Abstract
We have isolated overlapping RNA fragments which contain the region surrounding the ribonuclease III cleavage site between bacteriophage T7 genes 0.3 and 0.7. Although all of these fragments contain the site of cleavage, only certain fragments are correctly recognized and cleaved by RNase III. Analysis of the cleavage products of the fragments indicates that the enzyme produces a single endonucleolytic break at this site in the T7 early RNA precursor molecule. In addition, the 3'-terminal adenylic acid residues observed previously on the in vivo T7 early RNA species were not found in these fragments and, therefore, must represent a post-transcriptional, post-processing modification of the RNA.
Full text
PDF


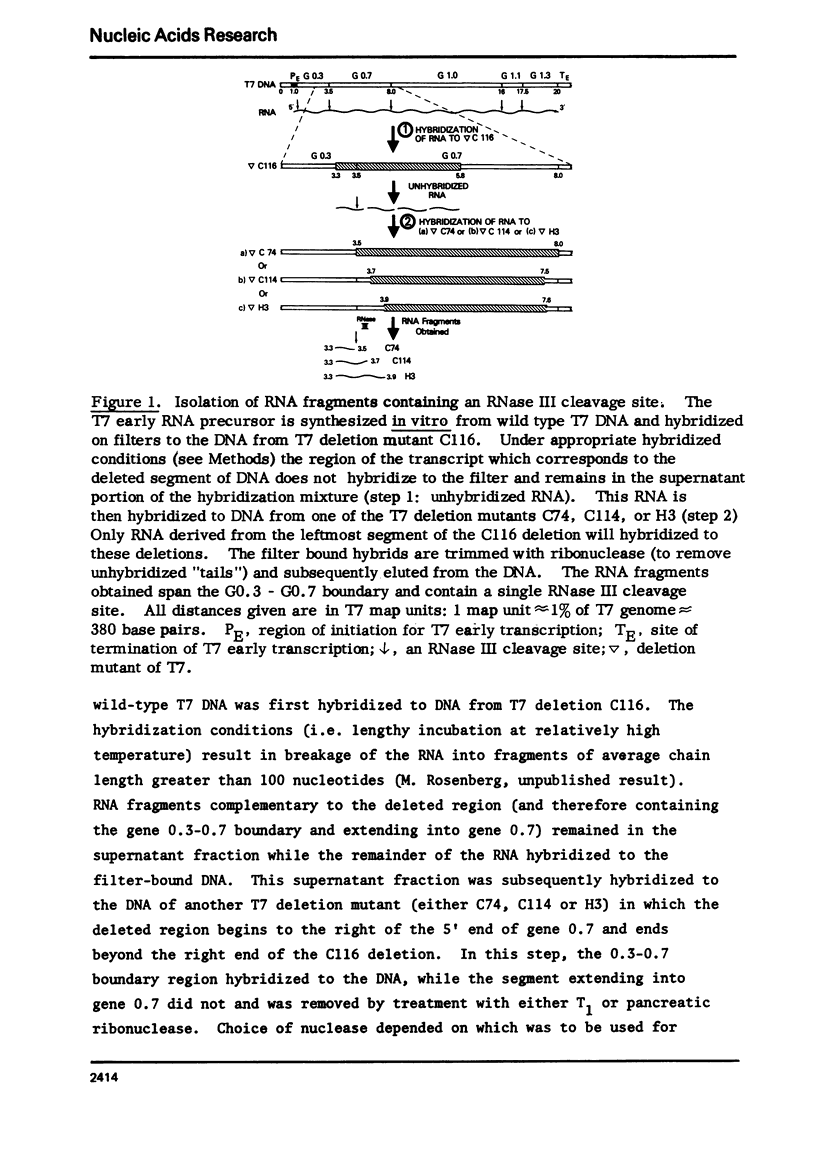


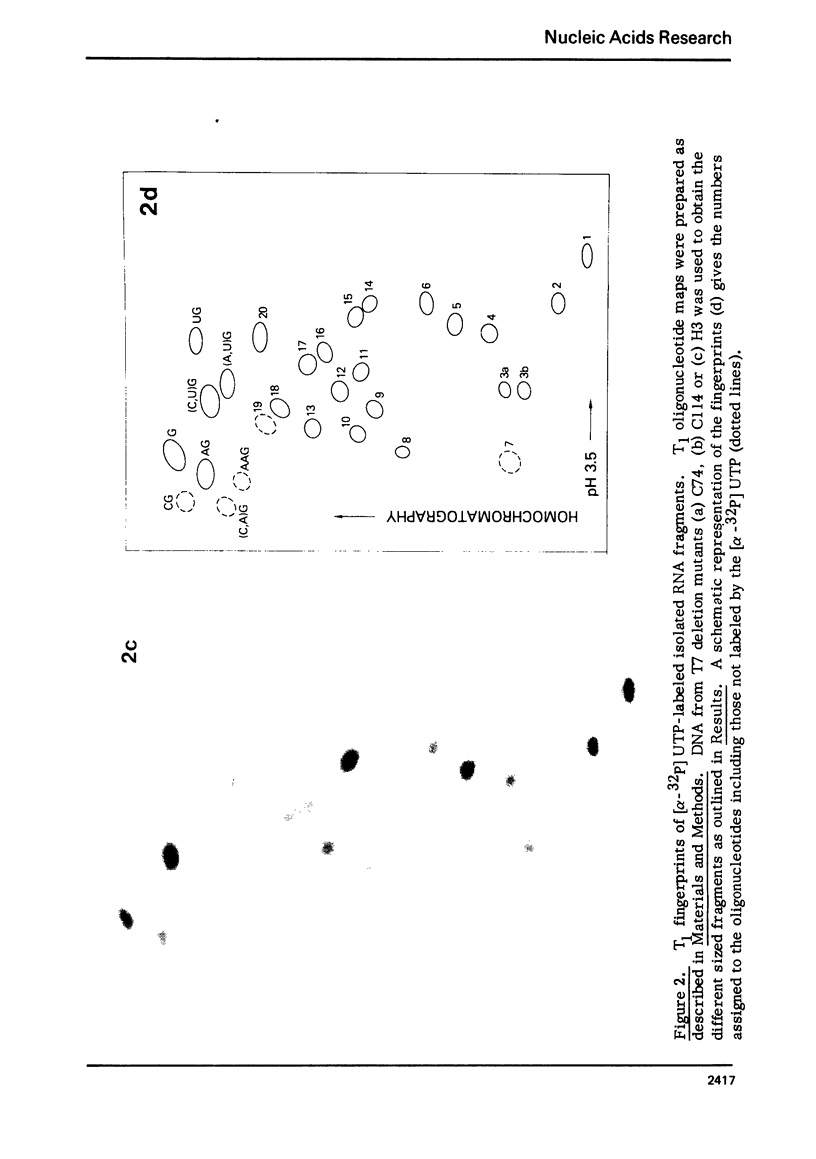
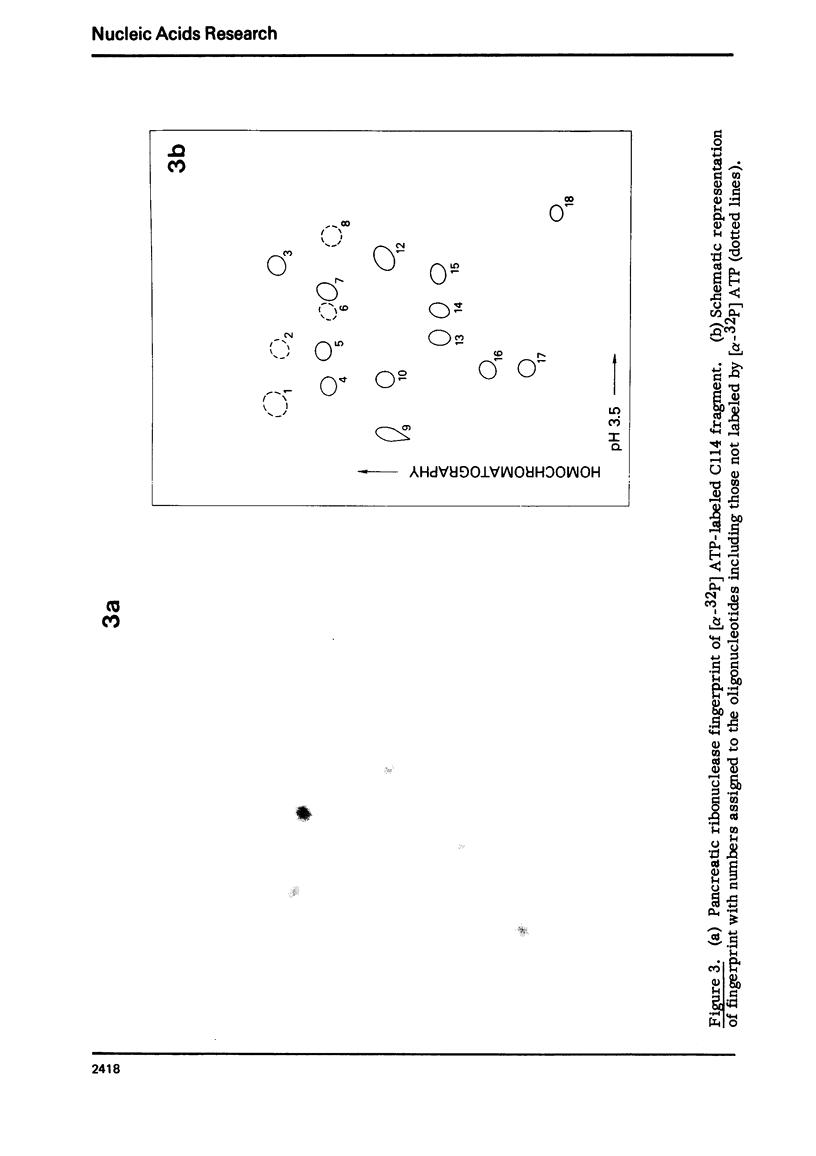
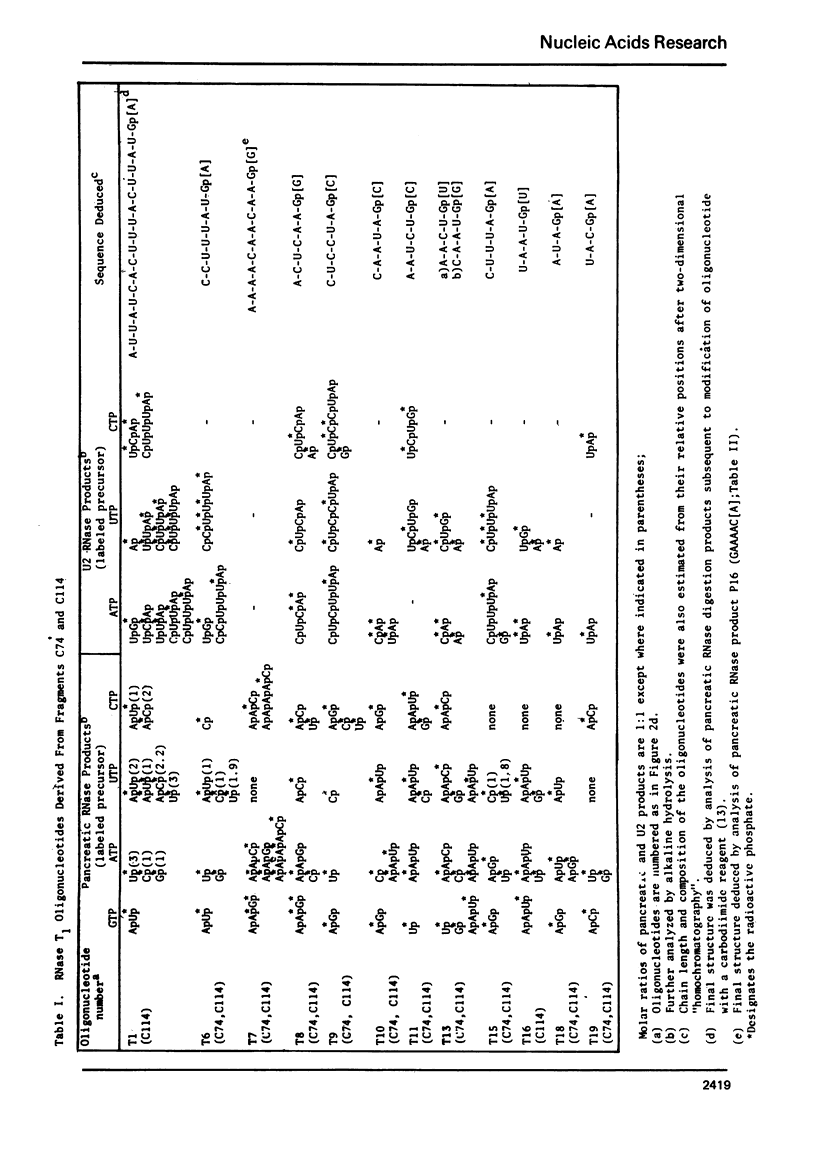
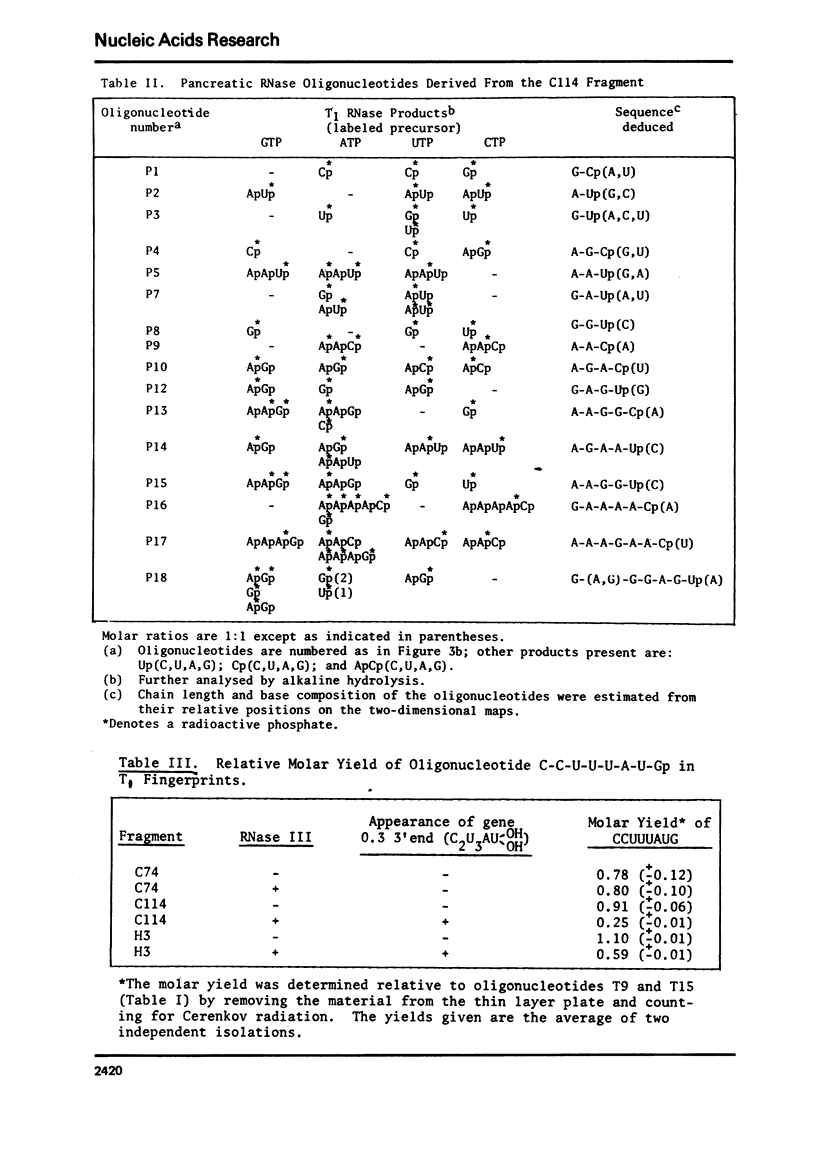

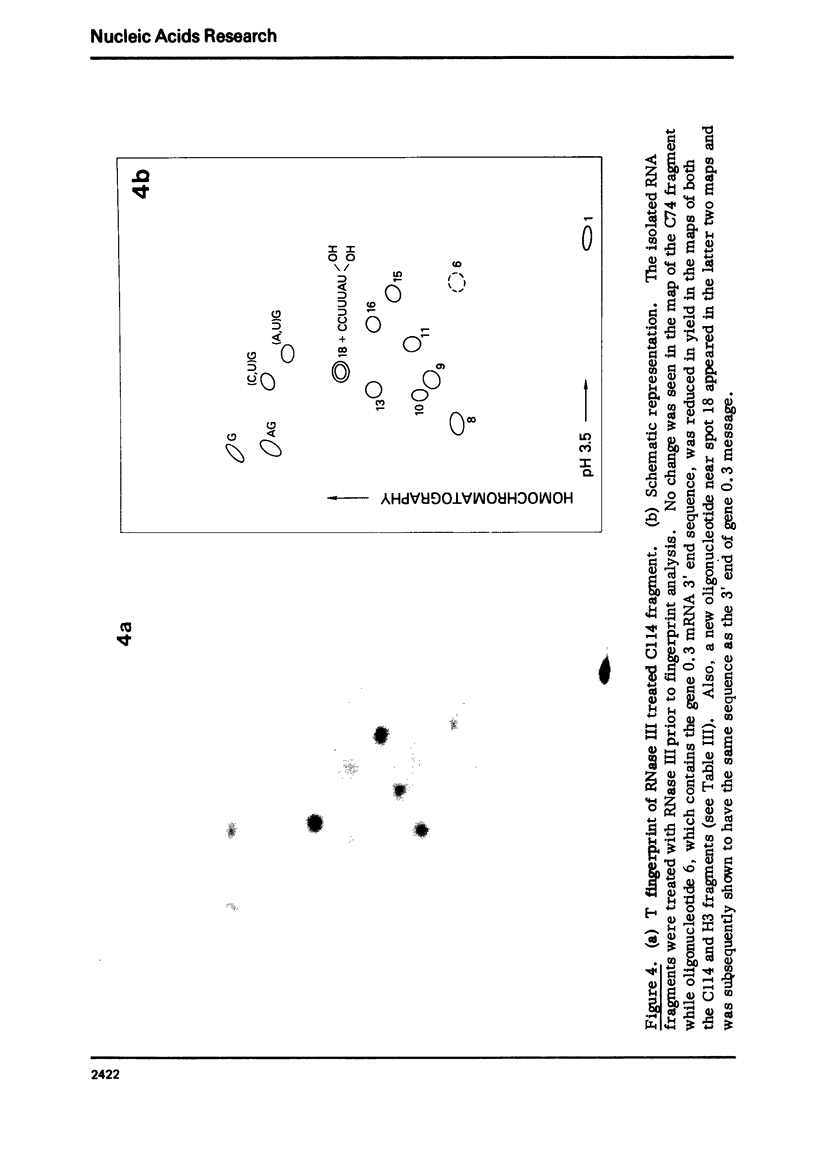




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Processing transcription, and translation of bacteriophage T7 messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):267–276. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Hyman R. W. Physical mapping of T7 messenger RNA. J Mol Biol. 1971 Oct 28;61(2):369–376. doi: 10.1016/0022-2836(71)90386-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Venkatesan S., Edmonds M. Polyadenylic acid sequences in E. coli messenger RNA. Nature. 1975 Jul 10;256(5513):144–146. doi: 10.1038/256144a0. [DOI] [PubMed] [Google Scholar]
- Paddock G. V., Abelson J. Nucleotide sequence determination of bacteriophage T4 species I ribonucleic acid. J Biol Chem. 1975 Jun 10;250(11):4185–4206. [PubMed] [Google Scholar]
- Paddock G. V., Fukada K., Abelson J., Robertson H. D. Cleavage of T4 species I ribonucleic acid by Escherichia coli ribonuclease III. Nucleic Acids Res. 1976 May;3(5):1351–1371. doi: 10.1093/nar/3.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Weissman S., deCrombrugghe B. Termination of transcription in bacteriophage lambda. Heterogeneous, 3'-terminal oligo-adenylate additions and the effects of rho factor. J Biol Chem. 1975 Jun 25;250(12):4755–4764. [PubMed] [Google Scholar]
- Rosenberg M., de Chrombrugghe B., Musso R. Determination of nucleotide sequences beyond the sites of transcriptional termination. Proc Natl Acad Sci U S A. 1976 Mar;73(3):717–721. doi: 10.1073/pnas.73.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Hedgpeth J. Oligo(A) not coded by DNA generating 3'-terminal heterogeneity in a lambda phage RNA. J Biol Chem. 1975 Jun 25;250(12):4818–4821. [PubMed] [Google Scholar]
- Srinivasan P. R., Ramanarayanan M., Rabbani E. Presence of polyriboadenylate sequences in pulse-labeled RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2910–2914. doi: 10.1073/pnas.72.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Brunovskis I., Hyman R. W. The process of infection with coliphage T7. VII. Characterization and mapping of the major in vivo transcription products of the early region. J Mol Biol. 1973 Mar 5;74(3):291–300. doi: 10.1016/0022-2836(73)90374-4. [DOI] [PubMed] [Google Scholar]