table 3). Patients who developed ESRD exhibited a significantly faster renal progression than those patients who did not (10.6 versus 2.1 ml/min per 1.73 m2 per year, P<0.001). Overall, patients with FSGS, MN, and IgAN did not differ significantly in renal progression, but all displayed a faster decline rate of eGFR than those patients with MCD.
Table 2.
Dialysis dependency and overall mortality in patients with different types of primary glomerulonephritis
| Outcome | Total (n=580) | FSGS (n=132) | IgAN (n=130) | MCD (n=109) | MN (n=209) | P Valuea |
|---|---|---|---|---|---|---|
| Dialysis dependency number (%) | 69 (11.9) | 34 (25.8) | 25 (19.2) | 1 (0.9) | 9 (4.3) | <0.001 |
| Overall mortality number (%) | 65 (11.2) | 19 (14.4) | 6 (4.6) | 4 (3.7) | 36 (17.2) | <0.001 |
FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy.
The P values were obtained from the chi-squared tests for testing the equality in population proportions among the FSGS, IgAN, MCD, and MN groups of patients.
Figure 2.
Renal survival in patients with the four most common types of primary glomerulonephritis. FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy.
Figure 3.
Patient survival in patients with the four most common types of primary glomerulonephritis. FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy.
Table 3.
Renal progression in patients with different types of primary glomerulonephritis
| eGFR Decline Rate | Total (n=417) | FSGS (n=106) | IgAN (n=94) | MCD (n=69) | MN (n=148) | P Value |
|---|---|---|---|---|---|---|
| Total (ml/min per 1.73 m2 per year) | 3.1±17.8 | 4.6±17.6 | 3.1±15.5 | 0.5±17.5 | 3.2±19.4 | 0.003 |
| Dialysis dependent patients (ml/min per 1.73 m2 per year) | 10.6±19.8 (n=50) | 11.3±20.3 (n=26) | 8.8±14.7 (n=19) | 7.0 (n=1) | 16.0±40.6 (n=4) | 0.88 |
| Non-dialysis dependent patients (ml/min per 1.73 m2 per year) | 2.1±17.3 (n=367) | 2.5±16.3 (n=80) | 1.7±15.4 (n=75) | 0.4±17.6 (n=68) | 2.9±18.7 (n=144) | 0.01 |
eGFR, estimated GFR; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy.
Predictors for Dialysis Dependence and Overall Mortality
A stepwise likelihood ratio model of the Cox proportional hazards method was used to identify independent predictors for dialysis dependence and all-cause mortality. Table 4 shows predictors for ESRD, including the FSGS group (hazard ratio [HR]=34.64, 95% confidence interval [CI]=2.68–447.38, P=0.007), IgAN patients with hypertension (HR=6.92, 95% CI=1.83–26.22, P=0.004), IgAN patients with higher proteinuria (HR=3.05, 95% CI=1.68–5.54, P<0.001), MN patients with higher proteinuria (HR=2.98, 95% CI=1.62–5.47, P<0.001), FSGS patients with higher proteinuria (HR=1.80, 95% CI=1.18–2.73, P=0.006), patients higher serum albumin (HR=1.74, 95% CI=1.17–2.60, P=0.007), patients higher serum creatinine (HR=1.49, 95% CI=1.25–1.78, P<0.001), and patients higher serum triglycerides (HR=1.003, 95% CI=1.001–1.004, P<0.001). By contrast, predictors against ESRD were male sex (HR=0.49, 95% CI=0.25–0.92, P=0.03) and higher hemoglobin (HR=0.64, 95% CI=0.54–0.77, P<0.001).
Table 4.
Multivariate analyses of predictors for time to dialysis
| Variablea | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Male | 0.49 | 0.25–0.92 | 0.03 |
| FSGS versus others | 34.64 | 2.68–447.37 | 0.007 |
| Baseline serum albumin | 1.74 | 1.17–2.60 | 0.007 |
| Baseline serum triglyceride | 1.003 | 1.001–1.004 | <0.001 |
| Baseline hemoglobin | 0.64 | 0.54–0.77 | <0.001 |
| Baseline serum Cre | 1.49 | 1.25–1.78 | <0.001 |
| IgAN × HTN | 6.92 | 1.83–26.22 | 0.004 |
| FSGS × Upro | 1.81 | 1.18–2.73 | 0.006 |
| IgAN × Upro | 3.05 | 1.68–5.54 | <0.001 |
| MN × Upro | 2.99 | 1.62–5.47 | <0.001 |
CI, confidence interval; FSGS, focal and segmental glomerulosclerosis; Cre, creatinine; IgAN, IgA nephropathy; HTN, hypertension; Upro, urine protein; MN, membranous nephropathy.
The symbol × indicates the interaction between two covariates, which could be literally interpreted as and or times in this table (for example, FSGS × Upro means FSGS times the amount of urine protein).
For overall mortality (Table 5), MN with higher proteinuria (HR=1.69, 95% CI=1.24–2.32, P=0.001), FSGS with higher serum creatinine (HR=1.46, 95% CI=1.19–1.80, P<0.001), and older age (HR=1.08, 95% CI=1.06–1.10, P<0.001) were independent predictors, whereas MN with high serum albumin (HR=0.54, 95% CI=0.33–0.08, P=0.01) was a strong protector. A longer time to dialysis (HR=1.21, 95% CI=0.99–1.48, P=0.06) and IgAN with hypertension (HR=2.81, 95% CI=0.88–9.05, P=0.08) were marginally significant risk factors for death from any cause. Finally, the average interval between onset of renal abnormalities and date of renal biopsy was obtained in 562 (97%) patients (Table 1) and used as a parameter in a separate set of multivariate analysis. The results showed that it was not an independent predictor for overall clinical outcomes (data not shown).
Table 5.
Multivariate analyses of predictors for time to death
| Variablea | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Age (years) | 1.08 | 1.06–1.10 | <0.001 |
| FSGS × baseline serum Cre | 1.46 | 1.19–1.80 | <0.001 |
| MN × Upro | 1.69 | 1.24–2.32 | 0.001 |
| MN × baseline serum albumin | 0.54 | 0.33–0.08 | 0.01 |
| IgAN × HTN | 2.81 | 0.88–9.05 | 0.08 |
| Time to dialysis (days) | 1.21 | 0.99–1.48 | 0.06 |
CI, confidence interval; FSGS, focal and segmental glomerulosclerosis; Cre, creatinine; MN, membranous nephropathy; Upro, urine protein; IgAN, IgA nephropathy; HTN, hypertension.
The symbol × indicates the interaction between two covariates, which could be literally interpreted as and or times in this table (for example, FSGS × serum Cre means FSGS times the amount of serum creatinine).
Discussion
This study is the first to provide the long-term outcomes of patients with primary GN in an ethnic Chinese population in Taiwan. In many Asian countries, GN remains one, if not the most, common cause of ESRD (11–13,15–18,20,22). In Taiwan, where CKD is endemic and the incidence of ESRD is the highest in the world, chronic GN is still the most common cause of prevalent ESRD and the second most common cause of newly diagnosed ESRD (19,20). As shown in Table 2, patients with MCD, as expected (25,26), fared the best in terms of renal outcome and patient survival, which was apparently because of the benign nature of this disease (i.e., good response to corticosteroids and young age). Patients with FSGS had the worst renal survival, which might be because of more renal insufficiency and hypertension at presentation. This observation is comparable with previous reports that have shown that 50% of FSGS patients develop ESRD within 8 years (27,28) and that the overall renal survival of FSGS is around 50%–80% (7). Nevertheless, our patients with FSGS had a better 5-year renal survival compared with Western countries (29,30).
Patients with FSGS were more prone to develop ESRD in the face of a relatively poor patient survival, suggesting that ESRD and death may not be competing outcomes in this disease entity. Also, many of our patients with IgAN lived to develop ESRD. These observations were consistent with the idea that most CKD patients in Asia tend to develop ESRD before dying from any cause (22,31,32). However, the outcomes of our patients with MN were similar to their Western counterparts, with more patients dying than developing ESRD (33,34).
In this cohort, MN patients were older and had more comorbidities such as diabetes and hypertension, which might contribute to the highest mortality rate. Conversely, patients with IgAN were younger and had fewer comorbidities. These characteristics might explain, at least in part, why the IgAN patients took longer to develop ESRD as opposed to their MN or FSGS counterparts. Furthermore, age was found to be a significant risk factor for overall death in this cohort. This finding could partially explain the observations that MN with higher proteinuria and lower serum albumin and FSGS with renal insufficiency were independent predictors for death from any cause given the relatively older age of the patients with these two types of GN.
Heavy proteinuria, renal insufficiency and hypertension are well known risk factors for the subsequent development of ESRD in patients with primary GN (5). Previous studies have reported that predictors for ESRD in IgAN consist of elevated serum creatinine, hypertension, and higher urine protein (35,36). In FSGS, severe anemia and poor treatment response (37,38) are predictors along with the above-mentioned factors, and in MN, advanced glomerular histopathology (stages III and IV), male sex, older age, nephrotic syndrome, hypertension, reduced GFR at presentation, and poor treatment response are predictors along with the above-mentioned factors (39,40). Our multivariate analysis also found that most of these factors were associated with the outcomes of primary GN across all histologic types, specifically renal insufficiency in FSGS, hypertension or higher proteinuria in IgAN, and higher proteinuria in MN. Additionally, diabetes and anemia have been considered as predictors for renal progression in primary MN, FSGS, and IgAN (7). However, in this study, we did not find diabetes to be a risk factor for ESRD, although we did notice that higher hemoglobin at diagnosis protected against the development of ESRD across all GN types.
Apart from the traditional risk factors, high serum albumin at diagnosis was identified as a novel predictor for ESRD in this study. Obviously, indications for renal biopsy in patients with high serum albumin could not be full-blown nephrotic syndrome but conditions such as renal insufficiency or persistent urinary abnormalities, both of which are strong predictors for renal progression (7,30,31). Indeed, the level of serum albumin was the highest in patients with IgAN who had ESRD rates that were the second highest in this cohort. However, hypertriglyceridemia was also a predictor for ESRD in our study. The impact of hypertriglyceridemia on the development of ESRD in GN patients has seldom been documented before (41). Hypertriglyceridemia is one of the components of metabolic syndrome. Previous studies in CKD patients have shown that, as the components of metabolic syndrome increase, the risk of ESRD rises proportionately (42,43). Whether this causal relationship found in CKD can be applied to primary GN awaits additional studies.
The influence of sex on GN progression remains uncertain. The work by Moranne et al. (7) found no association between sex and progression of GN, whereas the work by Cattran et al. (10) reported that women in North America with MN and FSGS but not IgAN had a better renal survival than their male counterparts. This sex benefit in outcome could be attributed to lower proteinuria and BP at presentation and throughout follow-up. In our cohort, we found that male patients were less prone to develop ESRD than women, suggesting that the impact of sex on GN outcome might vary by geography or ethnicity. Alternatively, because more men were diagnosed with MCD and MN, with rates of ESRD that were lower than the rates in men diagnosed with FSGS and IgAN, we could not exclude the existence of bias arising from overrepresentation of MCD and MN.
This study is unique in that it identifies predictors for clinical outcomes among a pool of the four most important and common types of primary GN. This study allowed for comparisons of the relative impact of such risk factors as proteinuria and hypertension on distinct glomerular pathologies, and it can help to decide the intensity of therapy targeting these risk factors according to specific histologic variants. However, the results accrued from this study are limited by the retrospective nature of the design and its location at a single medical center. Furthermore, there might exist two types of lead time bias in this study. One bias was the lead time bias from different timing of biopsy. However, most patients in this study received a renal biopsy within 2 weeks, when clinically significant manifestations (i.e., moderate to heavy proteinuria and renal insufficiency) were found. Also, we chose date of biopsy as our starting time point to minimize recall errors, and we found that intervals between onset of disease and date of biopsy did not predict overall outcomes. The other type of lead time bias arose from different timing of initiation of dialysis. In this study, the endpoint of ESRD was decided by the time of application for a Major Catastrophic Illness issued by the Bureau of National Health Insurance in Taiwan, which to our knowledge, is quite stringent (7) and meets the current international recommendations (8,9). Thus, the lead time bias was a potential limitation of our study, but its impact might be low. Another limitation was that renal tubular handling of creatinine is altered in patients with nephrotic syndrome (10), and therefore, any creatinine-based eGFR formula may not be as valid in patients with nephrotic syndrome. For example, the MDRD4 formula (simplified MDRD with four variables: creatinine, age, sex, and race), which was used in the study, tends to overestimate GFR as measured by inulin clearance in patients with nephrotic syndrome (44). Nevertheless, this uncertainty does not interfere with the predictive power of baseline creatinine for time to dialysis; more patients in our MCD and MN groups exhibited heavy proteinuria and hypoalbuminuria (and their eGFR might be falsely high), but their renal outcome still fared better than either the IgAN or FSGS group. Future multicenter, prospective cohort studies are needed to evaluate the external validity of this study and investigate whether therapeutic interventions targeting the predictors identified here can be of help in reducing ESRD and overall death.
In conclusion, we found that patients with FSGS and IgAN were more prone to develop ESRD than death from any cause. Most predictors for ESRD and overall mortality found in this ethnic Chinese cohort were similar to other studies. However, some risk factors linked with distinct glomerular pathologies displayed differential clinical outcomes. Thus, greater therapeutic efforts should be focused particularly on patients presenting with these clinicopathological features.
Disclosures
None.
Acknowledgments
This work was supported by grants from the National Taiwan University Hospital (98-S1352), the Bureau of Health Promotion (DOH98-HP-1111), the Ta-Tung Kidney Foundation, and the Mrs. Hsiu-Chin Lee Kidney Research Fund, Taipei, Taiwan.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lysaght MJ: Maintenance dialysis population dynamics: Current trends and long-term implications. J Am Soc Nephrol 13[Suppl 1]: S37–S40, 2002 [PubMed] [Google Scholar]
- 2.Moeller S, Gioberge S, Brown G: ESRD patients in 2001: Global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant 17: 2071–2076, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Francesco PS: Epidemiology of end-stage renal disease: International comparisons of dialysis. Kidney Int Suppl 57: S39–S45, 2000 [Google Scholar]
- 4.Rivera F, López-Gómez JM, Pérez-García R, Spanish Registry of Glomerulonephritis : Clinicopathologic correlations of renal pathology in Spain. Kidney Int 66: 898–904, 2004 [DOI] [PubMed] [Google Scholar]
- 5.D’Amico G: Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis 20: 315–323, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Deegens JK, Wetzels JF: Diagnosis and treatment of primary glomerular diseases. Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy. Minerva Urol Nefrol 57: 211–236, 2005 [PubMed] [Google Scholar]
- 7.Moranne O, Watier L, Rossert J, Stengel B, GN-Progress Study Group : Primary glomerulonephritis: An update on renal survival and determinants of progression. QJM 101: 215–224, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Heaf J, Løkkegaard H, Larsen S: The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant 14: 1889–1897, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Simon P, Ramee MP, Boulahrouz R, Stanescu C, Charasse C, Ang KS, Leonetti F, Cam G, Laruelle E, Autuly V, Rioux N: Epidemiologic data of primary glomerular diseases in western France. Kidney Int 66: 905–908, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW, Troyanov S, Genes, Gender and Glomerulonephritis Group : The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 23: 2247–2253, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cai GY, Chen XM: Immunoglobulin A nephropathy in China: Progress and challenges. Am J Nephrol 30: 268–273, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H: The changing spectrum of primary glomerular diseases within 15 years: A survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 24: 870–876, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Research Group on Progressive Chronic Renal Disease : Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Nephron 82: 205–213, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S: Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65: 1870–1876, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, Kang SW, Choi KH, Han DS, Jeong HJ, Lee HY: Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant 24: 2406–2410, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Woo KT, Chiang GS, Edmondson RP, Wu AY, Lee EJ, Pwee HS, Lim CH: Glomerulonephritis in Singapore: An overview. Ann Acad Med Singapore 15: 20–31, 1986 [PubMed] [Google Scholar]
- 17.Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA: Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol 13: 38–43, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Chan MK, Yin PD, Chan KW: Primary glomerulonephritis in Hong Kong. Int Urol Nephrol 20: 413–420, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Yang WC, Hwang SJ, Taiwan Society of Nephrology : Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: The impact of national health insurance. Nephrol Dial Transplant 23: 3977–3982, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Heaf J: The Danish Renal Biopsy Register. Kidney Int 66: 895–897, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Iseki K, Miyasato F, Uehara H, Tokuyama K, Toma S, Nishime K, Yoshi S, Shiohira Y, Oura T, Tozawa M, Fukiyama K: Outcome study of renal biopsy patients in Okinawa, Japan. Kidney Int 66: 914–919, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Greene T, Kusek J, Beck GJ, Group MS: A simplified equation to predict glomerular filtration rate from serum creatinine (Abstract). J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 25.Saha TC, Singh H: Minimal change disease: A review. South Med J 99: 1264–1270, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Korbet SM, Schwartz MM, Lewis EJ: Primary focal segmental glomerulosclerosis: Clinical course and response to therapy. Am J Kidney Dis 23: 773–783, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM: Focal segmental glomerular sclerosis in adults: Presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Deegens JK, Steenbergen EJ, Borm GF, Wetzels JF: Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population—epidemiology and outcome. Nephrol Dial Transplant 23: 186–192, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Chiu YL, Chien KL, Lin SL, Chen YM, Tsai TJ, Wu KD: Outcomes of stage 3-5 chronic kidney disease before end-stage renal disease at a single center in Taiwan. Nephron Clin Pract 109: c109–c118, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 33.O’Callaghan CA, Hicks J, Doll H, Sacks SH, Cameron JS: Characteristics and outcome of membranous nephropathy in older patients. Int Urol Nephrol 33: 157–165, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Cattran DC: Outcomes research in glomerulonephritis. Semin Nephrol 23: 340–354, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Shimizu A, Takei T, Uchida K, Tsuchiya K, Nitta K: Predictors of poor outcomes in steroid therapy for immunoglobulin A nephropathy. Nephrology (Carlton) 14: 521–526, 2009 [DOI] [PubMed] [Google Scholar]
- 36.D’Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Alaleh G, Hassan O, Abbas M: Prediction of kidney survival in children with primary focal segmental glomerulosclerosis (a two-center study). JRMS 12: 107–111, 2007 [Google Scholar]
- 38.Schnaper HW: Idiopathic focal segmental glomerulosclerosis. Semin Nephrol 23: 183–193, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Marx BE, Marx M: Prediction in idiopathic membranous nephropathy. Kidney Int 56: 666–673, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Glassock RJ: Diagnosis and natural course of membranous nephropathy. Semin Nephrol 23: 324–332, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Syrjänen J, Mustonen J, Pasternack A: Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 15: 34–42, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Lee PH, Chang HY, Tung CW, Hsu YC, Lei CC, Chang HH, Yang HF, Lu LC, Jong MC, Chen CY, Fang KY, Chao YS, Shih YH, Lin CL: Hypertriglyceridemia: An independent risk factor of chronic kidney disease in Taiwanese adults. Am J Med Sci 338: 185–189, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Hadjadj S, Duly-Bouhanick B, Bekherraz A, BrIdoux F, Gallois Y, Mauco G, Ebran J, Marre M: Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab 30: 43–51, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Hofstra JM, Willems JL, Wetzels JFM: Estimated glomerular filtration rate in the nephrotic syndrome. Nephrol Dial Transplant 26: 550–556, 2011 [DOI] [PubMed] [Google Scholar]