Abstract
Objective
We sought to determine if vitamin D status is associated with developing new T2 lesions or contrast-enhancing lesions on brain MRI in relapsing multiple sclerosis (MS).
Methods
EPIC is a five-year longitudinal MS cohort study at the University of California, San Francisco. Participants had clinical evaluations, brain MRI, and blood draws annually. From the overall cohort, we evaluated patients with clinically isolated syndrome or relapsing-remitting MS at baseline. In univariate and multivariate (adjusted for age, sex, ethnicity, smoking, and MS treatments) repeated measures analyses, annual 25-hydroxyvitamin D levels were evaluated for their association with subsequent new T2-weighted and gadolinium-enhancing T1-weighted lesions on brain MRI, clinical relapses, and disability (Expanded Disability Status Scale [EDSS]).
Results
2,362 3T brain MRI scans were acquired from 469 subjects. In multivariate analyses, each 10 ng/mL higher 25-hydroxyvitamin D was associated with a 15% lower risk of a new T2 lesion (incidence rate ratio [IRR]= 0.85, 95% CI [0.76, 0.95], p=0.004) and a 32% lower risk of a gadolinium-enhancing lesion (IRR=0.68, 95% CI [0.53, 0.87], p=0.002). Each 10 ng/mL higher vitamin D level was associated with lower subsequent disability (−0.047, 95% CI [−0.091, −0.003], p=0.037). Higher vitamin D levels were associated with lower, but not statistically significant, relapse risk. Except for the EDSS model, all associations were stronger when the within-person change in vitamin D level was the predictor.
Interpretation
Vitamin D levels are inversely associated with MS activity on brain MRI. These results provide further support for a randomized trial of vitamin D supplementation.
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disorder occurring in those who possess or are exposed to a combination of genetic and environmental risk factors. A few environmental MS risk factors have been identified: cigarette smoking, infection with Epstein Barr virus, and lower vitamin D levels.1–8 Lower vitamin D levels have recently been associated with increased relapse risk among patients with relapsing-remitting (RR) MS or clinically isolated syndrome (CIS).9,10 However, neither study evaluated if vitamin D levels are associated with brain magnetic resonance imaging (MRI) measures of inflammation. Brain MRI may provide a more objective measure of disease activity and is less susceptible to concerns about missed exacerbations or pseudoexacerbations. In this study, we capitalized on a large prospective cohort of patients with MS followed annually for five years to determine if vitamin D status is associated with MRI and clinical measures of inflammation or with disability progression.
METHODS
Subjects
EPIC is a five-year MS cohort study in which subjects had clinical and MRI evaluations and gave a blood sample annually. The study was approved by the UCSF Committee on Human Research; participants provided informed consent. White patients with MS (ages 18 to 70, Expanded Disability Status Scale [EDSS] score less than 8.0) were recruited for the study, primarily from the UCSF MS Center. A diagnosis of MS or CIS, made using the International Panel criteria, was required.11,12 Subjects with CIS needed three of four dissemination in space criteria on brain MRI.12 Exclusion criteria included relapse or treatment with corticosteroids within the past month, enrollment in a study of unapproved MS medications, inability to have an MRI, recent drug or alcohol abuse, or a medical condition that could introduce risks by participating. Recruitment began in July, 2004 with a planned enrollment of 500 individuals; subjects were added to compensate for drop-outs (the group enrolled by September 2005 was considered the “original cohort”; additional subjects were enrolled over the next five years). For the current study, we included those subjects with CIS or RRMS who were in the original cohort and had completed at least one follow-up, plus those who had completed or were scheduled to have completed at least two years of follow-up at the time the blood samples were prepared for allocation for vitamin D measurements (June 2010).
Clinical Assessments
Age, ethnicity (Hispanic or non-Hispanic), sex, disease duration, weight, self-reported height, smoking status, EDSS score, and use of disease-modifying therapy (DMT) and other medications and supplements were recorded at baseline. In follow-up, self-reported height and weight (typically self-reported) and EDSS scores were documented, and clinical relapses were recorded and confirmed by a UCSF study physician. The month and year at which a DMT was started or stopped were recorded. Supplement usage was assessed annually by asking patients an open-ended question about their use thereof in the prior 12 months.
Magnetic Resonance Imaging Protocol
Brain MRI scans were acquired on a single 3T GE Excite scanner (GE Healthcare Technologies) using an 8-channel phased array coil in reception and a whole body coil in transmission. Images were read (blinded from vitamin D levels) at baseline and annually throughout the study using simultaneously-viewed, axial dual-echo T2/PD-weighted images (TE1/TE2=20/90ms, TR=2000ms, 512×512×44 matrix, 240×240×132mm3 FOV, slice thickness=3mm, no gaps) to identify new brain lesions. Gadolinium enhancing T1-weighted lesions were identified from post-contrast T1-weighted conventional spin echo images (TE/TR=8/467ms, 256×256×44 matrix, 240×240×132 FOV, NEX=1) acquired 5 minutes after administering a single dose (0.1mM/kg) of contrast agent.
Vitamin D status
The total 25-hydroxyvitamin D concentration (also referred to herein as “vitamin D level [s]”) was measured from plasma (baseline) or serum (years 1 through 4) by batched chemiluminescent immunoassay (Heartlands Assays, Inc., Ames, IA). Levels are presented in ng/mL (1 ng/mL = 2.496 nmol/L). We used plasma instead of serum at baseline because insufficient quantities of serum remained to conduct the measurements, and plasma was not available at all remaining timepoints. In a subset of patients for whom serum and plasma was available at the same timepoint (n=15), we had previously measured vitamin D levels in both samples and found no meaningful difference (p=0.75 in a paired t test; Pearson correlation coefficient 0.94; Supplementary Figure 1. The intra-assay coefficient of variation is 8.1%.
Statistical Analyses
Outcomes included the number of new T2 lesions, the number of gadolinium-enhancing lesions, the number of clinical relapses, and the EDSS score. The primary predictor was the prior visit’s 25-hydroxyvitamin D level; as such, the vitamin D level used to predict the outcomes of interest preceded their occurrence. We also assessed vitamin D status as a categorical predictor (in quintiles). Repeated measures analyses were performed using Poisson generalized estimating equations with robust standard errors to generate incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for all outcomes except EDSS. An offset of the log time since the prior MRI or visit was included to account for the different time between follow-ups for those who missed visits. When disability was the outcome, we used a linear model with generalized estimating equations, with the estimates representing the average change in EDSS. In addition, multivariate models were generated and included age at visit, sex, ethnicity, baseline smoking status, and use of any DMT during each interval. DMT was also modeled as a categorical variable (none, first-line [interferon beta, glatiramer acetate and steroids], and second-line [broad-spectrum immunosuppressants, monoclonal antibodies, and fingolimod]). We also assessed if having had ≥ three months of DMT exposure in each interval was a confounder. We further explored if adding body mass index (BMI; kg/m2) at the beginning of each follow-up period, disease duration at the time of the visit, or HLA-DRB1 status (positive or negative for the *15:01 allele;13 possessing at least one copy was considered positive) to the models influenced the results. To isolate the effect of within-person changes in vitamin D, we also evaluated models in which we entered both the baseline vitamin D level as well as that participant’s change over baseline in vitamin D as predictors. The latter is not subject to confounding at the individual level. Finally, we assessed for interactions between vitamin D and DMT use, vitamin D and HLA-DRB1*15:01 status,14 and vitamin D and having one versus two copies of the HLA-DRB*15:01 allele among those who were HLA-DRB*15:01 positive. Since sun exposure and oral intake of vitamin D are causative of vitamin D status, these variables were not included in the models.
RESULTS
A total of 469 subjects with RRMS/CIS were included; 2,362 3T brain MRI scans were obtained during the study. Baseline characteristics are presented in Table 1. Seventy-five percent of the total group completed a year 5 visit. Of those not in the original cohort (n=31), only 3 patients had missed a visit or withdrawn from the study at the time the vitamin D levels were sampled.
Table 1.
Baseline characteristics of cohort subjects
| Characteristic | n=469 |
|---|---|
| Age in years, mean (±SD) | 42 ± 10 |
| Disease duration in years, median (± interquartile range) | 5 (0, 35) |
| Female sex, n (%) | 330 (70) |
| Hispanic ethnicity, n (%) | 24 (5) |
| HLA-DRB1 positive, n (%) | 214 (46) |
| Smoker, n (%) | 56 (12) |
| Body mass index in kg/m2, mean (±SD) | 25 ± 5 |
| Clinically isolated syndrome, n (%) | 90 (19) |
| Any MS treatment within prior year, n (%) | 298 (64) |
| Use of second-line therapy in past year, n (%) | 17 (4) |
| Use of multivitamin, n (%) | 224 (48) |
| Use of vitamin D supplements, n (%) | 40 (9) |
SD= standard deviation
The vitamin D levels, EDSS scores, and number of relapses, new T2 lesions, and gadolinium-enhancing T1-weighted lesions are presented in Table 2. The average vitamin D level by year 4 was 5.3 ng/mL higher than at baseline (95% CI [3.9, 6.8]; p value for increase throughout the study <0.0001). While only 9% were taking vitamin D at baseline, 43% were taking it by year 5 (p<0.0001; OR for year 5 versus baseline=8.5, 95% CI [6.0, 12.2]). Those who reported using vitamin D supplements within the past 12 months had an 8.7 ng/mL higher vitamin D level than those who did not (95% CI [6.6, 10.8], p<0.001).
Table 2.
Vitamin D levels and clinical and MRI activity during the study
| Baseline n=469 |
Year 1 n=447 |
Year 2 n=407 |
Year 3 n=353 |
Year 4 n=345 |
Year 5 n=350 |
|
|---|---|---|---|---|---|---|
| 25-hydroxyvitamin level (ng/mL) at prior visit, mean ± SD | N/A | 27.8 ± 10.7 | 27.8 ± 10.9 | 27.7 ± 11.5 | 30.7 ± 12.8 | 32.9 ± 15.8 |
| EDSS score, median (IQR) | 1.5 (0, 5) | 2 (0, 6.5) | 2 (0, 6) | 2 (0, 6) | 2 (0, 6.5) | 2 (0, 6) |
| Relapses since prior visit, median (IQR)*[mean ± SD] | 0 (0, 4) [0.6 ± 0.8] |
0 (0, 3) [0.3 ± 0.7] |
0 (0, 2) [0.3 ± 0.6] |
0 (0, 2) [0.2 ± 0.5] |
0 (0, 2) [0.2 ± 0.5] |
0 (0, 2) [0.1 ± 0.4] |
| New T2 lesions since last scan, median (IQR)[mean ± SD] | N/A | 0 (0, 24) [1.8 ± 5.0] |
1 (0, 16) [1.9 ± 3.8] |
0 (0, 4) [0.2 ± 0.7] |
0 (0, 4) [0.2 ± 0.7] |
0 (0, 5) [0.3 ± 1.0] |
| Gadolinium-enhancing lesions, median (IQR)[mean ± SD] | 0 (0,6) [0.3 ± 1.0] |
0 (0, 8) [0.3 ± 1.3] |
0 (0, 3) [0.1 ± 0.6] |
0 (0, 2) [0.1 ± 0.8] |
0 (0, 1) [0.0 ± 0.3] |
0 (0, 1) [0.0 ± 0.2] |
EDSS= Expanded Disability Status Scale; IQR= interquartile range; SD= standard deviation
Lesion counts do not account for the fact that some patients had missed visits, so the summary statistics may reflect activity over more than one year. In the statistical models, this concern is addressed by using an offset of the log time since the prior visit/scan. Vitamin D levels from the prior year were missing for one patient at year 1, two patients at year 3, and six patients at year 5. EDSS scores were missing for 2 subjects at year 1, 2, and 4, and for 3 subjects at year 3 and 5). Gadolinium-enhancing lesions could not be determined for one patient at baseline. In follow-up, of those for whom vitamin D levels were available, seven patients missed a brain MRI (at one time point each); 23 subjects were missing either gadolinium-enhancing lesion or new T2 lesion counts at one time point, while three patients were missing one of the measures at two time points.
For the baseline visit, the number of relapses in the year prior to the visit was documented.
Vitamin D and new MRI lesions
In univariate models, each 10 ng/mL higher vitamin D level was associated with a 15% lower risk of later developing new T2 lesions (IRR= 0.85, 95% CI [0.76, 0.95], p=0.005). The multivariate results were nearly identical (Table 3). Younger age and cigarette smoking were also associated with increased risk of new T2 lesions. The estimates did not meaningfully change when disease duration, BMI, or HLA-DRB1 status was added to the models, nor did they change if DMT was modeled as a categorical variable or if a person was considered “on” DMT if they had received it for at least three months of the prior interval. Even after adjusting for baseline vitamin D, each 10 ng/mL within-person increase in vitamin D was associated with a much lower risk of developing a new T2 lesion (IRR=0.67, 95% CI [0.55, 0.83], p<0.001).
Table 3.
Radiologic and clinical MS activity associated with vitamin D (multivariate models)
| New T2 lesions | Contrast-enhancing lesions | Relapse | Disability (EDSS) | |
|---|---|---|---|---|
| Vitamin D level (per 10 ng/mL greater) | 0.85 (0.76, 0.95) p=0.004 |
0.68 (0.53, 0.87) p=0.002 |
0.94 (0.86, 1.02) p=0.12 |
−0.047 (−0.091, −0.003) p=0.037 |
| Age (per year greater) | 0.93 (0.92, 0.95) p<0.001 |
0.92 (0.89, 0.95) p<0.001 |
0.97 (0.95, 0.98) p<0.001 |
0.05 (0.04, 0.06) p<0.001 |
| Female sex | 0.93 (0.59, 1.47) p=0.77 |
1.35 (0.69, 2.64) p=0.38 |
1.15 (0.89, 1.49) p=0.28 |
0.04 (−0.17, 0.25) p=0.71 |
| Hispanic ethnicity | 1.07 (0.52, 2.20) p=0.84 |
1.40 (0.38, 5.07) p=0.61 |
0.83 (0.47, 1.45) p=0.50 |
−0.16 (−0.44, 0.12) p=0.27 |
| Smoker at baseline | 1.83 (0.98, 3.40) p=0.056 |
1.00 (0.43, 2.35) p=0.999 |
1.43 (1.01, 2.03) p=0.046 |
0.09 (−0.17, 0.36) p=0.49 |
| Use of any DMT | 1.27 (0.85, 1.90) p=0.24 |
0.75 (0.33, 1.69) p=0.49 |
2.05 (1.47, 2.85) p<0.001 |
0.30(0.14, 0.46) p<0.001 |
Results are presented as incidence rate ratios (for first three columns) or as actual coefficients (for disability) with 95% confidence intervals and p values
DMT= disease-modifying therapy; EDSS= Expanded Disability Status Scale
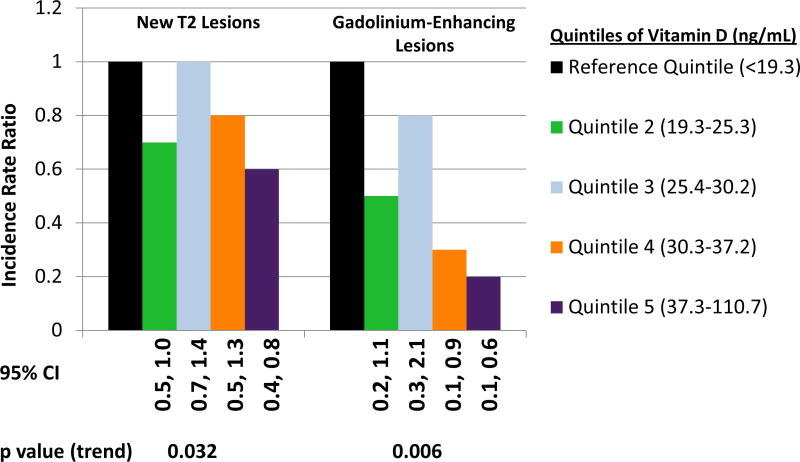
There was no statistically significant deviation from linearity in the association of vitamin D and new T2 lesions (p value for nonlinearity term=0.34). The association of quintiles of vitamin D levels and new T2 lesions is depicted in the Figure; the p value for the test of trend was 0.032. There was no apparent interaction of DMT with vitamin D levels (p=0.56), of HLA-DRB1 status with vitamin D levels (p=0.41) or, among those who were HLA-DRB1*15-positive, between the number of copies of HLA-DRB1*15 and vitamin D (p= 0.49).
Vitamin D levels were also strongly associated with the development of contrast-enhancing lesions on brain MRI. In univariate models, each 10 ng/mL higher vitamin D level was associated with nearly a one-third reduction in the risk of a subsequent contrast-enhancing lesion (IRR=0.68, 95% CI [0.54, 0.86], p=0.001). The results were similar in the multivariate models (Table 3). Younger age was also associated with an increased risk of contrast-enhancing lesions. Adding disease duration, BMI at the prior visit, or HLA-DRB1 status to the models did not meaningfully change the association of vitamin D with contrast-enhancing lesions. The results were unchanged if DMT was modeled as a categorical variable or if a person was considered “on” DMT if they had received it for at least three months of the prior interval. Even after adjusting for baseline vitamin D, each 10 ng/mL within-person increase in vitamin D was associated with a substantially lower risk of gadolinium-enhancing lesions (IRR=0.53, 95% CI [0.37, 0.75], p<0.001).
There was no statistically significant deviation from linearity in the association of vitamin D and contrast-enhancing lesions (p value for nonlinearity term=0.25). The relation of quintiles of vitamin D levels and gadolinium-enhancing lesions is depicted in Figure 1; the p value for the test of trend was 0.006. There was no apparent interaction between vitamin D levels and DMT use (p=0.29), but there was a potential interaction between HLA-DRB1 status and vitamin D levels (p=0.023). A 10 ng/mL higher vitamin D level was associated with an IRR for contrast-enhancing lesions of 0.57 (95% CI [0.41, 0.78], p<0.001) in HLA-DRB1 negative patients versus 0.89 (95% CI [0.69, 1.14], p=0.34) in HLA-DRB1 positive patients. The p value for the interaction term of vitamin D levels and the number of HLA-DRB1*15:01 alleles in those positive for HLA-DRB1 was 0.61.
Figure 1.
MRI Outcomes Associated with Quintiles of Vitamin D
Vitamin D and clinical outcomes
Higher vitamin D levels were also associated with lower risk of relapse in univariate models, although the p values did not demonstrate statistical significance. Each 10 ng/mL higher vitamin D level was associated with an IRR for relapse of 0.94 (95% CI [0.86, 1.02], p=0.13). The results were similar in the multivariate models (Table 3). Younger age and cigarette smoking were independently associated with greater relapse risk. Adding disease duration, BMI at the prior visit, or HLA-DRB1 status to the models did not influence the main results. Changing the way DMT was modeled did not meaningfully alter the association of interest. After adjusting for baseline vitamin D, each 10 ng/mL within-person increase in vitamin D was associated with a lower risk of relapse (IRR=0.88, 95% CI [0.77, 1.01], p=0.062).
There was no statistically significant deviation from linearity in the association of vitamin D and relapses (p value for nonlinearity term=0.12). While the highest quintile of vitamin D had the lowest risk in relapse models (IRR 0.92, 95% CI 0.66, 1.29), the p value for the test of trend was not significant (p=0.60). There was no apparent overall interaction between vitamin D levels and DMT (p=0.27) or between vitamin D levels and HLA-DRB1 status (p=0.74); however, among those who were HLA-DRB1*15:01 positive, the p value for the interaction term was 0.063. Each 10 ng/mL higher vitamin D level was associated with an IRR for relapse of 0.86 (95% CI [0.75, 0.99], p=0.040) in those with only one copy of HLA-DRB1*15:01 versus 1.12 (95% CI [0.89, 1.40], p=0.34) in those with two copies.
For disability, in the univariate models, each 10 ng/mL higher vitamin D level was associated with a 0.02-point lower subsequent EDSS score ( 95% CI [−0.07, 0.02], p=0.32). The results of the multivariate models were stronger; each 10 ng/mL higher vitamin D level was associated with lower subsequent disability (−0.047, 95% CI [−0.091, −0.003], p=0.037; see Table 3). Older age and DMT use were independently associated with higher EDSS. Adding disease duration, BMI at the prior visit, or HLA-DRB1 status to the models did not influence the results, nor did changing the way DMT was modeled meaningfully alter the association of interest. After adjusting for baseline vitamin D, each 10 ng/mL within-person increase in vitamin D level was associated with a 0.045-point higher subsequent EDSS score (95% CI [−0.004, 0.094], p=0.074. There was no statistically significant deviation from linearity in the association of vitamin D and disability (p value for nonlinearity term=0.44). Although the highest quintile of vitamin D had the lowest subsequent EDSS (−0.16, 95% [CI −0.32, 0.00]), the p value for the test of trend was not significant (p=0.29). There was no apparent overall interaction between vitamin D levels and DMT (p=0.20) or between vitamin D levels and HLA-DRB1 status (p=0.93)
DISCUSSION
Individuals with CIS/RRMS with higher vitamin D levels are at much lower risk of the subsequent development of new lesions and of gadolinium-enhancing lesions on brain MRI, even after accounting for potential confounding factors. Particularly important is that the within-person effect of vitamin D, which is less subject to confounding at the individual level, is even stronger. That a prior study did not find an association is probably because it was cross-sectional, while our study assessed the relation of vitamin D levels and subsequent outcomes.15 The results are clinically important since the development of new lesions has been associated with later disability or needing ambulatory assistance in a few studies.16–18 That vitamin D status is associated with MRI evidence of disease activity may thus have implications for subsequent disability.
The association of vitamin D levels with clinical relapses was less robust here than in two prior studies.9,10 It is likely that the method by which relapses were ascertained contributed to the weakness of this association, since some patients were seen by their regular UCSF neurologist between study visits, while others were not. A low number of clinical relapses during the study may also have contributed to a reduced ability to detect a vitamin D effect. Vitamin D levels did appear to be inversely associated with subsequent disability as assessed by the EDSS, although this association had an opposite trend in the models assessing the within-person change in vitamin D as the primary predictor. It is thus unclear whether vitamin D levels are truly associated with subsequent accrual of disability. The EDSS itself has several limitations as an outcome measure,19, 20 and the median EDSS did not substantially change over the five-year study, such that confirming an association may be difficult in this cohort. Further studies are required to better explore this relationship.
A vitamin D response element has been reported in the promoter of HLA-DRB1*15 and *16 haplotypes,14 although it may not be specific to those haplotypes or to HLA-DR1.21 Here, vitamin D was strongly associated with reduced development of contrast-enhancing lesions in those who were HLA-DRB1*15:01-negative, while the effect was less substantial in those who were positive for HLA-DRB1*15:01. Among those who were positive for HLA-DRB1*15:01, higher vitamin D levels were associated with a lower risk of relapse in those who had one copy of the allele but did not appear to correlate with relapse risk in those with two copies. It is possible that these differential results are spurious, especially since they are not consistent across all models. Further, the ability to detect interactions may be limited due to the sample size. However, it is possible that the effect of vitamin D may not be consistent among all individuals.
While not the primary aim of this paper, it is intriguing that those who were active smokers at entry in the study were at substantially greater risk of developing new T2 lesions and clinical relapses throughout the study. Smoking is a relatively well-established risk factor for MS, but its association with the course of the disease has been less definitively established.22–25 While our results do not provide evidence that smoking cessation reduces this risk, the finding may be useful to clinicians in providing advice about smoking cessation to patients with MS. A puzzling result was the independent association of DMT use with relapses. In two prior observational studies, one in a cohort of patients seen within a year of MS onset at UCSF 26 and the other in a pediatric-onset MS population,9 DMT use was not associated with prolonged time to early relapse or with reduced risk of relapse. It is likely that this finding is due in part to confounding by indication, namely that patients at greatest risk of relapses are those who are counseled to use DMT, creating an apparent (but non-causal) association.
Our study has some limitations. First, the results may not apply to the entire MS population, as the cohort included only white individuals with relapsing MS. Height, and at many timepoints weight, were self-reported; as such, the adjustment for BMI may not have been completely accurate. However, the mean BMI was relatively stable throughout the period of follow-up (data not shown), supporting that self-reports were likely fairly accurate.
Patients in our cohort, whether on their own or in consultation with their physician, have already begun supplementation with vitamin D, as evidenced by the increase in self-reported vitamin D intake throughout the course of the EPIC study. It is important to note that our results do not provide evidence that vitamin D supplementation is beneficial to individuals with MS. Observational studies, even when meticulously conducted, can still lead to incorrect conclusions, as seemingly beneficial associations in the observational setting may be shown to have no effect or harmful in the context of a randomized controlled trial.27–29 However, our findings provide further support for the role of vitamin D in MS inflammatory activity and for a randomized trial of vitamin D supplementation.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the UCSF MS Center providers for referring patients to the EPIC study as well as the study coordinators who worked on the project, including Jessica Bautista, Aracely Delgadillo, Michaela George, Refujia Gomez, Mickey Guzman, and Abigail Pablico. We thank Adam Santaniello for maintaining the database and Rosa Guerrero and Hourieh Mousavi for processing the blood samples. Finally, we thank the patients who participated in the study.
The study was funded by the NIH (EMM: K23NS067055; DP:R01NS062885), GlaxoSmithKline, and Biogen Idec. The sponsors did not participate in the design or conduct of the study, the data analysis and interpretation, or any aspect of the manuscript preparation or approval.
Footnotes
Drs. Mowry and Pelletier had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Several authors (CEM, AE, PAG, DB, RL, MCO, PQ, MB) report no conflicts of interest. Dr. Mowry is funded by NIH K23NS067055. Dr. Waubant has received has received honorarium for 2 educational presentations from TEVA. She is ad hoc consultant for Roche, Sanofi Aventis, and Actelion. She has received free study medication from Bioden Idec and Sanofi Aventis. She has received funding from the Marcled Foundation, the Nancy Davis Foundation, the National MS Society, and the NIH. She has received an educational grant from TEVA and Biogen Idec. Dr. Okuda has received funding for travel or speaker honoraria from the National MS Society, Multiple Sclerosis Association of America, TEVA Neuroscience, Biogen IDEC, and Acorda Therapeutics; and received research support from Pfizer, Inc. and EMD Serono, Inc. Dr. Hauser served as both a board member and consultant to BioMarin and Receptos, has served as a consultant to Novartis, has received travel accommodations from Roche, and his institution (UCSF) has received payment for lectures given to Wyeth-Pfizer. He has received personal compensation from Wiley Publishing for serving as Editor-In-Chief of the journal Annals of Neurology and from McGraw-Hill Publishers for serving as editor of Harrison’s Internal Medical textbook. He has received stock/stock options from Receptos, all of which were then transferred to his institution (UCSF). Dr. Pelletier has received research funding from Biogen-Idec and has received consulting fees from Synarc. Inc and CNS Imaging Consultant LLC.
Authors’ contributions: conception and design (EMM, EW, SLH, DP); acquisition of data (EMM, DTO, AE, RL, PG, DB, MO, PQ, MB, DP); analysis and interpretation of data (EMM, EW, CM, PG, DP); drafting of the manuscript (EMM, DP); critical revision of the manuscript (all authors); statistical analysis (EMM, CM); obtaining funding (EMM, DP, SLH); supervision (RL, PQ, DP).
References
- 1.Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epi. 2001;154:69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;61:1122–1124. doi: 10.1212/01.wnl.0000081305.66687.d2. [DOI] [PubMed] [Google Scholar]
- 3.Sundstrom P, Nystrom L, Hallmans G. Smoke exposure increases the risk for multiple sclerosis. Eur J Neurol. 2008;15:579–583. doi: 10.1111/j.1468-1331.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 4.Mikaeloff Y, Caridade G, Tardieu M, Suissa S KIDSEP study group. Parental smoking at home and the risk of childhood-onset multiple sclerosis. Brain. 2007;130:2539–2595. doi: 10.1093/brain/awm198. [DOI] [PubMed] [Google Scholar]
- 5.Myhr KM, Riise T, Barrett-Connor E, et al. Altered antibody pattern to Epstein-Barr virus but not to other herpesviruses in multiple sclerosis: a population based case-control study from western Norway. J Neurol Neurosurg Psychiatry. 1998;64:539–542. doi: 10.1136/jnnp.64.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin LI, Munger KL, Rubertone MV, Peck CA, Lennette ET, Spiegelman D, Ascherio A. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 7.Wandinger KP, Jabs W, Sikehaus A, et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–184. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 8.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 9.Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67:618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 10.Simpson S, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 11.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 13.Okuda DT, Srinivasan R, Oksenberg JR, et al. Genotype-phenotype correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1HMR spectroscopy and MRI measures. Brain. 2009;132:250–259. doi: 10.1093/brain/awn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramagopalan SV, Maugeri NJ, Handunnetthi L, et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009:e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock-Guttman B, Zivadinov R, Qu J, et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J Neurol Neurosurg Psych. 2011;82:189–195. doi: 10.1136/jnnp.2010.227942. [DOI] [PubMed] [Google Scholar]
- 16.Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 17.DiFilippo M, Andersonn VM, Altmann DR, et al. Brain atrophy and lesion load measures over 1 year relate to clinical status after 6 years in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2010;81:204–208. doi: 10.1136/jnnp.2009.171769. [DOI] [PubMed] [Google Scholar]
- 18.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131:808–817. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 19.Sharrack B, Hughes RA, Soudain S, Dunn G. The psychometric properties of clinical rating scales used in multiple sclerosis. Brain. 1999;122:141–159. doi: 10.1093/brain/122.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Hobart J, Kalkers N, Barkhof F, Uitdehaag B, Polman C, Thompson A. Outcome measures for multiple sclerosis clinical trials: relative measurement precision of the Expanded Disability Status Scale and Multiple Sclerosis Functional Composite. Mult Scler. 2004;10:41–46. doi: 10.1191/1352458504ms983oa. [DOI] [PubMed] [Google Scholar]
- 21.Stewart CA, Horton R, Allcokc RJN, et al. Complete MHC haplotype sequencing for common disease gene mapping. Genome Res. 2004;14:1176–1187. doi: 10.1101/gr.2188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiPauli F, Reindl M, Ehling R, et al. Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler. 2008;14:1026–1030. doi: 10.1177/1352458508093679. [DOI] [PubMed] [Google Scholar]
- 23.Hernan MA, Jick SS, Logroscino G, Olek MJ, Ascherio A, Jick H. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128:1461–1465. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- 24.Koch M, van Hartern A, Uyttenboogaart M, DeKeyser J. Cigarette smoking and progression in multiple sclerosis. Neurology. 2007;69:1515–1520. doi: 10.1212/01.wnl.0000277658.78381.db. [DOI] [PubMed] [Google Scholar]
- 25.Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66:858–864. doi: 10.1001/archneurol.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mowry EM, Pesic M, Grimes B, Deen SR, Bacchetti P, Waubant E. Clinical predictors of early second event in patients with clinically isolated syndrome. J Neurol. 2009;256:1061–1066. doi: 10.1007/s00415-009-5063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the women’s antioxidant and cardiovascular study. Circulation. 2009;119:2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei W, Rong Y, Jinming L, Yongjun L, Hui Z. Effect of homocysteine interventions on the risk of cardiocerebrovascular events: a meta-analysis of randomised controlled trials. Int J Clin Pract. 2010;64:208–215. doi: 10.1111/j.1742-1241.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90:429–437. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.