Abstract
Objective
Embolism from a proximal source to the retinal circulation could be a sign of embolism from the same source to the hemispheric circulation. We sought to determine the frequency of acute brain infarcts on diffusion-weighted imaging (DWI) in patients with monocular visual loss of presumed ischemic origin (MVL).
Methods
We retrospectively studied 129 consecutive patients with MVL secondary to retinal ischemia. All patients underwent DWI, comprehensive ophthalmologic and neurologic examination, and diagnostic evaluations for the underlying etiology. Statistical analyses explored univariable and multivariable predictors of DWI evidence of acute brain infarcts.
Results
DWI revealed concurrent acute brain infarct(s) in 31 of the 129 patients (24%). The probability of positive DWI was higher in embolic versus non-embolic MVL (28% vs. 8%, p=0.04), in MVL characterized by permanent visual loss versus transient symptoms (33% vs. 18%, p=0.04), and in MVL associated with concurrent hemispheric symptoms versus isolated MVL (53% vs. 20%, p<0.01). Patients with positive DWI were more likely to harbor a major underlying etiology as compared to those with normal DWI (OR 3.7, 95% CI 1.5–9.4).
Interpretation
This study demonstrates that MVL does not always represent an isolated disease of the retina; approximately one out of every four patients with MVL demonstrates acute brain infarcts on DWI. Since patients with concurrent brain infarcts are more likely to exhibit a cardiac or vascular source of embolism, imaging evidence of brain injury in patients with MVL may be a useful marker to guide the timing and extent of the diagnostic examinations.
INTRODUCTION
The distribution of embolic material within a vascular tree depends on several factors including angulation of bifurcations, relative flow within each vascular branch, and physical properties of the embolic material.1, 2 The ophthalmic artery arises at a perpendicular angle from the supraclinoid internal carotid artery (ICA) and is only one fifth of the diameter of the ICA.3, 4 Since suspended particles in the blood tend to remain in the axial stream,5, 6 the probability of emboli entering into the ophthalmic artery is lower than that of traveling into the hemispheric circulation. Therefore, if an embolus happens to enter into the ophthalmic system, many others may be distributed to the hemispheric system and possibly cause acute infarcts detectable by brain imaging. In this study, we sought to identify the prevalence of acute ischemic lesions on diffusion-weighted imaging (DWI) in patients with monocular visual loss (MVL) caused by ischemia in the retinal circulation.
PATIENTS AND METHODS
We studied a consecutive set of patients who presented to our Emergency Department with the chief complaint of MVL between the years 2000 and 2008. All patients underwent a comprehensive ophthalmologic evaluation by a neurologist or ophthalmologist. Ophthalmologic evaluation included assessment of the visual acuity, visual fields, pupils, anterior segment, ocular motility, and dilated fundoscopic examinations. Brain imaging was obtained in all patients with no obvious ocular etiologies and optic nerve disorders. The presumed mechanism of MVL was considered to be ischemia within the retinal arterial system when there were primarily “negative” symptoms denoting loss of function (monocular loss of vision including blurring, fogging, dimming partial or complete blackness), altitudinal field defect, visual loss lasting 2–30 minutes, or when typical angiographic or fundoscopic evidence of retinal ischemia (cherry red spots, arterial plaques, absence of vascular filling) were seen.7 Exclusively “positive” symptoms (stars, bright lights, colors) were not deemed to be related to an ischemic event. The study protocol was approved by the local institutional review board.
The standard evaluation in patients with MVL secondary to retinal ischemia at our center included blood tests (blood cell counts, blood chemistry, erythrocyte sedimentation rate, activated partial thromboplastin time, and prothrombin time). brain imaging, EKG, echocardiography, Holter monitoring if EKG and echocardiography did not reveal a cardiac source, imaging of the brain and intra- and extra-cranial vessels (ultrasonography, CT-angiography, or MR-angiography), and other specific tests such as CSF analysis, antiphospholipid antibodies, haemostatic markers, genetic markers, and vasculitis markers depending upon the level of suspicion of a particular etiology. Hypercoagulability markers including homocysteine level, protein C and S levels and activity, antithrombin III level, and fibrinogen level were studied in 82% of the patients. Stroke etiology was classified using the automated Causative Classification of Stroke (CCS) software.8 The CCS system provided etiologic stroke subtypes in five domains (large artery atherosclerosis, cardio-aortic embolism, small artery occlusion, other rare causes, and undetermined causes). The system further classified each subtype into three levels of confidence based on relative strength of associations between MVL and the underlying pathology as “evident”, “probable”, and “possible”. An etiology was considered “major” when final subtype assignment was either evident or probable and “minor” when it was possible. The CCS system classified multiple competing evident etiologies into the category of “undetermined-unclassified”. For the purpose of this study, undetermined-unclassified etiology was also considered to be a major etiology.
Brain MRI included T1-, T2-, and FLAIR-sequences and diffusion-weighted images (DWI). MRI was performed on 1.5 Tesla scanners (GE Signa; GE Medical Systems, Milwaukee, WI; or Siemens Sonata; Siemens Medical Solutions, Erlangen, Germany). DWI was obtained using echo-planar imaging with a repetition time of 6000–10000 ms, an echo time of 78–101 ms, a field of view of 22×22 cm, image matrix of 128×128, slice thickness 5–6 mm with a 1-mm gap, and b-values of 0 s/mm2 and 1000 s/mm2. All images were evaluated by a neuroradiologist for the presence of acute ischemic lesions. Lesions that were hyperintense on DWI and hypo- or normointense on the apparent diffusion coefficient maps were considered acute ischemic lesions. Since small infarcts remain visible on DWI only for 7–14 days,9 the analyses were restricted to patients who underwent DWI within seven days of symptom onset. The choice of imaging in this study was DWI because DWI posed the unique ability to distinguish acute infarcts from chronic lesions suggesting a link between brain infarct and index clinical event.10 Ischemic lesions on DWI were manually outlined and lesion volumes were calculated using MRIcro software (University of Nottingham, UK).
We classified MVL that lasted less than 24 hours as “transient”, and longer than 24 hours as “permanent”. The site of retinal vascular occlusion was classified as “proximal” (central retinal artery) and “distal” (branch retinal artery) based on retinal angiography findings and the extent of visual field loss. Complete monocular visual field loss with or without central sparing denoted a “proximal” occlusion whereas incomplete visual field loss such as altitudinal field defects designated a “distal” occlusion. We classified the mechanism of MVL into “embolism-probable” and “embolism-uncertain” categories. Embolism was considered to be the probable mechanism when there was angiographic or fundoscopic evidence of embolic material in the retinal circulation or when there was a major cardiac or vascular embolic source in the absence of a concurrent alternative causative mechanism but no history of recurrent episodes of short lasting stereotypic MVL events accompanied by hypotensive episodes, severe proximal arterial stenoses, or increased retinal oxygen demand.11 The presumed mechanism was considered “embolism-uncertain” in all MVL events not conforming to the criteria described for “embolismprobable”.
Statistical analyses explored the relationship between probability of acute ischemic brain lesions or major stroke etiology and clinical MVL characteristics. Differences in baseline categorical and continuous variables among study groups were compared with Chi-square test and Mann-Whitney U or Student t-test, respectively. We constructed logistic regression models to identify independent predictors of acute infarcts on DWI as well as predictors of underlying major stroke etiology. These models included baseline patient features, clinical characteristic of monocular visual symptoms, and imaging findings with a univariate p value < 0.1 as covariates. Standard regression diagnostics were used to assess logistic regression assumptions. All numerical variables were expressed as mean ± standard deviation (SD) or median (interquartile range). Associations were presented as odds ratios (OR) with corresponding 95% confidence intervals (95% CI). A level of p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.5.
RESULTS
A total of 397 patients were admitted to the emergency department with the complaint of MVL during the study period. We excluded 69 patients in whom the initial examination revealed binocular visual field loss. We further excluded 178 patients in whom the final diagnosis was an ocular or neurological condition other than retinal ischemia. These included intraocular pathologies (n=37), brain or orbital tumor (n=33), migraine (n=25), syncope (n=25), and other neurological conditions (such as multiple sclerosis, aneurysm, arterio-venous malformation, hydrocephalus, etc. n=58). Of the remaining 150 patients with MVL of presumed retinal artery ischemia, 19 were excluded due to unavailability of DWI (MRI not obtained at the discretion of the treating physician in 10 and contraindications to MRI in 9 patients). Two other patients were excluded because of severe motion artifacts on MRI that prevented reliable interpretation of the images. The remaining 129 patients comprised the study population. The MVL characteristics, vascular risk factors, and diagnostic evaluation findings were not different between the target study population and the 21 patients excluded due to lack of MRI.
The baseline characteristics of the study population are summarized in the Table 1. There was an acute ischemic lesion on DWI in 31 patients (24%). Brain infarcts were in an appropriate location to the index MVL in all but three patients; the DWI lesion was in the posterior circulation in 1 and in the contralateral hemisphere in 2 of the 3 patients with inappropriate lesions. DWI lesions were typically very small; their volume changed between 0.1ml and 1ml. The mean volume was 0.2ml (95% confidence intervals: 0.1ml – 0.7ml) (Figure). The brain lesions were multiple in 20 (65%) and single in 11 (35%) patients; there were 2 lesions in 3, 3 lesions in 2, and 4 or more lesions in 15 patients. Infarcts were exclusively located in deep gray matter in 2, subcortical white matter in 3, cortex in 8, and both cortex and subcortical white matter in 18 patients. The probability of concurrent acute infarcts on DWI was higher in embolic as compared non-embolic MVL (28% vs. 8%, p=0.04) and in permanent as compared to transient MVL (33% vs. 18%, p=0.04) (Table 2). Monocular visual symptoms occurred simultaneously with hemispheric symptoms in 17 patients. DWI was more often positive in patients with accompanying hemispheric symptoms as compared to patients presenting with isolated MVL (53% vs. 20%, p<0.01). A logistic regression model revealed the presence of hemispheric symptoms as the only significant variable associated with acute ischemic lesions on DWI (OR=4.5, 95% CI 1.5–13.5; p<0.01).
Table 1.
Baseline characteristics of the study population
| Overall population N=129 |
DWI | Major Etiology | |||||
|---|---|---|---|---|---|---|---|
| Positive N=31 |
Negative N=98 |
p | Positive N=71 |
Negative N=58 |
p | ||
| Age, years (mean ± SD) | 64 ± 16 | 67 ± 15 | 63 ± 16 | P=0.26 | 65 ± 15 | 54 ± 17 | P=0.62 |
| Female gender, n (%) | 61 | 16 (26) | 45 (74) | P=0.58 | 29 (48) | 32 (52) | P=0.11 |
| Hypertension, n (%) | 76 | 20 (26) | 56 (74) | P=0.47 | 46 (61) | 30 (29) | P=0.13 |
| Diabetes mellitus, n (%) | 10 | 2 (20) | 8 (80) | P=1.00 | 4 (40) | 6 (60) | P=0.34 |
| Prior stroke or TIA, n (%) | 54 | 15 (28) | 39 (72) | P=0.40 | 33 (61) | 21 (39) | P=0.24 |
| Current smoking, n (%) | 29 | 8 (28) | 21 (72) | P=0.61 | 23 (79) | 6 (21) | P<0.01 |
| Mean arterial Blood Pressure, median, IQR | 96 (88–107) | 95 (84–105) | 97 (88–108) | P=0.51 | 95 (88–106) | 97 (88–109) | P=0.62 |
| Prior stereotypical MVL attacks, n (%) | 42 | 7 (17) | 35 (83) | P=0.17 | 23 (55) | 19 (45) | P=0.97 |
| Cardiovascular intervention within 14 days prior to MVL, n (%) | 9 | 4 (44) | 5 (56) | P=0.14 | 8 (89) | 1 (11) | P=0.03 |
| Time from symptom onset to MRI, hours (median, IQR) | 38 (23–60) | 43 (29–68) | 33 (20–58) | P=0.21 | 36 (24–62) | 29 (15–57) | p=0.19 |
“Major” etiology refers to high risk mechanisms such as atherosclerotic plaques causing greater than 50% stenosis, high risk cardiac sources, arterial dissection, vasculitis, etc. These are the mechanisms classified by the CCS system into known categories with an evident or probable level of confidence or into the category of undetermined-unclassified that designates multiple competing high-risk etiologies8.
Figure 1.
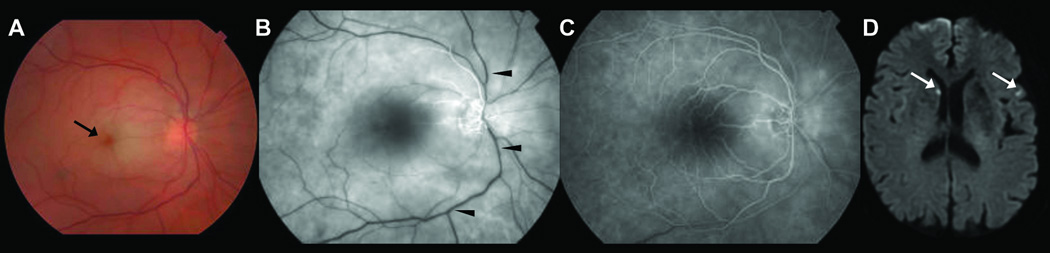
Retinal and brain images of a 69 year-old woman with paroxysmal atrial fibrillation who presented with isolated sudden visual loss in the right eye. Direct fundoscopic examination of the right eye reveals a cherry red spot (black arrow) with edema of the macula (A). Fluorescein fundoscopic and angiographic images at 28 seconds shows delayed arterial filling (B) which essentially normalizes by 65 seconds (C) consistent with retinal arterial occlusion (black arrowheads); Diffusion-weighted images of the brain reveal two discrete punctate acute infarcts, one in the right caudate head and the other one in the left temporal lobe (white arrows).
Table 2.
Clinical and imaging characteristics of the study population
| Overall population |
DWI | Major Etiology | |||||
|---|---|---|---|---|---|---|---|
| Positive N=31 |
Negative N=98 |
p | Positive N=71 |
Negative N=58 |
P | ||
| Site of retinal occlusion, n (%) | |||||||
| Distal | 61 | 12 (20) | 49 (80) | P=0.27 | 33 (54) | 28 (46) | P=0.84 |
| Proximal | 68 | 19 (28) | 49 (72) | 38 (56) | 30 (44) | ||
| Visual symptoms, n (%) | |||||||
| Isolated MVL | 112 | 22 (20) | 90 (80) | P<0.01 | 58 (52) | 54 (48) | P=0.07 |
| Isolated transient MVL | 66 | 9 (14) | 57 (86) | 30 (45) | 36 (54) | ||
| Isolated permanent MVL | 46 | 13 (28) | 33 (72) | 28 (61) | 18 (29) | ||
| MVL with concurrent hemispheric symptoms | 17 | 9 (53) | 8 (47) | 13 (76) | 4 (24) | ||
| Tempo of visual symptoms, n (%) | 129 | ||||||
| Transient MVL | 74 | 13 (18) | 61 (82) | P=0.05 | 37 (50) | 37 (50) | P=0.18 |
| Permanent MVL | 55 | 18 (33) | 37 (67) | 34 (62) | 21 (38) | ||
| Duration of transient visual symptoms, minutes (median, IQR) | 10 (4–30) | 20 (5–120) | 9 (3–30) | P=0.13 | 5 (3–15) | 13 (5–56) | p=0.21 |
| Angiography/fundoscopy findings | |||||||
| Embolic occlusion in retinal circulation | 55 | 17 (31) | 38 (69) | P=0.12 | 34 (62) | 21 (38) | P=0.18 |
| Normal retinal circulation | 74 | 14 (19) | 60 (81) | 37 (50) | 37 (50) | ||
| Mechanism, n (%) | |||||||
| Embolism-probable | 103 | 29 (28) | 74 (72) | P=0.04 | 55 (53) | 48 (47) | P=0.74 |
| Embolism-uncertain | 26 | 2 (8) | 24 (92) | 16 (62) | 10 (38) | ||
| Etiologic subtype, n (%) | |||||||
| Large artery atherosclerosis | 41 | 11 (27) | 30 (63) | P=0.22 | 41 (100) | 0 (0) | P<0.01 |
| Cardio-aortic embolism | 35 | 11 (31) | 24 (69) | 11 (31) | 24 (69) | ||
| Small artery occlusion | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Other uncommon causes | 18 | 5 (28) | 13 (72) | 18 (100) | 0 (0) | ||
| Undetermined causes | 35 | 4 (11) | 31 (89) | 1 (3) | 34 (97) | ||
| DWI, n (%) | |||||||
| Positive | 31 | - | - | - | 24 (77) | 7 (23) | p<0.01 |
| Negative | 98 | - | - | 47 (48) | 51 (52) | ||
Isolated visual symptoms refer to MVL unaccompanied by hemispheric symptoms.
All patients had vascular imaging studies (CT-angiography in 64, MR-angiography in 102, Doppler ultrasound 67 patients) and electrocardiography whereas 104 patients had echocardiography. Diagnostic investigations revealed an underlying etiology in 94 patients (Table 2). The probability of positive DWI was higher in patients with an identified etiology as compared to patients with undetermined etiology (34% vs. 12%, p<0.01). Seventy-one of the 94 patients with an identified etiology were classified to have a “major” etiology. Patients with a positive DWI more often harbored a major etiology as compared to those with normal DWI (OR 3.7, 95% CI 1.5–9.4). A logistic regression model demonstrated that DWI evidence of acute infarct on DWI was the only independent predictor of underlying major etiology (OR 3.3, 95%CI 1.3–8.4; p=0.02).
DISCUSSION
Patients with MVL often present to physicians with isolated visual symptoms. The present study demonstrates that MVL does not always represent an isolated disruption of the retinal circulation; approximately one out of every four patients with MVL presumed to be caused by ischemia in the retinal arterial system demonstrate DWI positive brain infarcts that have occurred either concurrently or closely related in time. These infarcts are typically small, often multiple, frequently occur in the hemisphere ipsilateral to the involved eye, and tend to remain asymptomatic. The probability of concurrent brain infarcts is higher in MVL presumed to be caused by an embolic mechanism as compared to non-embolic events and in permanent visual loss as compared to transient symptoms. The current study also shows that the identification of concurrent acute brain infarcts in a patient with MVL is important because it suggests the patient has an elevated risk of harboring a major underlying etiology that might be amenable to urgent treatment; patients with concurrent brain infarcts had approximately 50% higher risk of having a major etiology as compared to patients with normal brain imaging.
The course of the embolic material after it enters into the carotid circulation is one of the key determinants of the clinical symptoms – retinal, cerebral or both – that the patient is going to present with. It has been suggested that embolic particles enter into the ophthalmic artery, the first major branch off of the internal carotid artery, only when they are “small” enough to travel along the walls of the internal carotid artery.12 Suspended particles in the bloodstream are distributed along a radial gradient where large particles are more concentrated in the axial stream and small particles are in the peripheral streamline (Fahraeus-Lindqvist effect).5, 13 In a human cadaveric perfusion model, selective distribution to regions supplied by the most axial stream (watershed zones) was observed when particles around 200 micrometers were injected into the ICA.14 In contrast, there was no selective distribution to axial branches when particles less than 150 micrometers were injected. In another experiment, in an in-vitro model of unevenly bifurcating system, perfusion with 200 micrometer particles resulted in lower concentration of particles in smaller branches while particles less than 100 micrometers showed no relative concentration shift across branches of different size.14 Published evidence, thus, suggests that the critical size that determines selective distribution of embolic material into an unevenly branching artery (such as the ophthalmic artery) is around 150 micrometers. The size of most of the major arteries in the retinal circulation is, however, larger than 150 micrometers; the vascular diameter is 1600 micrometers (1500–1800 micrometers) at the origin of the ophthalmic artery from the ICA, 200 micrometers (100–400 micrometers) at the proximal central retinal artery, and 110 micrometers (60–160) at the major retinal branch arteries.3, 15 Thus, embolic particles that are large enough to preferentially travel in the axial streamline along the ICA can sometimes lodge into the retinal circulation causing concurrent retinal symptoms and brain lesions. In contrast, emboli small enough to travel in the peripheral streamline are preferentially distributed to the ophthalmic artery, occlude branch retinal arteries, and often cause partial, transient, and isolated MVL. It is possible that such small particles are also distributed into the hemispheric circulation. Nevertheless, they only rarely cause infarcts detectable by MRI possibly due to rapid spontaneous resolution or rich pial anastomoses,16 while their counterparts entering into the ophthalmic circulation lead to retinal symptoms.
Strengths of the present study include consecutive recruitment of subjects, stringent ascertainment of the diagnosis of MVL by detailed ophthalmologic and neurological assessment, and thorough diagnostic investigation for the underlying mechanism of MVL. Limitations include retrospective design and failure to obtain brain imaging in every patient. Although we collected data retrospectively through chart reviews, this is unlikely to have caused a systematic bias towards selection of a particular population because all patients admitted with the complaint of MVL underwent a standard battery of laboratory tests and physical examination. Even though we excluded 21 patients due to unavailability of DWI, baseline patient characteristics and MVL features (listed in Table 1) were similar between patients with and without MRI, arguing against a potential selection bias. Incidental small DWI lesions can occur in up to 15% of patients with diffuse small vessel disease associated with ischemic leukoaraiosis, amyloid angiopathy, or CADASIL. 17, 18 Nonetheless, the presence of a temporal relationship with MVL, the existence of a high-risk cardiac or arterial mechanism in the majority, and inappropriate location of most infarcts for small vessel disease make it unlikely that DWI lesions observed in the current study were incidental. Finally, it should be acknowledged that contamination of studies such as the present one by retinal ischemic events due to non-embolic mechanisms (such as hemodynamic failure or retinal vasospasm) is inevitable and such contamination obviously blurs the relationship between MVL and concurrent brain infarcts.
Our findings have implications for a wide range of physicians (including but not limited to primary care physicians, emergency physicians, ophthalmologists, neuro-ophthalmologists, internists, neurologists) involved in the care of patients with MVL by highlighting the need for urgency in obtaining brain imaging and performing a full diagnostic work-up in patients suspected to have had ischemic MVL. Although recent American Heart Association/American Stroke Association guidelines recommend that all patients with suspected brain or retinal ischemia should undergo urgent brain imaging and etiologic testing,19 the current practice in MVL does not a priori require brain imaging as a part of the diagnostic work-up.20, 21 A recent survey among US physicians shows that there is significant reluctance even in referring patients with MVL due to central retinal artery occlusion to an emergency room for urgent assessment;22 only 35% of ophthalmologists and 73% of neurologist reported sending such patients for immediate evaluation. In a study conducted in the Netherlands, only 72% of patients with transient MVL were referred by general practitioners to a specialist.23 Our findings suggest that patients presenting with symptoms consistent with ischemic MVL, whether it is isolated or accompanied by other neurologic symptoms, should be referred urgently for brain imaging to exclude concurrent brain ischemia. Given that up to 12% of untreated patients with brain ischemia develop a recurrent stroke in two weeks,24 urgent work-up of imaging positive patients with MVL would facilitate timely identification of the underlying etiology, early institution of specific preventive treatments, and reduction in risk a subsequent devastating stroke. Future studies are needed to elucidate whether MVL associated with concurrent brain infarcts poses a higher risk of subsequent stroke as compared to MVL without accompanying acute brain infarcts.
ACKNOWLEDGEMENTS
Dr Ay was funded by NIH grant R01-NS059710. Dr. Helenius was partly supported by the Finnish Cultural Foundation, Paulo Foundation, Orion-Farmos Foundation, Paavo Nurmi Foundation, Emil Aaltonen Foundation, and Finnish Foundation for Cardiovascular Research. Dr. Goldstein was supported by NIH grant K23-NS059774.
REFERENCES
- 1.Bushi D, Grad Y, Einav S, Yodfat O, Nishri B, Tanne D. Hemodynamic evaluation of embolic trajectory in an arterial bifurcation: An in-vitro experimental model. Stroke. 2005;36:2696–2700. doi: 10.1161/01.STR.0000190097.08862.9a. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind D, Babikian V, Llanes J, Lim S, Villablanca J, Wijman C, Saver J. Ct angiography reveals anatomic features that account for the distribution of emboli in the anterior cerebral circulation. Stroke. 2001;32:335. [Google Scholar]
- 3.Perrini P, Cardia A, Fraser K, Lanzino G. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg. 2007;106:142–150. doi: 10.3171/jns.2007.106.1.142. [DOI] [PubMed] [Google Scholar]
- 4.Rhoton AJ. The supratentorial arteries. Neurosurgery. 2002;51(4 Suppl):S53–S120. [PubMed] [Google Scholar]
- 5.Pollanen M. A hempelian explanatory shift in neuropathology: A study in the history and logic of medicine. Can Bull Med Hist. 1991;8:65–76. doi: 10.3138/cbmh.8.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Pollanen M. Behaviour of suspended particles at bifurcations: Implications for embolism. Phys Med Biol. 1991;36:397–401. doi: 10.1088/0031-9155/36/3/008. [DOI] [PubMed] [Google Scholar]
- 7.Murtha T, Stasheff S. Visual dysfunction in retinal and optic nerve disease. Neurol Clin. 2003;21:445–481. doi: 10.1016/s0733-8619(02)00108-1. [DOI] [PubMed] [Google Scholar]
- 8.Ay H, Benner T, Arsava E, Furie K, Singhal A, Jensen M, Ayata C, Towfighi A, Smith E, Chong J, Koroshetz W, Sorensen A. A computerized algorithm for etiologic classification of ischemic stroke: The causative classification of stroke system. Stroke. 2007;38:2979–2984. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 9.Copen W, Schwamm L, González R, Wu O, Harmath C, Schaefer P, Koroshetz W, Sorensen A. Ischemic stroke: Effects of etiology and patient age on the time course of the core apparent diffusion coefficient. Radiology. 2001;221:27–34. doi: 10.1148/radiol.2211001397. [DOI] [PubMed] [Google Scholar]
- 10.Ay H, Oliveira-Filho J, Buonanno F, Schaefer P, Furie K, Chang Y, Rordorf G, Schwamm L, Gonzalez R, Koroshetz W. 'Footprints' of transient ischemic attacks: A diffusion-weighted mri study. Cerebrovasc Dis. 2002;14:177–186. doi: 10.1159/000065682. [DOI] [PubMed] [Google Scholar]
- 11.Babikian V, Wijman C, Koleini B, Malik S, Goyal N, Matjucha I. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovasc Dis. 2001;12:108–113. doi: 10.1159/000047689. [DOI] [PubMed] [Google Scholar]
- 12.Mead G, Lewis S, Wardlaw J, Dennis M. Comparison of risk factors in patients with transient and prolonged eye and brain ischemic syndromes. Stroke. 2002;33:2383–2390. doi: 10.1161/01.str.0000029827.93497.97. [DOI] [PubMed] [Google Scholar]
- 13.Fahraeus R, Lindquist T. The viscosity of the blood in narrow capillary tubes. American Journal of Physiology. 1931;96:562–568. [Google Scholar]
- 14.Pollanen M, Deck J. The mechanism of embolic watershed infarction: Experimental studies. Can J Neurol Sci. 1990;17:395–398. doi: 10.1017/s031716710003095x. [DOI] [PubMed] [Google Scholar]
- 15.Dorner G, Polska E, Garhöfer G, Zawinka C, Frank B, Schmetterer L. Calculation of the diameter of the central retinal artery from noninvasive measurements in humans. Curr Eye Res. 2002;25:341–345. doi: 10.1076/ceyr.25.6.341.14231. [DOI] [PubMed] [Google Scholar]
- 16.Edvinsson L, MacKenzie E. General and comparative anatomy of the cerebral circulation. In: Edvinsson L, Krause D, editors. Cerebral blood flow and metabolism. Lippincott Wiilams and Wilkins; 2002. pp. 3–29. [Google Scholar]
- 17.O'Sullivan M, Rich P, Barrick T, Clark C, Markus H. Frequency of subclinical lacunar infarcts in ischemic leukoaraiosis and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. AJNR Am J Neuroradiol. 2003;24:1348–1354. [PMC free article] [PubMed] [Google Scholar]
- 18.Kimberly W, Gilson A, Rost N, Rosand J, Viswanathan A, Smith E, Greenberg S. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easton J, Saver J, Albers G, Alberts M, Chaturvedi S, Feldmann EH, TS, Higashida R, Johnston S, Kidwell C, Lutsep H, Miller E, Sacco R, American HA American SASC, Council oCSaA, Council oCRaI, Council oCN, Interdisciplinary CoPVD. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the american heart association/american stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; and the interdisciplinary council on peripheral vascular disease. The american academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 20.The AFSG. Current management of amaurosis fugax. Stroke. 1990;21:201–208. doi: 10.1161/01.str.21.2.201. [DOI] [PubMed] [Google Scholar]
- 21.Biousse V, Trobe J. Transient monocular visual loss. Am J Ophthalmol. 2005;140:717–721. doi: 10.1016/j.ajo.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Atkins E, Bruce B, Newman N, Biousse V. Translation of clinical studies to clinical practice: Survey on the treatment of central retinal artery occlusion. Am J Ophthalmology. 2009;148:172–173. doi: 10.1016/j.ajo.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Donders R, Kappelle L, Algra A, van Dijk G, van Gijn J. How do general practitioners diagnose and manage patients with transient monocular loss of vision of sudden onset? J Neurol. 1999;246:1145–1150. doi: 10.1007/s004150050533. [DOI] [PubMed] [Google Scholar]
- 24.Cerebral ESG. Immediate anticoagulation of embolic stroke: Brain hemorrhage and management options. Stroke. 1984;15:779–789. doi: 10.1161/01.str.15.5.779. [DOI] [PubMed] [Google Scholar]