Abstract
The plant-specific NAC (NAM, ATAF1/2, and CUC2) transcription factors (TFs) play important roles in plant growth, development, and stress responses. However, the precise role of NAC TFs in relation to fruit ripening is poorly understood. In this study, six NAC genes, designated MaNAC1–MaNAC6, were isolated and characterized from banana fruit. Subcellular localization showed that MaNAC1–MaNAC5 proteins localized preferentially to the nucleus, while MaNAC6 was distributed throughout the entire cell. A transactivation assay in yeast demonstrated that MaNAC4 and MaNAC6, as well as their C-terminal regions, possessed trans-activation activity. Gene expression profiles in fruit with four different ripening characteristics, including natural, ethylene-induced, 1-methylcyclopropene (1-MCP)-delayed, and a combination of 1-MCP with ethylene treatment, revealed that the MaNAC genes were differentially expressed in peel and pulp during post-harvest ripening. MaNAC1 and MaNAC2 were apparently upregulated by ethylene in peel and pulp, consistent with the increase in ethylene production. In contrast, MaNAC3 in peel and pulp and MaNAC5 in peel were constitutively expressed, and transcripts of MaNAC4 in peel and pulp and MaNAC6 in peel decreased, while MaNAC5 or MaNAC6 in pulp increased slightly during fruit ripening. Furthermore, the MaNAC2 promoter was activated after ethylene application, further enhancing the involvement of MaNAC2 in fruit ripening. More importantly, yeast two-hybrid and bimolecular fluorescence complementation analyses confirmed that MaNAC1/2 physically interacted with a downstream component of ethylene signalling, ethylene insensitive 3 (EIN3)-like protein, termed MaEIL5, which was downregulated during ripening. Taken together, these results suggest that MaNACs such as MaNAC1/MaNAC2, may be involved in banana fruit ripening via interaction with ethylene signalling components.
Key words: banana, EIL, fruit ripening, interaction, NAC, regulation
Introduction
Fruit ripening is a complex and genetically programmed process that results in marked changes in colour, flavour, aroma, texture, and nutritional content of the flesh (Giovannoni, 2004). These changes are the result of the coordinated activation of multiple genetic and biochemical pathways, which are influenced by internal and external cues, including regulation by many critical transcription factors (TFs) (Martel et al., 2011). Numerous plant TFs such as apetalous (AP2), basic leucine zipper domain (bZIP), myeloblastosis oncogene (MYB), myelocytomatosis oncogene (MYC), WRKY, Mcm1-Agamous-Deficiens-Srf (MADS), heat-shock TF (HSF), and several classes of Cys2/His2-type zinc finger proteins, have been identified and characterized according to their DNA-binding motifs (Yamasaki et al., 2008). The NAC, named after NAM (no apical meristem), ATAF1,2, and CUC2 (cup-shaped cotyledon), constitutes one of the largest families of plant-specific TFs, with 106 members in Arabidopsis (Gong et al., 2004), 149 members in rice (Xiong et al., 2005), and 101 members in the soybean genome (Pinheiro et al., 2009). The distinguishing characteristic of NAC TFs is possession of a highly conserved N-terminal NAC domain, which is divided into five conserved subdomains (A–E), and a variable transcriptional regulation C terminus, which is able to activate or repress the transcription of multiple target genes (Duval et al., 2002; Hao et al., 2010).
NAC TFs have been implicated to contribute to diverse and vital physiological processes, including shoot apical meristem development (Hibara et al., 2003), floral morphogenesis (Sablowski and Meyerowitz, 1998), leaf formation and senescence (Berger et al., 2009; Balazadeh et al., 2011; Zhang and Gan, 2012), embryo development (Larsson et al., 2011), seed development (Verza et al., 2011), flavonoid biosynthesis (Morishita et al., 2009), lateral root development (He et al., 2005), grain nutrient remobilization (Waters et al., 2009), shoot branching determination (Mao et al., 2007), and secondary cell-wall biosynthesis (Zhao et al., 2010; Zhong et al., 2010). Moreover, some NAC TFs have been reported to participate in hormone signalling mediated by auxin (Xie et al., 2000), ethylene (He et al., 2005), abscisic acid (Fujita et al., 2004; Gao et al., 2010), gibberellic acid (Aida et al., 1997; Kim et al., 2008), salicylic acid (Delessert et al., 2005), methyl jasmonate (Bu et al., 2008; Yoshii et al., 2010), and cytokinin (Kim et al., 2007), as well as in the response to biotic and abiotic stresses such as fungus infection, drought, cold, high salinity, and mechanical wounding (He et al., 2005; Meng et al., 2009; Xia et al., 2010; Nakashima et al., 2012). These stress inducible NAC TFs have been shown to bind to cis-regulatory elements, termed NACRSs (NAC recognition sequences), upstream of other stress responsive genes and activate their expression (Tran et al., 2004). Furthermore, a few NAC TFs are differentially regulated by various pathways, including microRNA-mediated cleavage of mRNAs and ubiquitination-mediated proteolysis (Greve et al., 2003; Berger et al., 2009). Although the functions of NAC TFs in many plants have been studied extensively, the involvement of NAC TFs in relation to ripening of economic fruits has generally received less attention.
Banana (Musa acuminata, AAA group), a typical climacteric fruit, is one of the most important food crops after rice, maize, and wheat in tropical and subtropical countries. It comprises an important part in the diet of millions of people around the world. Commercially, it is a very prosperous crop in the world trade. The post-harvest physiology of banana fruit is characterized by a pre-climacteric phase, followed by a peak in ethylene production that orchestrates ripening-associated processes, including climacteric respiration, pulp softening, peel degreening, and the production of aroma compounds (Clendennen and May, 1997). However, the physiological climacteric attribute of banana fruit results in a short post-harvest life of 10–15 d at ambient temperature (Bapat et al., 2010). In addition, unlike many other climacteric fruits, the peel and pulp of banana fruit exhibit different ethylene biosynthesis patterns (Dominguez and Vendrell, 1993; Elitzur et al., 2010). For these reasons, it is imperative to gain a better understanding of the mechanism of fruit ripening, as this will aid in improving the quality and storage potential of this fruit. Some genes associated with ethylene biosynthesis and perception pathways have been identified in banana fruit, including 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, ACC oxidase, ethylene receptor, CTR1 orthologue, and ethylene insensitive 3-like (EIL) genes (Clendennen et al., 1997; Liu et al., 1999; Mbéguié-A-Mbeguié et al., 2008). However, there is little information about how ripening-related TFs regulate the ripening of banana fruit in relation to ethylene signalling. Recently, several MaMADS TFs were reported to be induced by ethylene, suggesting that the MADS TF may participate in banana fruit ripening (Liu et al., 2009; Elitzur et al., 2010). Moreover, current research into fruit ripening is likely to focus on the identification of new candidate genes, as well as understanding the interactions among the already characterized components (Kumar et al., 2012). NOR (non-ripening) is an NAC-domain TF whose mutation results in a non-ripening phenotype including ethylene synthesis, increased respiration, carotenoid accumulation, softening, and aroma volatile production (Martel et al., 2011). Furthermore, NAC is also ethylene inducible (He et al., 2005; Xia et al., 2010) and may represent a TF downstream of the ethylene insensitive 2 gene (EIN2) in parallel with EIN3 (Kim et al., 2009; Al-Daoud and Cameron, 2011). Thus, it is interesting to study the transcriptional regulatory network concerning NAC in relation to the ethylene signal during fruit ripening. In the present study, six NAC TFs were isolated and characterized from banana fruit, and the expression patterns of six MaNAC genes in the fruit of four different ripening characteristics, including natural, ethylene-induced, 1-methylcyclopropene (1-MCP, a competitive inhibitor of ethylene action)-delayed, and a combination of 1-MCP with ethylene treatment (1-MCP+ethylene), were analysed by real-time quantitative PCR. In addition, the promoter of one ripening-related NAC, MaNAC2, was isolated and its response to ethylene was also investigated. More importantly, the direct interactions between MaNAC1/MaNAC2 and MaEIL5, a downstream component of ethylene signalling, were detected by yeast two-hybrid and the bimolecular fluorescence complementation (BiFC) assays. Our results suggest that MaNAC genes may be involved in banana fruit ripening via interaction with ethylene signalling components.
Materials and methods
Plant materials and treatments
Pre-climacteric banana (Musa acuminata, AAA group, cv. Carvendish) fruit at the 75–80% plump stage were obtained from a local commercial plantation near Guangzhou, south-eastern China. The hands were separated into individual fingers, and fruit of uniform weight, shape, and maturity, as well as being free of visual defects, were selected. The fruit were first surface sterilized by dipping in a 1% hypochloride solution for 1min and then immersed in 0.05% Sporgon (with 46% Prochloraz–Mn; Aventis, Valencia, Spain) for 3min to prevent fungal disease. They were then allowed to air dry at 25 °C for 2h and treated as follows.
The selected banana fruit were randomly divided into four groups of 150 fingers each for the following treatments. Fifteen unsealed polyethylene plastic bags (0.01mm in thickness) of ten fingers each were used for each treatment, and samples were taken based on the rate of ethylene production and fruit firmness change during ripening. Group 1 (control, non-conditioned for natural ripening) fruit were stored directly at 22 °C and 90% relative humidity for 25 days; samples were taken at 0, 1, 3, 5, 7, 15, 19, 21, and 25 days until the fruit ripened completely. Fruit of group 2 were treated with 100 µl l–1 ethylene for 18h in a closed chamber and then stored at 22 °C for 7 days; samples were taken at 0, 1, 3, 5, and 7 days of storage. Fruit of group 3 were treated with 0.5 µl l–1 1-MCP for 18h and then stored at 22 °C for 35 days; samples were taken at 0, 1, 3, 5, 7, 21, 25, 30, and 35 days of storage. Fruit of group 4 were treated with 100 µl l–1 ethylene followed by 0.5 µl l–1 1-MCP for 18h, and then stored at 22 °C for 30 days and samples were taken at 0, 1, 3, 5, 7, 15, 17, 19, 21, 25, 28, and 30 days of storage.
All of the samples were frozen in liquid nitrogen immediately after sampling, and stored at –80 °C for further use. All assessments were conducted using three biological replicates.
Fruit ripening evaluations
Fruit ripening was evaluated using the following two parameters: ethylene production and fruit firmness. Ethylene production was determined in three replicates each containing three fruit at the given sampling times. The three fruit were placed in a 2 l flask and capped with a rubber stopper for 2h at 22 °C. A 1ml sample of headspace gas was sampled and analysed for ethylene using gas chromatography (Model GC-17A; Shimadzu Co., Kyoto, Japan) fitted with a flame ionization detector and an activated alumina column (200 cm×0.3cm), with an injector temperature of 120 °C, column temperature of 60 °C, and detector temperature of 60 °C, according to the method of Wang et al. (2006).
Fruit firmness was measured using a penetrometer (Model Instron 5542; Instron Co., USA) equipped with a cylindrical flat-surfaced plunger (6mm diameter). A small slice of fruit skin was removed and firmness was then recorded from three different fruit, with three different points per fruit; means were expressed as newtons (N).
RNA extraction, gene isolation, and sequence analysis
Frozen tissues were ground in liquid nitrogen using a mortar and pestle. Total RNA was extracted using the hot borate method of Wan and Wilkins (1994). Potentially contaminating DNA was eliminated by treatment with DNAse I digestion using an RNAse-free kit (Promega Madison, WI, USA). The DNA-free total RNA was then used as template for RT-PCR. The first-strand cDNA of the product was subjected to PCR amplification.
Six NAC genes, termed MaNAC1–MaNAC6, were isolated from our transcriptome database obtained using a high-throughput Solexa/Illumina sequencing platform (Beijing Genomics Institute, Shenzhen, China) and the sequences were first verified by recloning and resequencing. Two of the six NAC genes, MaNAC3 and MaNAC4, were full-length sequences in the database, with complete start and stop codes, while full-length sequences of the other four NAC genes, including MaNAC1, MaNAC2, MaNAC5 and MaNAC 6, were obtained by 3’- or 5’-rapid amplification of cDNA ends (RACE) using a RACE kit (TaKaRa Biotechnology, Dalian, PR China) according to the manufacturer’s instructions. The specific primers used for RACE are provided in Supplementary Table S1 at JXB online.
Alignments were carried out on CLUSTALW version 1.83 and GeneDoc software, and a phylogenetic tree was constructed using the neighbour-joining method in the MEGA5 program and visualized by TreeView software. The theoretical isoelectric points (pIs) and mass values for mature peptides were calculated using the PeptideMass program (http://web.expasy.org/peptide_mass/).
Subcellular localization of MaNAC proteins
The coding sequences of MaNAC1–MaNAC6 without the stop codon were amplified by PCR (primers are listed in Supplementary Table S2 at JXB online) and subcloned into the pBI221-GFP vector, in frame with the green fluorescent protein (GFP) sequence, resulting in 35S::gene–GFP vectors under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The fusion constructs and the control GFP vector were used for transient assays using a modified polyethylene glycol (PEG) transfection method with tobacco BY-2 suspension culture cell protoplasts as described previously (Abel and Theologis, 1994). GFP fluorescence was observed with a fluorescence microscope (Zeiss Axioskop 2 Plus). All transient expression assays were repeated at least three times.
Transcriptional activation analysis in yeast cells
The coding regions of MaNAC1–MaNAC6 were cloned into the pGBKT7 vector (Clontech, USA) to create the pGBKT7-MaNAC1 to -6 constructs, respectively. Following the protocol of the manufacturer, pGBKT7-MaNAC1 to -6, the positive control pGBKT7-53+pGADT7-T, and the negative control pGBKT7 plasmids were used to transform the AH109 yeast strain using the lithium acetate method. The transformed strains were streaked onto minimal medium without Trp (SD/–Trp) or SD/–Trp–His–Ade plates, and the transactivation activity of each protein was evaluated according to their growth status and the activity of α-galactosidase. For mapping the activation domain, different truncated derivatives were constructed and transformed into the yeast strain for transcription activation activity as described above. The primers used for transcriptional activation analysis are listed in Supplementary Table S3 at JXB online.
Quantitative real-time PCR analysis
Isolation of total RNA from the samples and synthesis of first-strand cDNA were performed as described above. The synthesized cDNA was diluted 1:40 with water, and 2 µl of the diluted cDNA was used as a template for quantitative real-time PCR analysis (qPCR). PCRs were performed in a total volume of 20 µl, containing 1 µl of each primer (10 µM; final concentration 200nM) and 10 µl of SYBR® Green PCR Supermix (Bio-Rad Laboratories) on a Bio-Rad CFX96 Real-Time PCR System according to the manufacturer’s instructions. The qPCR program included an initial denaturation step at 94 °C for 5min, followed by 40 cycles of 10 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C. No-template controls for each primer pair were included in each run. The oligonucleotide primers for qPCR analysis were designed on the basis of the 3’-untranslated region using Primer 5.0 software. The sequences of all primers used for qPCR analysis are described in Supplementary Table S4 at JXB online. RPS2 (ribosomal protein 2) and CAC (clathrin adaptor complex) were selected as reference genes under different series samples according to our previous study on the selection of reliable reference genes for expression study by qPCR in banana fruit (L. Chen et al., 2011). Each assay using the gene-specific primers amplified a single product of the correct size with high PCR efficiency (90–110%). All qPCRs were normalized using the cycle threshold (C t) value corresponding to the reference gene. The relative expression levels of the target gene were calculated using the formula 2–ΔΔCt (Livak and Schmittgen, 2001). The values represent the mean of three biological replicates.
Promoter isolation and its activity assay
Genomic DNA was extracted from banana leaves using a DNeasy Plant Mini kit (Qiagen). Genomic DNA (2 µg for each) was digested separately with eight restriction enzymes (DraI, Ecl136II, EcoRV, HpaI, MscI, StuI, SspI, and ScaI). After enzyme digestion, all products were cleaned up using a DNA Clean and Concentration kit (Zymo Research) and then ligated to an adaptor (Clontech).
The 5’-upstream region of the MaNAC2 gene was isolated using a Genome Walker kit (Clontech) with nested PCR. The nested PCR analysis was performed with two sets of primers, including two adaptor primers that were obtained from the kit and two gene-specific primers that were designed by Primer 5.0, as listed in Supplementary Table S5 at JXB online. The amplification product was cloned into the pGEM-T Easy vector (Promega) and sequenced. Conserved cis-element motifs of the promoter were predicted using the PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html) and Plant-CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html) databases.
For the promoter activity assay, the MaNAC2 promoter region (~1.5kb) was amplified by PCR using the specific primers listed in Supplementary Table S6 at JXB online. The PCR product was inserted into the vector pUC-GFP by replacing the CaMV 35S promoter to generate the construct PMaNAC2::GFP containing the GFP-coding region under the control of the MaNAC2 promoter. The construct PMaNAC2::GFP and the positive control 35S::GFP were transfected into tobacco BY-2 protoplasts using a PEG method as described above. Transfected protoplasts were subjected to 0 (control) or 0.8 mmol l–1 ethrel treatment and then incubated at 23 °C for 14h. The GFP signal was detected before and after ethylene treatment using a fluorescence microscope (Zeiss Axioskop 2 Plus).
Yeast two-hybrid (Y2H) assay
Y2H assays were performed using the Matchmaker™ Gold Yeast Two-Hybrid System (Clontech). The coding regions of MaNAC1–MaNAC6 and MaEIL5 were cloned into pGADT7 and pGBKT7 to fuse with the activation domain (AD) and DNA-binding domain (DBD), respectively, to create different baits and preys (primers are shown in Supplementary Table S7 at JXB online). MaEIL5 did not show any transcriptional activation activity in yeast cells (data not shown). Different pairs of bait and prey constructs were then co-transformed into yeast strain Gold Y2H using the lithium acetate method, and yeast cells were grown on a SD/–Leu/–Trp) according to the manufacturer’s protocol (Clontech) for 3 d. Transformed colonies were plated onto minimal medium quadruple dropout (SD medium with –Leu/–Trp/–His/–Ade) containing 125 µM Aureobasidin A and 4mg ml–1 X-α-Gal at 30 °C to test for possible interactions between MaNAC1–MaNAC6 and MaEIL5 according to their growth status and the activity of α-galactosidase.
BiFC assay
To generate constructs for the BiFC assays, the full-length coding sequences of MaNAC1, MaNAC2 and MaEIL5 (without their stop codons) were subcloned into the pUC-pSPYNE or pUC-pSPYCE vector obtained from the laboratory of K. Harter and J. Kudla (Walter et al., 2004). Expression of target genes alone was used as negative controls. The resulting constructs were used for transient assays using a modified PEG transfection of tobacco BY-2 suspension culture cell protoplasts as described previously (Abel and Theologis, 1994). The transformed protoplasts were then grown in liquid MS medium containing 0.4M sucrose for 24–48h, and transfected cells were imaged using a fluorescence microscope with a yellow fluorescent protein (YFP) filter (Zeiss Axioskop 2 Plus). The primers used in the BiFC assay are listed in Supplementary Table S8 in JXB online.
Statistical analysis
The experiment was arranged in a completely randomized design. Each sample time point per treatment comprised three independent biological replicates. Data are plotted on figures as means ±standard error (SE).
Results
Isolation and sequence analysis of MaNAC genes from banana fruit
Six novel putative NAC full-length cDNAs were isolated from banana fruit and designated as MaNAC1–MaNAC6. MaNAC1–MaNAC6 were predicted to encode proteins of 283, 293, 284, 257, 302, and 278 aa, with calculated molecular weights of 31.3, 33.2, 31.9, 29.1, 33.8, and 31.1kDa and pI values of 8.71, 6.55, 9.20, 6.83, 7.60, and 5.43, respectively. Alignment of the full-length deduced proteins of the MaNACs clearly showed that the proteins contained the NAC conserved domain in their N-terminal regions, which was further divided into five subdomains (A–E) (Ooka et al., 2003) (Fig. 1). However, the C-terminal regions showed no significant similarity to that of any other members of NAC family (Fig. 1). In addition, although all the MaNACs proteins had the conserved NAC domain, the similarities among the six MaNACs varied from 20% (MaNAC1 and MaNAC4) to 66% (MaNAC2 and MaNAC4) (Supplementary Table S9 at JXB online).
Fig. 1.
Amino acid sequence alignment of the MaNACs proteins with other plant NAC proteins. MaNACs were aligned with Arabidopsis ANAC019 (At1g52890.1), ANAC042 (At2g43000.1), ANAC043 (At2g46770.1), and ANAC073 (At4g28500.1), and rice ONAC022 (AK107090). Identical and similar amino acids are indicated by black and grey shading, respectively. Gaps were introduced to optimize alignment. The five highly conserved amino acid motifs (A–E) and the nuclear localization signal (NLS) are indicated by black and grey lines, respectively.
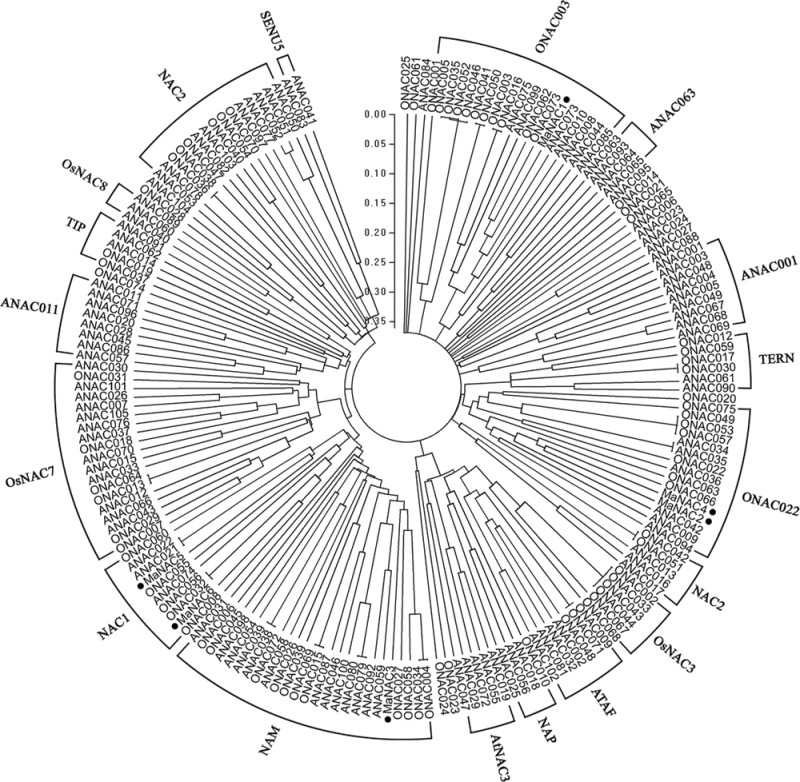
To examine the phylogenetic relationship among the NAC domain proteins in banana, Arabidopsis and rice, a phylogenetic tree was constructed based on their translated amino acid sequences. As shown in Fig. 2, the whole NAC proteins of Arabidopsis and rice could be classified into two major groups, I and II, which comprised 14 and four subgroups, respectively (Ooka et al., 2003). Based on the tree, the banana six NAC genes were clustered into four subfamilies. MaNAC2 and MaNAC4 belonged to ONAC022, and MaNAC5 and MaNAC6 belonged to NAC1, whereas MaNAC1 and MaNAC3 belonged to ONAC003 and NAM, respectively (Fig. 2). Collectively, these data suggested that MaNAC1–MaNAC6 may exhibit diverse functions.
Fig. 2.
Phylogenetic tree of NACs. Six banana MaNACs (black circles) were aligned with those of the Arabidopsis and rice NAC families as designated by Ooka et al. (2003). Multiple alignment was carried using CLUSTALW and the phylogenetic tree was constructed with MEGA5.0 using a bootstrap test of phylogeny with minimum evolution test and default parameters. The GenBank accession numbers of the Arabidopsis and rice NAC proteins are listed as Supplementary Data at JXB online.
Subcellular localization of the MaNAC proteins
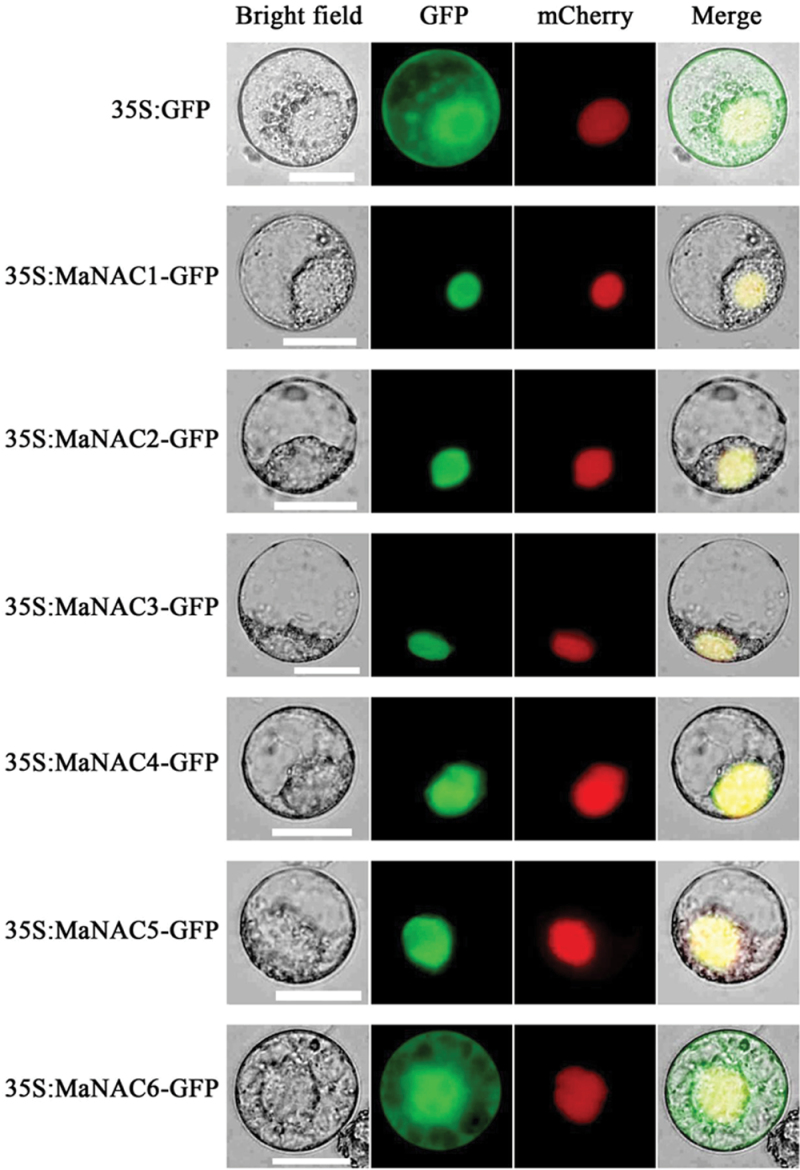
NAC proteins are TFs that are usually located in the nucleus; however, some NAC proteins are also membrane associated (Seo et al., 2010; Hao et al., 2011). To validate the subcellular localizations of the MaNAC1–MaNAC6 proteins, the full-length coding sequences of MaNAC1–MaNAC6 were fused in frame with the GFP gene. Transient expression of these constructs in tobacco BY-2 protoplasts showed that the fluorescence of MaNAC1–MaNAC5 was localized exclusively in the nucleus (Fig. 3). By contrast, the fluorescence from the GFP control was distributed rather evenly within the cells. Interestingly, the fluorescence of MaNAC6 was not targeted exclusively to the nucleus, and the GFP signal was also found in the cytoplasm and cell membrane (Fig. 3), although it contained a nuclear localization sequence (NLS).
Fig. 3.
Subcellular localization of MaNACs in tobacco BY-2 protoplasts. Protoplasts were transiently transformed with MaNAC–GFP constructs or GFP vector using a modified PEG method. GFP fluorescence was observed with a fluorescence microscope. VirD2NLS-mCherry was included in each transfection to serve as a control for successful transfection, as well as for nuclear localization. Images were taken in a dark field for green fluorescence, while the outline of the cell and the merged were photographed in a bright field. Bars, 25 µm.
Transcriptional activation ability of MaNAC proteins in yeast
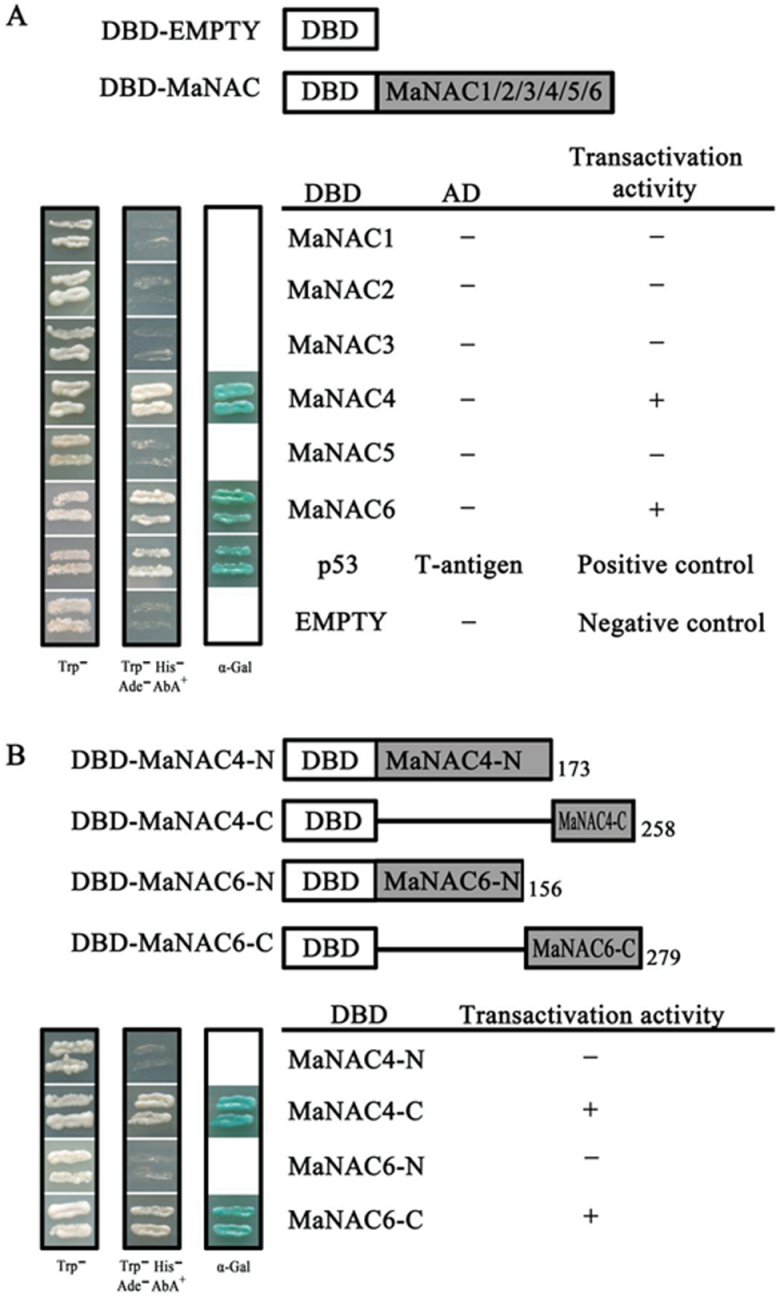
It has been reported that the N-terminal regions of NAC proteins show DNA-binding ability while the C-terminal regions function as a transcriptional activation domain (Olsen et al., 2005). To investigate the abilities of MaNAC1–MaNAC6 to activate transcription, a transient expression assay using a GAL4-responsive reporter system in yeast cells was performed. First, the full-length coding regions of MaNAC1–MaNAC6 were fused to the GAL4 DBD to generate pGBKT7-MaNAC1 to -6 fusion plasmids (DBD–MaNAC1 to -6) (Fig. 4A). The plasmids were then transformed into yeast strain AH109 and the transformants were assayed for their ability to activate transcription from the GAL4 upstream activation sequence and to promote yeast growth in medium lacking histidine. The transformants containing pGBKT7-53+pGADT7-T (DBD–P53+T-antigen) and pGBKT7 vectors (empty) were used as positive and negative controls, respectively. Fig. 4A showed that the transformed yeast cells harbouring DBD–MaNAC4, DBD–MaNAC6 and DBD–P53+T-antigen (positive control) grew well in SD medium lacking tryptophan, histidine, and adenine, and showed α-galactosidase activity, whereas cells containing DBD–MaNAC1, DBD–MaNAC2, DBD–MaNAC3, DBD–MaNAC5, or pGBKT7 (negative control) showed no α-galactosidase activity. To identify further the transcriptional activation domains of MaNAC4 and MaNAC6, the N- and C-terminal regions of MaNAC4 and MaNAC6 were fused to the GAL4 DBD (Fig. 4B) and transformed into yeast strain AH109. As shown in Fig. 4B, yeast cells harbouring DBD–MaNAC4-C and DBD–MaNAC6-C showed α-galactosidase activity. These data confirmed that MaNAC4 and MaNAC6 exhibited transcriptional activation ability in their C-terminal regions.
Fig. 4.
Transcriptional activation of MaNACs in yeast. (A) The coding regions of MaNAC1–MaNAC6 were cloned into the pGBKT7 (GAL4 DBD) vector to create the DBD–MaNAC1 to -6 constructs, respectively. (B) Truncation analysis of transcriptional activation of MaNAC4 and MaNAC6 for mapping activation domain. C- and N-terminal derivatives of MaNAC4 and MaNAC6 were fused with the pGBKT7 vector to create the DBD–MaNAC4 and -6 constructs, respectively. The numbers on the right indicate the last residues of the polypeptides. In (A) and (B), all of the constructs mentioned above, together with the positive control (p-53+T-antigen) and negative control (pGBKT7) were transformed into yeast strain AH109. Yeast clones transformed with different constructs were grown on SD plates without tryptophan or without tryptophan, histidine, and adenine but containing 125 µM Aureobasidin A for 3 d at 30 °C. Transcription activation was monitored by the detection of yeast growth and an α-galactosidase (α-Gal) assay.
Changes in fruit firmness and ethylene production in fruit with four different ripening characteristics
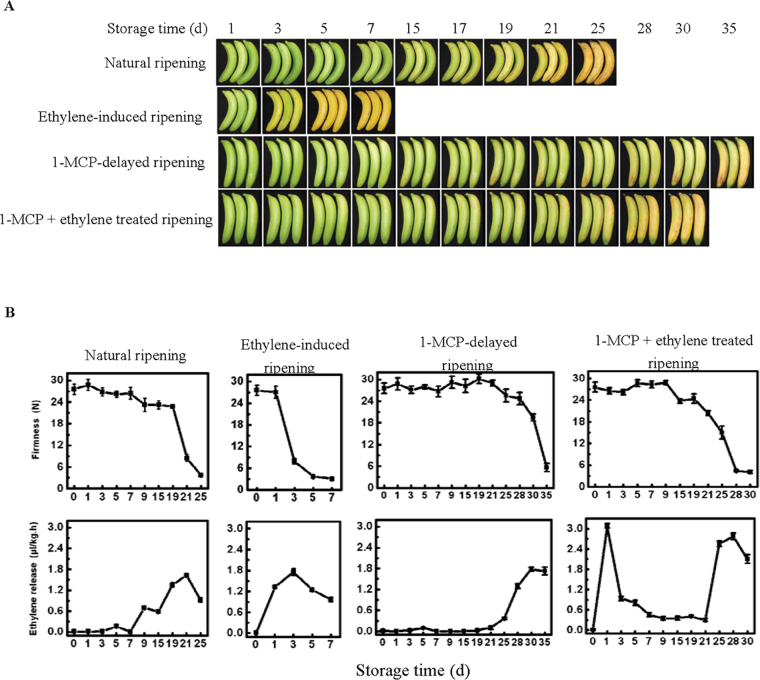
Changes in banana fruit firmness and ethylene production in fruit with four different ripening characteristics, comprising natural, ethylene-induced, 1-MCP-delayed, and a combination of 1-MCP with ethylene treatment, were shown in Fig. 5. The firmness of fruit ripened naturally began to decline at day 19, reaching a firmness of ~4 N at day 25, with ethylene production obviously increased at day 15, reaching a maximum at day 21 and then decreasing at day 25. The decline in fruit firmness and increase in ethylene production were substantially faster in ethylene-treated fruit, reaching the same ripening stage at day 7 as natural ripening at day 35, and ethylene production in ethylene-treated fruit began to be detected at 1 d of treatment, peaking at day 3. In contrast, treatment with 1-MCP delayed fruit softening and the increase in ethylene production, its firmness began to decline at day 28 reaching ~5 N at day 35, and ethylene production was detected at day 25 and reached a peak at day 30. However, application of ethylene to the 1-MCP-treated fruit promoted a decline in the firmness and an increase in ethylene evolution, the firmness of the fruit began to decline at day 21, reaching to ~4N at day 30, and ethylene production started to increase at day 25 and reached a peak at day 28. It should be noted that the higher ethylene production of 1-MCP+ethylene-treated fruit at day 1 might be the residual ethylene of the treatment.
Fig. 5.
Photograph of fruit with four different ripening characteristics, comprising natural (control), ethylene-induced, 1-MCP-delayed, and a combination of 1-MCP+ethylene treated ripening (A), and changes in fruit firmness and ethylene production (B) during ripening. Each value represents the mean ±SE of three replicates.
Expression of MaNAC and MaEIL genes in peel and pulp during fruit ripening
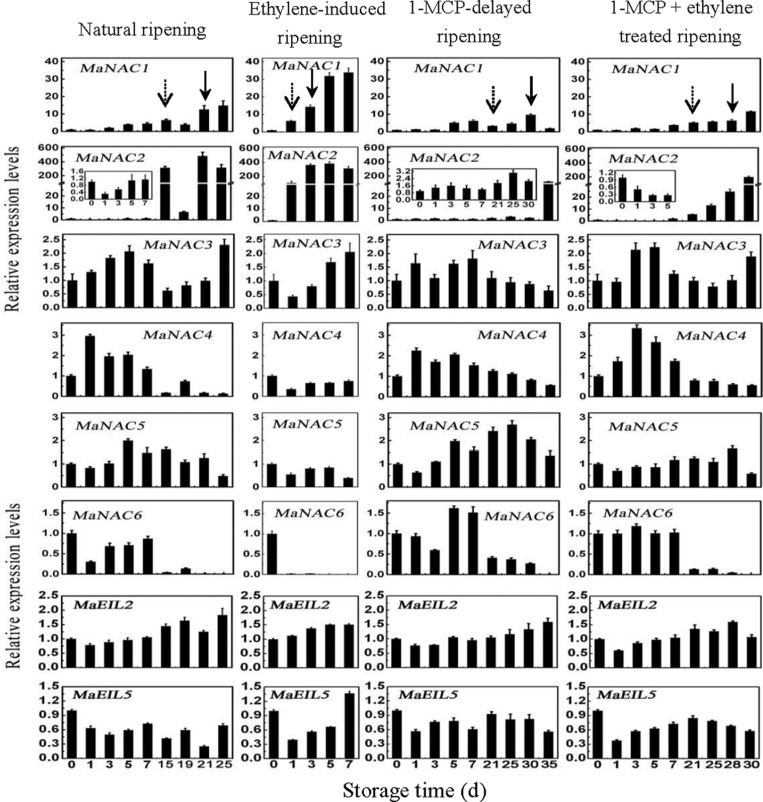
To understand the possible role of MaNAC1–MaNAC6 in banana fruit ripening, the expression patterns of MaNAC1–MaNAC6 in peel and pulp of fruit with the four different ripening characteristics described above were investigated by qPCR. As shown in Fig. 6, in peel tissues, among the six MaNAC genes, MaNAC1 and MaNAC2 were strongly induced at 1 d after ethylene treatment, with ~30- and ~350-fold increases at day 3, respectively. Their transcript levels in peel were low during early storage, but clearly began to increase with ethylene evolution. In contrast, MaNAC4 and MaNAC6 transcript levels decreased following ethylene treatment and ethylene evolution, and their transcript levels in peel decreased following the increase in ethylene production, with a more remarkable decrease for MaNAC6. In addition, MaNAC3 and MaNAC5 were expressed constitutively and their transcripts changed only slightly during ripening.
Fig. 6.
Expression of MaNAC and MaEIL genes in banana fruit peel with four different ripening characteristics: natural (control), ethylene-induced, 1-MCP-delayed, and a combination of 1-MCP and ethylene-treated ripening. The expression levels of each gene are expressed as a ratio relative to the harvest time (0 d of control), which was set at 1. Each value represents the mean ±SE of three replicates. The broken arrow and full arrow represent the time point of ethylene production beginning to increase and its peak for each treatment, respectively.
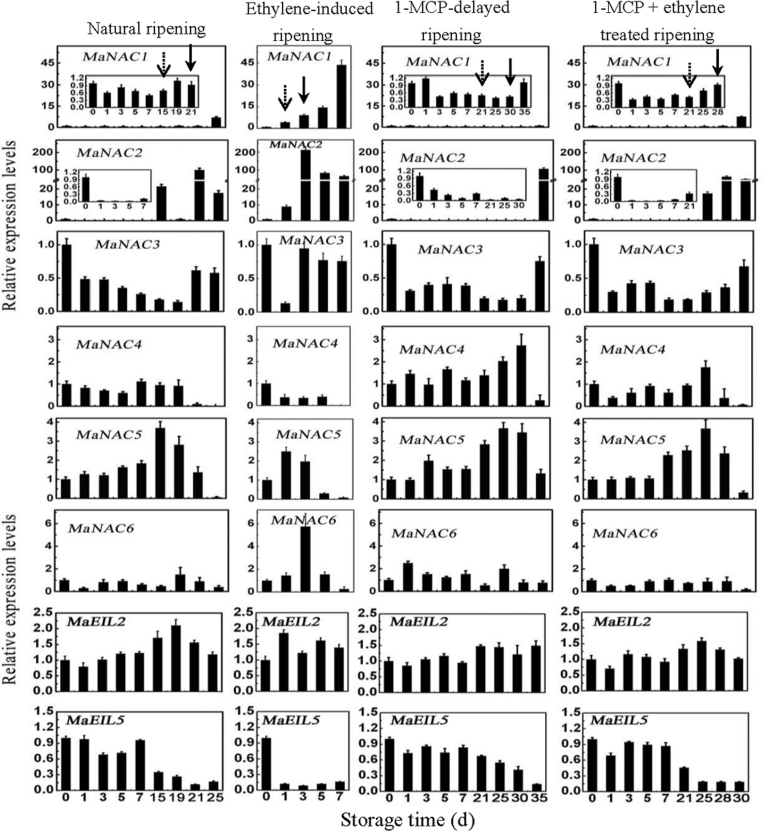
In pulp tissues, as shown in Fig. 7, expression of MaNAC1, MaNAC2, MaNAC3, and MaNAC4 showed a similar change in pattern to their expression in peel. MaNAC1 and MaNAC2 were ethylene induced and their transcript levels in natural, ethylene-induced, 1-MCP-delayed ripening, or a combination of 1-MCP with ethylene-treated fruit clearly increased with ethylene evolution. The MaNAC3 transcript level changed slightly and MaNAC4 transcript decreased following ethylene production increase during the ripening stage. Unlike the expression patterns in peel, expression of MaNAC5 and MaNAC6 was slightly enhanced after ethylene treatment, and their transcript levels in natural, 1-MCP-delayed ripening, or a combination of 1-MCP with ethylene-treated fruit also increased following the increase in ethylene production. These results indicated that MaNAC genes are differentially expressed in peel and pulp during post-harvest ripening, and that MaNAC1 and MaNAC2 may be more related to fruit ripening.
Fig. 7.
. Expression of MaNAC and MaEIL genes in banana fruit pulp with four different ripening characteristics: natural (control), ethylene-induced, 1-MCP-delayed, and a combination of 1-MCP and ethylene-treated ripening. The expression levels of each gene are expressed as a ratio relative to the harvest time (0 d of control), which was set at 1. Each value represents the mean ±SE of three replicates. The broken arrow and full arrow represent the time point of ethylene production beginning to increase and its peak for each treatment, respectively.
Five EIN-like genes, designated MaEIL1–MaEIL4, and AB266321 (termed MaEIL5 in the present work) have been isolated from banana fruit in a previous study, and it was shown that, in pulp tissue, MaEIL2 was a unique ripening- and ethylene-induced gene, while the MaEIL1, MaEIL4, and AB266321 (MaEIL5) genes were downregulated during ripening (Mbéguié-A-Mbeguié et al., 2008). As shown in Figs 6 and 7, similar to the previous report, MaEIL2 in peel and pulp was ethylene induced and its transcript level increased, while that of MaEIL5 in peel and pulp clearly decreased once ethylene evolution could be detected during ripening. MaEIL1and MaEIL4 transcript levels in pulp also decreased during ripening but the changes were not as obvious as that of MaEIL5 (data not shown).
The promoter activity of MaNAC2 is induced by ethylene
Gene expression profiles of the MaNAC genes during post-harvest fruit ripening suggested that MaNAC2 was ethylene inducible and might be involved in fruit ripening. To better establish the mechanisms by which MaNAC2 expression is modulated, a 1529bp upstream sequence from the start codon of MaNAC2 was isolated from the genome of Musa acuminata using a genome-walking PCR method. Analysis of the promoter using the PLACE and Plant-CARE databases, as illustrated in Table 1, revealed that 15 distinct putative cis-acting elements were identified in the promoter region. Besides the core cis-acting elements like the TATA-box and CAAT-box, other potential cis-regulatory elements involved in the activation of abiotic stress (cold, water, and heat) and hormone-responsive (GA and SA) genes were also found. Interestingly, two sites for the ethylene-responsive element, ATTTCAAA, with one nucleotide change found in the promoter at positions –11 (ATTACAAA) and –680 (ATTTCACAA) upstream of ATG, could probably govern expression in response to ethylene.
Table 1 .
Main regulatory motifs found within the promoter sequence of MaNAC2
| Factor or site name | Site | Signal sequence | Function |
| MYB1AT | 181(+); 147(+); 451(–); 900(–) | WAACA | MYB recognition site found in the promoters of the dehydration-responsive gene rd22 and many other genes |
| MYB2AT | 1018(–) | TAACTG | Binding site for regulation of genes that are responsive to water stress |
| MYBCORE | 162(+); 394(+); 633(+); 1018(+); 159 (–) | CNGTTR | Binding site for regulation of genes that are responsive to water stress |
| MYCCONSENSUSAT | 375(+); 481(+); 499(+); 1239(+); 1523(+); 375(–); 481(–); 499(–); 1239(–); 1523(–) | CANNTG | Regulates the transcription of CBF/DREB1 genes in the cold |
| HSE | 646(–); 972(+); 886(+); 973(+) | AAAAAATTTC | Cis-acting element involved in heat stress responsiveness |
| GARE | 603(+) | TCTGTTG | Gibberellin-responsive element |
| LTRE1HVBLT49 | 1491(–) | CCGAAA | Cis-acting element involved in low-temperature responsiveness |
| LTRECOREATCOR15 | 1154(–) | CCGAC | Core of low temperature responsive element (LTRE) of cor15a gene |
| GAREAT | 866(+) | TAACAAR | Gibberellin-responsive element |
| P-box | 670(–) | GCCTTTTGAGT | Gibberellin-responsive element |
| MBS | 989(–) | TAACTG | MYB-binding site involved in drought inducibility |
| TC-rich repeats | 444(+); 970(–);850(–) | ATTTTCTCCA | Cis-acting element involved in defense and stress responsiveness |
| TCA element | 1223(+); 1226(–) | CAGAAAAGGA | Cis-acting element involved in salicylic acid responsiveness |
| WBOXNTERF3 | 425(–);1290(–);1275(–);1335(–) | TGACY | May be involved in activation of ERF3 gene by wounding |
| WRKY71OS | 77(+); 359(+); 695(+); 104(–); 426(–);1276(–); 1281(–); 1291(–); 1336(–) | TGAC | Binding site of rice WRKY71, a transcriptional repressor of the gibberellin signaling pathway |
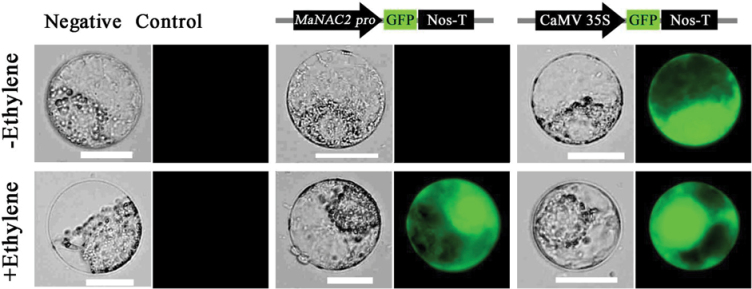
To analyse MaNAC2 promoter activity in response to ethylene, we conducted a transient protoplast assay using a GFP reporter construct containing the MaNAC2 promoter (MaNAC2pro::GFP). As shown in Fig. 8, in tobacco BY-2 protoplast transfected with MaNAC2pro::GFP, GFP signals were not detected before ethylene treatment but were observed after ethylene treatment. As a positive control, a GFP signal was constitutively observed before or after ethylene treatment in protoplasts transfected with the CaMV 35S promoter-driven construct (35S::GFP), and no GFP signal was detected in protoplasts that were not transfected. Similar results were also obtained in Arabidopsis mesophyll protoplasts (Supplementary Fig. S1 at JXB online). The results that ethylene induced the MaNAC2 promoter activity further support the suggestion that MaNAC2 is ethylene inducible and might be involved in fruit ripening.
Fig. 8.
MaNAC2 promoter activity in response to ethylene. GFP reporter constructs containing the MaNAC2 promoter (MaNAC2pro::GFP) and the CaMV 35S promoter (35S::GFP, positive control) were transiently transformed into tobacco BY-2 protoplasts using a modified PEG method and test for ethylene induction. Non-transformed protoplasts were used as a negative control. After incubation for 12h, GFP fluorescence was observed by fluorescence microscopy. Bar, 25 µm. The experiment was repeated at least three times.
MaNAC1 and MaNAC2 physically interact with MaEIL5
Several reports have suggested that NAC TFs are involved in ethylene signalling pathways through interaction with ethylene signalling components, such as EIN2 (He et al., 2005; Kim et al., 2009; Al-Daoud and Cameron, 2011). Based on the expression characteristics of the MaNAC and MaEIL genes during fruit ripening, it is thought that possible interactions between MaNAC and MaEIL proteins might exist.
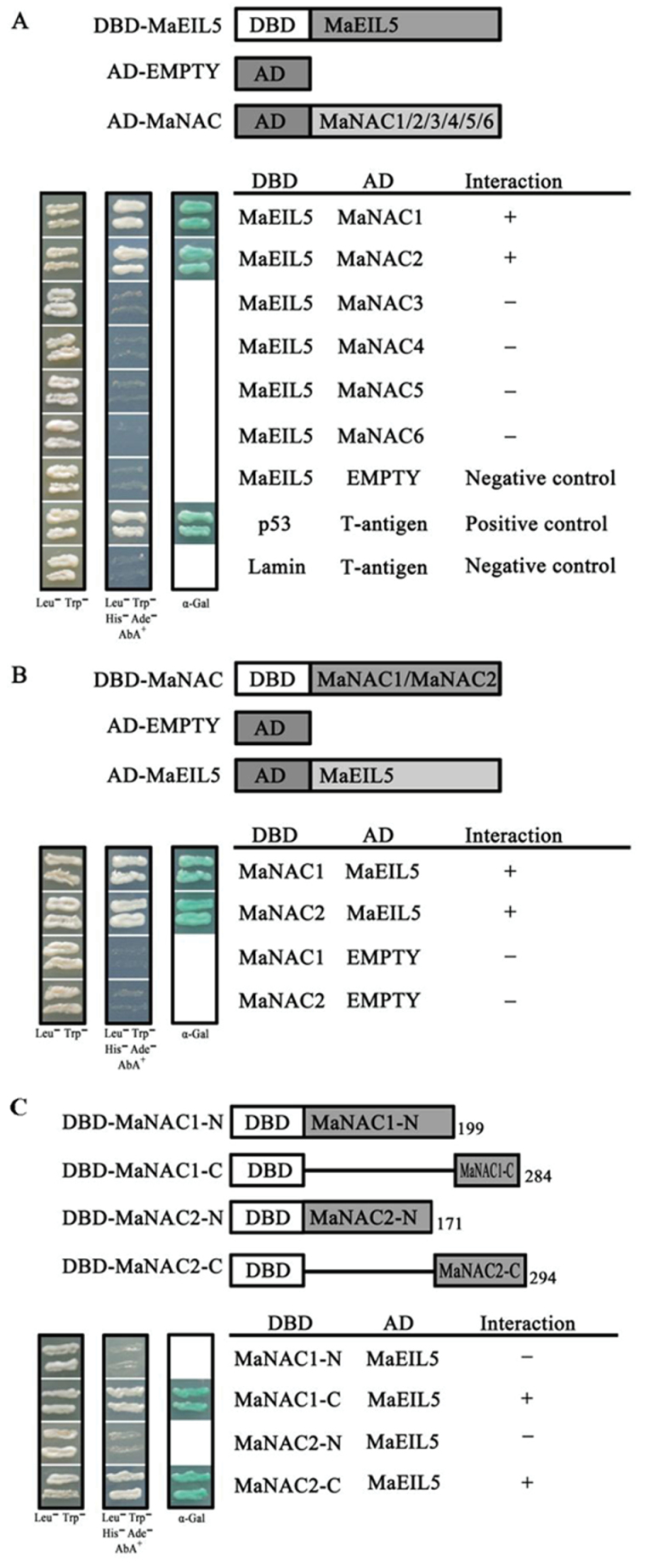
In order to investigate whether MaNACs physically interact with MaEILs, the Matchmaker™ Gold Yeast Two-Hybrid System was used to investigate the interactions between MaNACs and MaEILs. MaNAC1–MaNAC6 and MaEIL5 coding sequences were subcloned into pGADT7 and pGBKT7 vectors for a Y2H assay, as MaNAC4 and MaNAC6 showed transactivation activity in yeast when fused with the DBD (Fig. 4). As shown in Fig. 9A, yeast cells co-transformed the positive control (pGBKT7-53+pGADT7-T) and MaNAC1 or MaNAC2 with MaEIL5, could grow on selective medium (synthetic medium lacking tryptophan, leucine, histidine, and adenine) supplement with the toxic drug Aureobasidin A, and turned blue in the presence of the chromagenic substrate X-α-Gal. However, yeast cells harbouring MaNAC3, MaNAC4, MaNAC5, or MaNAC6 with MaEIL5, and the negative controls, could not grow on the selective medium and did not turn blue under the same conditions (Fig. 9A). Interactions between MaNAC1 or MaNAC2 and MaEIL5 were also observed, when MaNAC1 or MaNAC2 and MaEIL5 coding sequences were subcloned into pGBKT7 and pGADT7, respectively (Fig. 9B). Moreover, the C terminus of MaNAC1 or MaNAC2 was sufficient for the interaction with MaEIL5 (Fig. 9C). In addition, we also examined the interactions between MaNAC1–MaNAC6 and MaEIL2 but detected no interactions among them (data not shown).
Fig. 9.
. Physical interactions between MaNAC proteins and MaEIL5 detected in Y2H assays. (A) The coding regions of MaNAC1–MaNAC6 were cloned into the pGADT7 vector to create the AD–MaNAC1 to -6 constructs, while the coding region of MaEIL5 was cloned into the pGBKT7 vector to create the DBD–MaEIL5 construct. Gold Y2H yeast strains were co-transformed with DBD–MaEIL5 and AD–MaNAC1 to -6, respectively. (B) The coding regions of MaNAC1/2 were cloned into the pGBKT7 vector to create the DBD–MaNAC1 and -2 constructs, while the coding region of MaEIL5 was cloned into the pGADT7 vector to create the AD–MaEIL5 construct. Gold Y2H yeast strains were co-transformed with DBD–MaNAC1 or -2 and AD–MaEIL5, respectively. (C) The N and C termini of MaNAC1 and MaNAC2 were tested for interaction with MaEIL5. Gold Y2H yeast strains were co-transformed with DBD–MaNAC1 or -2 derivatives and AD–MaEIL5, respectively. In (A), (B) and (C), the ability of yeast cells to grow on synthetic medium lacking tryptophan, leucine, histidine, and adenine but containing 125 µM Aureobasidin A, and to turn blue in the presence of the chromagenic substrate X-α-Gal, was scored as a positive interaction. Yeast cells transformed with pGBKT7-53+pGADT7-T, DBD–MaEIL5+pGADT7-T, or pGBKT7-Lam+pGADT7-T were included as positive or negative controls, respectively.
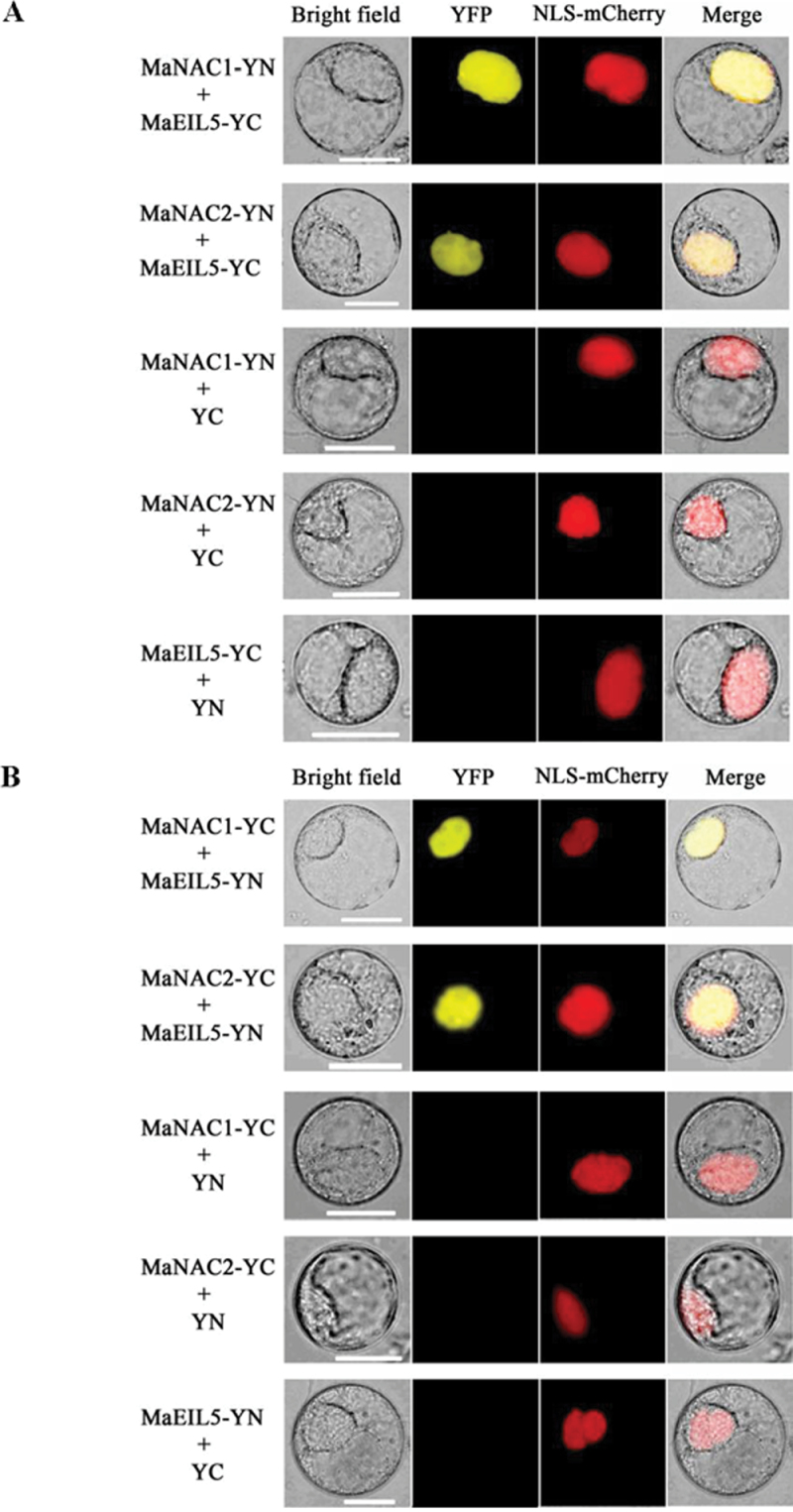
To further confirm the interactions between MaNAC1/MaNAC2 and MaEIL5 observed in the Y2H assays, we performed BiFC assays in tobacco BY-2 protoplasts. MaNAC1 or MaNAC2 tagged with pSPYNE (split YFP N-terminal fragment expression) and MaEIL5 tagged with pSPYCE (split YFP C-terminal fragment expression) were transiently co-expressed in tobacco BY-2 protoplasts following PEG transfection. As shown in Fig. 10A, robust YFP fluorescent signal was detected in the nucleus of BY-2 cells expressing MaNAC1-pSPYNE or MaNAC2-pSPYNE and MaEIL5-pSPYCE, while no YFP fluorescent signal was observed either in the cells expressing MaNAC1/MaNAC2-pSPYNE with only the pSPYCE or in those expressing MaEIL5-pSPYCE with only the pSPYNE (Fig. 10A). Similar results were also observed when MaNAC1/MaNAC2-pSPYCE was co-transfected with MaEIL5-pSPYNE (Fig. 10B). The BiFC results not only demonstrated the in vivo interaction among the three proteins tested but also showed the specific localization of the interacting proteins in the nucleus, which was consistent with the subcellular localization of MaNAC1, MaNAC2, and MaEIL5 in the nuclear compartment (Fig. 3 and Supplementary Fig. S2 at JXB online).
Fig. 10.
BiFC visualization of the MaNAC1/MaNAC2 and MaEIL5 interaction in transiently co-expressed tobacco BY-2 protoplasts. (A) MaNAC1/ MaNAC2 and MaEIL5 proteins were fused with the N (YN) and C (YC) termini of YFP, respectively. (B) MaNAC1/MaNAC2 and MaEIL5 proteins were fused with the C (YC) and N (YN) terminus of YFP, respectively. Expression of MaNAC1/MaNAC2 or MaEIL5 alone was used as a negative control. VirD2NLS-mCherry was included in each transfection to serve as a control for successful transfection, as well as for nuclear localization. YFP fluorescence is yellow; the merged image is a digital merge of bright field and fluorescent images. Bar, 25 µm.
Discussion
Identification of banana MaNAC genes
NAC is a family of plant-specific TFs that have been identified in various species (Berger et al., 2009; Meng et al., 2009; Zhong et al., 2010). There are approximately 100 NAC TFs in both the Arabidopsis and rice genomes (Gong et al., 2004; Xiong et al., 2005). From our transcriptome database, there are at least six putative proteins that exhibit a significant sequence identity with Arabidopsis AtNAC and rice OsNAC homologues, further confirming the notion that NAC proteins are encoded by a large multigene family (Ooka et al., 2003). The predicted MaNAC paralogues were 20–66% identical to each other at the amino acid level (Supplementary Table S9). Alignment of the six MaNACs proteins showed that they shared a highly conserved N-terminal DBD, termed the NAC domain (Ooka et al., 2003), which contains five consensus subdomains (Fig. 1). The NAC domain may function as a potential NLS (Duval et al., 2002; Hegedus et al., 2003; Ernst et al., 2004) and most isolated NAC proteins are localized in the nucleus (Pinheiro et al., 2009; Hao et al., 2011). Similarly, the subcellular localizations of the MaNACs indicated that MaNAC1–MaNAC5 were all localized in the nucleus, while MaNAC6 was distributed throughout the whole cell, including nucleus, cytoplasm, and cell membrane (Fig. 3). Thirteen members of the membrane-associated type of NAC proteins have been found in Arabidopsis (Kim et al., 2007). These NACs, which are larger in molecular weight than non-membrane-associated NAC TFs (~320 residues), have residue numbers ranging from 335 to 652, because of their C-terminal extensions, which contain the transmembrane motif (Morishita et al., 2009). In eukaryotic cells, molecular mechanisms that regulate the localization of TFs have been investigated extensively, one of which is cytoplasmic sequestration caused by the presence of a membrane anchor (Lee and Hannink, 2003). Following specific cues, membrane-bound precursors are proteolytically cleaved, allowing nuclear localization of the TF (Morishita et al., 2009). One Arabidopsis NAC protein, AtNAC78, has been located in the nucleus and cytoplasm in onion epidermal cells when fused with its full-length sequence (including the transmembrane motif), but was located in the nucleus when the putative transmembrane motif was deleted (Morishita et al., 2009). However, the same is not true for MaNAC6, which includes a similar C-terminal extension to the other five MaNACs (Fig. 1). Thus, the mechanism and role of MaNAC6 localization within whole cells need to be elucidated further. In addition, the C-terminal regions of MaNAC1–MaNAC6 proteins are highly variable (Fig. 1). It has been reported that the non-conserved C-terminal regions of several NAC proteins, but not NAC domains, function as transcriptional activation domains (Hegedus et al., 2003; Fujita et al., 2004; Kim et al., 2006), which may confer the regulation diversities of transcriptional activation activity. Our data also showed that, among the six MaNAC proteins, only MaNAC4 and MaNAC6 exhibited transcriptional activation ability in their C-terminal regions, and the NAC domain in their N-terminals did not activate reporter genes in yeast cells (Fig. 4). In addition, it should be pointed out that as the largest TF family in plants, other NAC genes may exist in banana fruit and their functions also need to be elucidated.
Arabidopsis thaliana and Oryza sativa NAC proteins have been classified previously into two groups based on similarities in the NAC domain structures (Ooka et al., 2003). Group I is divided into 14 subgroups, namely TERN, ONAC022, SENU5, NAP, AtNAC3, ATAF, OsNAC3, NAC2, ANAC011, TIP, OsNAC8, OsNAC7, NAC1, and NAM, while the remaining four subgroups, comprising ANAC011, ONAC003, ONAC001, and ANAC063, constitute group II (Ooka et al., 2003). Although many reported NAC TFs are known for their diverse functions, the functions of the majority of members in this family are still unclear. Generally, NAC genes with the same function show a tendency to fall into one subgroup. For example, Arabidopsis ANAC072 (RD26), ANAC019, and ANAC055, which have been found to be involved in the response to various environmental stresses, are clustered into subgroup AtNAC3 (Tran et al., 2004; Fujita et al., 2004). Subgroup NAM encompasses NAM and CUC2, which have been shown to function in shoot apical meristem formation and development (Souer et al., 1996; Aida et al., 1997). SND1 (ANAC012), together with NST1 (ANAC043) and VND6 (ANAC101), are grouped into subgroup OsNAC7, which has been proposed to regulate secondary wall synthesis (Zhong et al., 2010). Additionally, the membrane-associated NAC proteins that harbour a transmembrane motif with a predicted α-helix in the far C-terminal region have been placed phylogenetically into subgroups ANAC001, TIP, and NAC2 (Kim et al., 2006). The members of these subgroups are highly expressed in vegetative organs that are more susceptible to abiotic stress conditions (Kim et al., 2006). In the present work, the six banana MaNAC genes fell into four subgroups, in which MaNAC1 and MaNAC3 belonged to ONAC003 and NAM, respectively, while MaNAC2 and MaNAC4 belonged to ONAC022, and MaNAC5 and MaNAC6 belonged to NAC1. These results suggest that MaNAC genes may have diverse functions in banana.
Possible roles of MaNAC TFs in relation to banana fruit ripening
The regulation of fruit ripening has been well studied, and ripening is known to be influenced by hormones, light, temperature, and developmental gene regulation (Klee and Giovannoni, 2011). However, critical TFs involving in fruit ripening were identified only recently, even in tomato fruit (Martel et al., 2011; Qin et al., 2012). One of the tomato MADS-box TFs, RIPENING INHIBITOR (RIN), exhibited elevated expression at the onset of ripening (Vrebalov et al., 2002). Moreover, recently, it has been confirmed that RIN interacts with the promoters of genes involved in the major pathways associated with observed and well-studied ripening phenotypes and phenomena, including the transcriptional control network involved in overall ripening regulation, ethylene biosynthesis, ethylene perception, the downstream ethylene response, cell-wall metabolism, carotenoid biosynthesis, and aroma formation (Martel et al., 2011; Qin et al., 2012). MADS-box TFs have also been suggested to be involved in the ripening of other fruit, such as strawberry (Seymour et al., 2011), apple (Yao et al., 1999), banana (Elitzur et al., 2010), and avocado (Hershkovitz et al., 2011). Interestingly, although banana MaMADS2 showed a high sequence similarity to tomato LeMADS-RIN, complementation analysis demonstrated that it could not complement a ripening rin mutant in tomato (Elitzur et al., 2010). In addition, other TFs, such as FaSPT, which encodes a basic helix–loop–helix TF, and FaASR, a TF involved in abscisic acid signal transduction, are related to strawberry ripening (Tisza et al., 2010; J.Y. Chen et al., 2011).
Little information about plant-specific NAC TFs associated with fruit ripening is available. In the present work, gene expression profiles in fruit with four different ripening characteristics – natural, ethylene-induced, 1-MCP-delayed, and 1-MCP and ethylene-treated ripening – revealed that banana MaNAC genes were differentially expressed in peel and pulp during ripening. MaNAC1 and MaNAC2 were apparently upregulated by ethylene in peel and pulp, consistent with the increase in ethylene production (Figs 6 and 7). In contrast, MaNAC3 in peel and pulp and MaNAC5 in peel were expressed constitutively, and transcripts of MaNAC4 in peel and pulp and MaNAC6 in peel decreased, while MaNAC5 or MaNAC6 in pulp slightly increased during fruit ripening (Figs 6 and 7). These results suggest that the transcription complexes created during ripening are constantly changing and MaNAC1/MaNAC2 expression may be more related to fruit ripening. Moreover, the MaNAC2 promoter, when transiently expressed in protoplasts, was activated after ethylene application, further confirming that MaNAC2 is ethylene inducible and may be involved in fruit ripening (Fig. 8 and Supplementary Fig. S1). Other plant NAC TFs, including Arabidopsis AtNAC2 (He et al., 2005), pepper CaNAC1 (Oh et al., 2005), rice OsNAC19 (Lin et al., 2007), and wheat TaNAC4 (Xia et al., 2010), are also ethylene inducible, supporting the notion that NAC TFs might be a common downstream component of the ethylene signalling pathway. In tomato, NOR (non-ripening) is a NAC-domain TF and fruit carrying its mutation, nor, cannot ripen even after exogenous ethylene application, suggesting an important role of NOR in regulating fruit ripening (Cara and Giovannoni, 2008). Thus, it will be interesting to investigate whether MaNAC1/MaNAC2 is able to complement ripening in a tomato nor mutant.
Interactions between MaNAC1/MaNAC2 and MaEIL5
NAC TFs have been found not only to bind to DNA but also to interact with other proteins, including several NAC proteins interacting with a RING protein (Greve et al., 2003), a TCP-domain TF (Weir et al., 2004), and a SNF1-related kinase (Kleinow et al., 2009). Moreover, some NAC proteins can interact with other NACs or with themselves to function by forming homodimers and/or heterodimers in plants, such as Arabidopsis NAC1 and ANA019 (Xie et al., 2000; Ernst et al., 2004), Brassica BnNAC14 with BnNAC3, BnNAC5-8, BnNAC5-11, and BnNAC485 (Hegedus et al., 2003), and rice OsNAC5 with OsNAC6, OsNAC9, OsNAC10, and itself (Jeong et al., 2009). Several groups have reported that NAC may represent a TF downstream of EIN2 but in parallel with EIN3, which is also a TF downstream of EIN2 (He et al., 2005; Kim et al., 2009; Al-Daoud and Cameron, 2011). In the present study, banana MaNAC1/MaNAC2 physically interacted with an EIN3-like protein, MaEIL5, in Y2H and BiFC assays (Figs 9 and 10). EIN3 and EIN3-like (EIL) proteins are key TFs in ethylene signalling, and play important roles in regulating fruit ripening (Mbéguié-A-Mbeguié et al., 2008; Yin et al., 2010). Our results, together with previous reports, clearly suggest that NAC TFs are involved in ethylene signalling pathways through interaction with ethylene signalling components. However, it should be pointed out that the MaEIL5 transcript level decreased following ethylene evolution during ripening (Figs 6 and 7). Generally, EILs act as positive regulators of ethylene response, but levels of EIL genes show no increase during fruit ripening, implying that changes in EIL mRNA levels may be not necessary to induce an ethylene response (Tieman et al., 2001). At the same time, EILs may be regulated at the post-transcriptional or post-translational level, as demonstrated by Potuschak et al. (2003), who showed that EBF1/EBF2 physically interacted with EIN3/EIL1 and probably targeted these proteins for degradation. Accordingly, it is imperative to study the protein modifications of EILs during banana fruit ripening in the future. In addition, it will be interesting to find why only MaNAC1/MaNAC2, which showed no transcription activation ability in yeast cells, could interact with MaEIL5, while MaNAC4/MaNAC6 with transcription activation ability, could not interact with MaEIL5 (Figs 4 and 9), and why none of the six MaNACs interacted with MaEIL2 (data not shown), whose transcript level increased during fruit ripening (Figs 6 and 7).
In summary, six banana fruit MaNAC genes were isolated and characterized. Gene expression profiles in fruit with four different ripening characteristics revealed that MaNAC genes are expressed differentially in peel and pulp during ripening. More importantly, MaNAC1/MaNAC2 physically interacted with MaEIL5. Taken together, these results suggest that MaNACs such as MaNAC1 and MaNAC2 may be involved in banana fruit ripening via interaction with ethylene signalling components. To the best of our knowledge, this is the first report that NACs interact physically with EILs, and our findings reveal a novel mechanism of NAC TFs participating in ethylene signalling.
© 2011 The Authors.
Supplementary Material
Supplementary data
Supplementary data are available at JXB online.
Supplementary data 1
Table S1. Primers used for RACE.
Table S2. Primers used for fusing GFP.
Table S3. Primers used for subcloning into pGBKT7.
Table S4. Primers used for quantitative real-time PCR analysis.
Table S5. Primers used for promoter isolation of MaNAC2.
Table S6. Primers used for MaNAC2 promoter fusing with GFP.
Table S7. Primers used for Yeast Two-Hybrid analysis.
Table S8. Primers used for BiFC assays.
Table S9. Sequence similarities among the different MaNACs genes.
Figure S1. MaNAC2 promoter activity in response to ethylene in Arabidopsis mesophyll protoplasts.
Figure S2. Subcellular localization of MaEIL5 in tobacco BY-2 protoplasts.
Supplementary data 2
GenBank accession numbers of Arabidopsis and rice NAC proteins used for phylogenetic tree of NACs.
Acknowledgements
We thank Professor Jörg Kudla (Institut für Biologie und Biotechnologie der Pflanzen, Universität Münster) and Professor Seiichiro Hasezawa (Department of Integrated Biosciences, The University of Tokyo) for the generous gift of BiFC vectors and tobacco BY-2 suspension cells, respectively. This work was supported in part by the National Public Benefit of Agricultural Research Foundation of China (grant no. 200903044-5), China Agriculture Research System (grant no. CARS-32-02A), National Natural Science Foundation of China (grant no. 31160406), and Guangdong Modern Agricultural Industry Technology System (grant no. LNSG2011-12).
Glossary
| Abbreviations | Expansion |
|---|---|
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| AD | activation domain |
| BiFC | bimolecular fluorescence complementation |
| CaMV | cauliflower mosaic virus |
| DBD | DNA-binding domain |
| EIN3 | ethylene insensitive 3 |
| EIL | ethylene insensitive 3-like |
| GFP | green fluorescent protein |
| 1-MCP | 1-methylcyclopropene |
| MADS | Mcm1-Agamous-Deficiens-S |
| NAC | NAM ATAF1/2 and CUC2 |
| PEG | polyethylene glycol |
| NLS | nuclear localization signal |
| pI | isoelectric point |
| qPCR | quantitative real-time PCR |
| TF | transcription factor |
| Y2H | yeast two-hybrid |
| YFP | yellow fluorescent protein |
References
- Abel S, Theologis A. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression The Plant Journal 5 421–427 [DOI] [PubMed] [Google Scholar]
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant The Plant Cell 9 841–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daoud F, Cameron RK. 2011. ANAC055 and ANAC092 contribute non-redundantly in an EIN2-dependent manner to age-related resistance in Arabidopsis Physiological and Molecular Plant Pathology 76 212–222 [Google Scholar]
- Balazadeh S, Kwasneewski M, Caldana C, Mehrnia M, Zanor MI, Xue GP, Mueller-Roeber B. 2011. ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana Molecular Plant 4 346–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat VA, Trivedi PK, Ghosh A, Sane VA. 2010. Ripening of fleshy fruit: molecular insight and the role of ethylene Biotechnology Advances 28 94–107 [DOI] [PubMed] [Google Scholar]
- Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, Zinder M, Samach A, Eshed Y, Ori N. 2009. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves Development 136 823–832 [DOI] [PubMed] [Google Scholar]
- Bu Q, Jiang H, Li CB, Zhai Q, Zhang J, Wu X, Sun J, Xie Q, Li C. 2008. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses Cell Research 18 756–767 [DOI] [PubMed] [Google Scholar]
- Cara B, Giovannoni JJ. 2008. Molecular biology of ethylene during tomato fruit development and maturation Plant Science 175 106–113 [Google Scholar]
- Chen JY, Liu DJ, Jiang YM, Zhao ML, Shan W, Kuang JF, Lu WJ. Molecular characterization of a strawberry FaASR in relation to fruit ripening. PloS ONE. 2011;6:24649. doi: 10.1371/journal.pone.0024649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhong HY, Kuang JF, Li JG, Lu WJ, Chen JY. 2011. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234 377–390 [DOI] [PubMed] [Google Scholar]
- Clendennen SK, Kipp PB, May GD. 1997. The role of ethylene in banana fruit ripening.In: Kanelis AK, ed. Biology and biotechnology of the plant hormone ethylene Dordrecht:Kluwer Academic Publishers; 141–148 [Google Scholar]
- Clendennen SK, May GD. 1997. Differential gene expression in ripening banana fruit. Plant Physiology 115 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delessert C, Kazan K, Wilson IW, Van Der Straeten D, Manners J, Dennis ES, Dolferus R. 2005. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. The Plant Journal 43 745–757 [DOI] [PubMed] [Google Scholar]
- Dominguez M, Vendrell M. 1993. Ethylene biosynthesis in banana fruit: evolution of EFE activity and ACC levels in peel and pulp during ripening. Journal of Horticultural Science 68 63–70 [Google Scholar]
- Duval M, Hsieh TF, Kim SY, Thomas TL. 2002. Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily. Plant Molecular Biology 50 237–248 [DOI] [PubMed] [Google Scholar]
- Elitzur T, Vrebalov J, Giovannoni JJ, Goldschmidt EE, Friedman H. 2010. The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. Journal of Experimental Botany 61 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst HA, Olsen AN, Skriver K, Larsen S, Leggio LL. 2004. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Reports 5 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LSP, Yamaguchi-Shinozaki K, Shinozaki K. 2004. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway The Plant Journal 39 863–876 [DOI] [PubMed] [Google Scholar]
- Gao F, Xiong AS, Peng RH, Jin XF, Xu J, Zhu B, Chen JM, Yao QH. 2010. OsNAC52, a rice NAC transcription factor, potentially responds to ABA and confers drought tolerance in transgenic plants. Plant Cell Tissue and Organ Culture 100 255–262 [Google Scholar]
- Giovannoni JJ. 2004. Genetic regulation of fruit development and ripening. The Plant Cell 16 S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Shen YP, Ma LG, et al. 2004. Genome-wide ORFeome cloning and analysis of Arabidopsis transcription factor genes. Plant Physiology 135 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve K, Cour TL, Jensen MK, Poulsen FM, Skriver K. 2003. Interactions between plant RING-H2 and plant-specific NAC (NAM/ATAF1/2/CUC2) proteins: RING-H2 molecular specificity and cellular localization. Biochemical Journal 371 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YJ, Song QX, Chen HW, Zou HF, Wei W, Kang XS, Ma B, Zhang WK, Zhang JS, Chen SY. 2010. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta 232 1033–1043 [DOI] [PubMed] [Google Scholar]
- Hao YJ, Wei W, Song QX, et al. 2011. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. The Plant Journal 68 302–313 [DOI] [PubMed] [Google Scholar]
- He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. 2005. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. The Plant Journal 44 903–916 [DOI] [PubMed] [Google Scholar]
- Hegedus D, Yu M, Baldwin D, Margaret G, Sharpe A, Parkin I, Whitwill S, Lydiate D. 2003. Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress. Plant Molecular Biology 53 383–397 [DOI] [PubMed] [Google Scholar]
- Hershkovitz V, Friedman H, Goldschmidt EE, Feygenberg O, Pesis E. 2011. Effect of seed on ripening control components during avocado fruit development. Journal of Plant Physiology 168 2177–2183 [DOI] [PubMed] [Google Scholar]
- Hibara K, Takada S, Tasaka M. 2003. CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation. The Plant Journal 36 687–696 [DOI] [PubMed] [Google Scholar]
- Jeong JS, Park YT, Jung H, Park SH, Kim JK. 2009. Rice NAC proteins act as homodimers and heterodimers. Plant Biotechnology Report 3 127–134 [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. 2009. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323 1053–1057 [DOI] [PubMed] [Google Scholar]
- Kim SG, Lee AK, Yoon HK, Park CM. 2008. A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. The Plant Journal 55 77–88 [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim SG, Kim YS, Seo PJ, Bae M, Yoon HK, Park CM. 2007. Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Research 35 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kim SG, Park JE, Park HY, Lim MH, Chua NH, Park CM. 2006. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. The Plant Cell 18 3132–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics 45 41–59 [DOI] [PubMed] [Google Scholar]
- Kleinow T, Himbert S, Krenz B, Jeske H, Koncz C. 2009. NAC domain transcription factor ATAF1 interacts with SNF1-related kinases and silencing of its subfamily causes severe developmental defects in Arabidopsis. Plant Science 177 360–370 [Google Scholar]
- Kumar R, Sharma MK, Kapoor S, Tyagi AK, Sharma AK. 2012. Transcriptome analysis of rin mutant fruit and in silico analysis of promoters of differentially regulated genes provides insight into LeMADS-RIN-regulated ethylene-dependent as well as ethylene-independent aspects of ripening in tomato. Molecular Genetics and Genomics 287 189–203 [DOI] [PubMed] [Google Scholar]
- Larsson E, Sitbon F, Sundström J, von Arnold S. NAC regulation of embryo development in conifers. BMC Proceedings. 2011;5:67. [Google Scholar]
- Lee SH, Hannink M. 2003. Molecular mechanisms that regulate transcription factor localization suggest new targets for drug development. Advanced Drug Delivery Reviews 55 717–731 [DOI] [PubMed] [Google Scholar]
- Lin R, Zhao W, Meng X, Wang M, Peng Y. 2007. Rice gene OsNAC19 encodes a novel NAC-domain transcription factor and responds to infection by Magnaporthe grisea. Plant Science 172 120–130 [Google Scholar]
- Liu J, Xu B, Hu L, Li M, Su W, Wu J, Yang J, Jin Z. 2009. Involvement of a banana MADS-box transcription factor gene in ethylene-induced fruit ripening. Plant Cell Reports 28 103–111 [DOI] [PubMed] [Google Scholar]
- Liu X, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A, Liu XJ. 1999. Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiology 121 1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Mao C, Ding W, Wu Y, Yu J, He X, Shou H, Wu P. 2007. Overexpression of a NAC-domain protein promotes shoot branching in rice. New Phytologist 176 288–298 [DOI] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ. 2011. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiology 157 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC. 2008. EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine) Physiologia Plantarum 133 435–448 [DOI] [PubMed] [Google Scholar]
- Meng C, Cai C, Zhang T, Guo W. 2009. Characterization of six novel NAC genes and their responses to abiotic stresses in Gossypium hirsutum L. Plant Science 176 352–359 [Google Scholar]
- Morishita T, Kojima Y, Maruta T, Nishizawa-Yokoi A, Yabuta Y, Shigeoka S. 2009. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoids biosynthesis under high-light. Plant and Cell, Physiology 50 2210–2222 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2012. NAC transcription factors in plant abiotic stress responses. Biochimica et Biophysica Acta 1819 97–103 [DOI] [PubMed] [Google Scholar]
- Oh SK, Lee S, Yu SH, Choi D. 2005. Expression of a novel NAC domain-containing transcription factor (CaNAC1) is preferentially associated with incompatible interactions between chili pepper and pathogens. Planta 222 876–887 [DOI] [PubMed] [Google Scholar]
- Olsen AN, Ernst HA, Leggio LL, Skriver K. 2005. NAC transcription factors: structurally distinct, functionally diverse. Trends in Plant Science 10 79–87 [DOI] [PubMed] [Google Scholar]
- Ooka H, Satoh K, Doi K, et al. 2003. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Research 10 239–247 [DOI] [PubMed] [Google Scholar]
- Pinheiro GL, Marques CS, Costa MD, Reis PA, Alves MS, Carvalho CM, Fietto LG, Fontes EP. 2009. Complete inventory of soybean NAC transcription factors: sequence conservation and expression analysis uncover their distinct roles in stress response. Gene 444 10–23 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. 2003. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115 679–689 [DOI] [PubMed] [Google Scholar]
- Qin G, Wang Y, Cao B, Wang W, Tian S. 2012. Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. The Plant Journal 70 243–255 [DOI] [PubMed] [Google Scholar]
- Sablowski RW, Meyerowitz EM. 1998. A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92 93–103 [DOI] [PubMed] [Google Scholar]
- Seo PJ, Kim MJ, Park JY, Kim SY, Jeon J, Lee YH, Kim J, Park CM. 2010. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. The Plant Journal 61 661–671 [DOI] [PubMed] [Google Scholar]
- Seymour G, Ryder C, Cevik V, Hammond J, Popovich A, King G, Vrebalov J, Giovannoni JJ, Manning K. 2011. A SEPALLATA gene is involved in the development and ripening of strawberry (Fragaria × ananassa Duch.) fruit, a non-climacteric tissue Journal of Experimental Botany 62 1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souer E, Houwelingen AV, Kloos D, Mol J, Koes R. 1996. The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordial boundaries. Cell 85 159–170 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. 2001. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. The Plant Journal 26 47–58 [DOI] [PubMed] [Google Scholar]
- Tisza V, Kovács L, Balogh A, Heszky L, Kiss E. 2010. Characterization of FaSPT, a SPATULA gene encoding a bHLH transcriptional factor from the non-climacteric strawberry fruit. Plant Physiology and Biochemistry 48 822–826 [DOI] [PubMed] [Google Scholar]
- Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell 16 2481–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verza NC, Figueira TRS, Sousa SM, Arruda P. 2011. Transcription factor profiling identifies an aleurone-preferred NAC family member involved in maize seed development. Annals of Applied Biology 158 115–129 [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. 2002. A MADS-box gene necessary for fruit ripening at the tomato Ripening-Inhibitor (Rin) locus. Science 296 343–346 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schutze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal 40 428–438 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lu W, Jiang Y, Luo Y, Jiang W, Joyce D. 2006. Expression of ethylene-related expansin genes in cool-stored ripening banana fruit. Plant Science 170 962–967 [Google Scholar]
- Wan CY, Wilkins TA. 1994. A modified hot borate method significantly enhances the yield of high quality RNA from cotton (Gossypium hirsutum L.) Analytical Biochemistry 223 7–12 [DOI] [PubMed] [Google Scholar]
- Waters BM, Uauy C, Dubcovsky J, Grusak MA. 2009. Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. Journal of Experimental Botany 60 4263–4274 [DOI] [PubMed] [Google Scholar]
- Weir I, Lu J, Cook H, Causier B, Schwarz-Sommer Z, Davies B. 2004. CUPULIFORMIS establishes lateral organ boundaries in Antirrhinum. Development 131 915–922 [DOI] [PubMed] [Google Scholar]
- Xia N, Zhang G, Sun YF, et al. 2010. TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiological and Molecular Plant Pathology 74 394–402 [Google Scholar]
- Xie Q, Frugis G, Colgan D, Chua NH. 2000. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes & Development 14 3024–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Liu T, Tian C, Sun S, Li J, Chen M. 2005. Transcription factors in rice: a genome-wide comparative analysis between monocots and eudicots. Plant Molecular Biology 59 191–203 [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Kigawa T, Inoue M, Watanabe S, Tateno M, Seki M, Shinozaki K, Yokoyama S. 2008. Structures and evolutionary origins of plant-specific transcription factor DNA-binding domains. Plant Physiology and Biochemistry 46 394–401 [DOI] [PubMed] [Google Scholar]
- Yao J, Kvarnheden A, Morris B. 1999. Seven MADS-box genes in apple are expressed in different parts of the fruit. Journal of the American Society for Horticultural Science 124 8–13 [Google Scholar]
- Yin X, Allan AC, Chen K, Ferguson IB. 2010. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiology 153 1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii M, Yamazaki M, Rakwal R, Kishi-Kaboshi M, Miyao A, Hirochika H. 2010. The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling. The Plant Journal 61 804–815 [DOI] [PubMed] [Google Scholar]
- Zhang K, Gan SS. 2012. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiology 158 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Gallego-Giraldo L, Wang H, Zeng Y, Ding SY, Chen F, Dixon RA. 2010. NAC transcription factor orchestrates multiple features of cell wall development in Medicago Truncatula. The Plant Journal 63 100–114 [DOI] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH. 2010. Functional characterization of poplar wood-associated NAC domain transcription factors. Plant Physiology 152 1044–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.