Abstract
A split-rooted containerized system was developed by approach grafting two, 1-year-old apple (Malus×domestica Borkh. cv ‘Gala’) trees to investigate the effect of soil moisture heterogeneity and total soil moisture content (θv) on tree water relations, gas exchange, and leaf abscisic acid (ABA) concentration [ABAleaf]. Four irrigation treatments comprising a 2×2 factorial experiment of irrigation volume and placement were imposed over a 30-day period: control (C) [>100% of crop evapotranspiration (ETc)] applied to both containers; PRD100 (>100% ETc) applied to one container only; and two treatments receiving 50% ETc applied to either one (PRD50) or both containers (DI50). Irrigation between PRD (partial rootzone drying) root compartments was alternated when θv reached ~35% of field capacity. Maximum daily sap flow of the irrigated roots of PRD100 exceeded that of C roots throughout the experimental period. Pre-dawn water potential (Ψpd) was similar between C and PRD100; however, daily water use and mid-day gas exchange of PRD100 was 30% lower. Slightly higher [ABAleaf] was observed in PRD100, but the effect was not significant and could not explain the observed reductions in leaf gas exchange. Both 50% ETc treatments had similar, but lower θv, Ψpd, and gas exchange, and higher [ABAleaf] than C and PRD100. Regardless of treatment, the container having the lower θv of a split-rooted system correlated poorly with [ABAleaf], but when θv of both containers or θv of the container possessing the higher soil moisture was used, the relationship markedly improved. These results imply that apple canopy gas exchange and [ABAleaf] are responsive to the total soil water environment.
Abbreviations:
- A
assimilation
- [ABAleaf]
leaf ABA concentration
- Bd
bulk density
- DI
deficit irrigation
- DOY
day of year
- dw
dry weight
- E
transpiration
- ETc
crop evapotranspiration
- FC
field capacity
- gs
stomatal conductance
- LA
leaf area
- PAR
photosynthetic active radiation
- PRD
partial rootzone drying
- Ψpd
pre-dawn leaf water potential
- θv
volumetric soil moisture content
- θw
, gravimetric soil moisture content
- TCA
trunk cross-sectional area
- TDR
time-domain reflectometry
- WUE
-
water use efficiency.
©2012 The Author(s).
Key words: Chemical signalling, deficit irrigation, Malus×domestica, partial rootzone drying, PRD, water use efficiency
Introduction
There is evidence that roots produce chemical signals during soil drying which modify growth of the shoots prior to detectable changes in leaf water status (see reviews by Davies and Zhang, 1991; Sauter et al., 2001; Hartung et al., 2002; Dodd, 2003). Partial stomatal closure has been closely associated with an increase of foliar abscisic acid concentration [ABAleaf] during episodes of soil drying (Loveys, 1984). Direct evidence that root synthesis of ABA increases with declining soil moisture exists for several species (Davies and Zhang, 1989; Zhang et al., 1987). That root-derived ABA travels in the xylem to elicit stomatal effects has also been documented (Zhang and Davies, 1991); though the response varies with different species (Loveys et al., 1987).
Experimental drying of one half of maize (Zea mays L.) root systems provided early indications that non-hydraulic signals were responsible for stomatal regulation of split-rooted plants (i.e. exposure of roots to heterogeneous soil drying) within 2 d of partial root drying (Blackman and Davies, 1985); though partial stomatal closure was not attributed to [ABAleaf]. Elevated [ABA] in both epidermis and roots from drying soil of split-rooted Commelina communis L. plants occurred following 3 d of partial soil drying (Zhang et al., 1987). In an elegant study, Gowing et al. (1990) observed an unidentified root-sourced signal that reduced growth and transpiration of half-dried apple plants; these physiological effects disappeared when roots inhabiting dry containers were removed. Control of vegetative vigour was also observed when drying half of split-rooted peach (Prunus persica L.) (Tan and Buttery, 1982) and grapevine (Vitis vinifera) (Dry et al., 1996, 2000a, b). The latter investigations formed the framework of an agricultural irrigation strategy termed partial rootzone drying (PRD). In PRD, deficit irrigation is imposed by irrigating only a fraction of the rootzone per series of irrigation events. Stomata are regulated via chemical signals that preclude, or more probably, interact with hydraulic signals (Comstock, 2002) derived from the portion of roots inhabiting the drying soil. Irrigation is typically alternated between dry and wet rootzones in an effort to maintain a consistent signal. Early PRD investigations (Dry et al., 1996, 2000a, b) did not compare PRD with equally water-limited treatments where water was uniformly distributed to the entire rootzone. Subsequent experiments have been unable to relate the agronomic performance of divergent taxa to irrigation placement when comparing PRD and deficit irrigation (DI) using apple (Leib et al., 2006), common bean (Phaseolus vulgaris L.) (Wakrim et al., 2005), potato (Solanum tuberosum L.) (Liu et al., 2006), grapevines (Gu et al., 2004; Rodrigues et al., 2008), and tomato (Solanum lycopersicum Mill.) (Zegbe-Dominguez et al., 2003). A synopsis of the literature showed that yield ratios (PRD to DI) typically exceeded 1 for experiments performed on perennial horticulture crops, although the ratio was not significant for non-containerized studies (Dodd, 2009).
Stomata of apple seedlings responded rapidly to exogenous ABA under non-limiting growing conditions (Bingham, 1972). Further, a 20-fold increase in mature leaf [ABA] occurred as the water potential (Ψ) of water-stressed, potted apple trees declined from –0.4MPa to –2.6MPa (Bingham, 1972). ABA levels in apple leaves increased linearly with decreasing turgor potential following a 4 d water stress treatment (Davies and Lakso, 1978). Yet there is disparity between results of highly controlled apple split-root studies (Gowing et al., 1990) (demonstrating the capacity for apple to produce, and respond to chemical signals, but without directly measuring specific signals such as ABA), and field studies (TCE and HWC, unpublished; Leib et al., 2006), which have not observed effects of PRD on reproductive or vegetative growth relative to other DI regimes. It is possible that the signal:noise ratio associated with field conditions (i.e. high variability of the soil–plant–atmosphere continuum) is too great for consistent signal generation and action. Further, stomata of field-grown crops (specifically those with rough, discontinuous canopies) can be more sensitive to high vapour pressure deficit (VPD) (Pérez-Pérez et al., 2012) and plant water status (Rodrigues et al., 2008) than soil water availability.
In 2003, a unique system was developed to evaluate PRD by connecting two containerized apple trees via an approach graft. The split-root system (two root zones: one canopy) offered the potential to observe the proportion of sap flow and soil moisture of separate root zones of a split-rooted unit relative to canopy gas exchange, water relations, and [ABAleaf] in a factorial design with two levels of irrigation volume and two levels of irrigation placement. Dodd (2007) developed a novel two root–one shoot system using grafted tomato plants which was later applied to sunflower (Dodd et al., 2008a) to measure the relative contribution of individual roots to total plant sap flow and leaf xylem ABA.
The present study was designed to test the responsiveness of a scaled-up experimental unit (well-developed, 2-year-old apple trees with roots split between two 30 litre containers) to PRD. Approach grafting provided the opportunity to fix the volume of soil for root exploration, and more precisely control the soil moisture content of the separate containers. Furthermore, split-rooted plants enabled measurement of sap flow from both rootzones and the canopy. The hypothesis was tested that differences in soil moisture between individual pots of split-rooted apple trees and among treatments would account for differences in canopy gas exchange, and that PRD would augment apple [ABAleaf]. Additionally, there was a technical objective of determining whether miniature sap flow sensors inserted into components of spit-rooted apple trees could estimate whole-tree water use and characterize the relative root activity of split-rooted units during partial soil drying.
Materials and methods
One-year-old ‘Gale Gala’ apple trees grafted onto ‘Malling 7’ (‘M.7’) rootstocks were potted into 30 litre containers in a retractable-roof greenhouse. Thirty split-rooted units were formed by approach grafting two trees ~120cm above their scion–rootstock graft unions in late June of 2003. All split-rooted systems were allowed to callus and grow for the remainder of the year. Following bud-break in spring of 2004, one canopy was removed above the approach graft. Trees were fully watered until the beginning of the experimental period. Twenty similar units were randomly assigned treatments in a complete randomized design comprising four irrigation treatments each with five replications: (i) control (C), both containers irrigated once daily with >100% of their daily crop evapotranspiration (ETc; see below); (ii) PRD100, one container irrigated once daily with >100% of daily ETc, the other receiving no irrigation; (iii) DI50, both containers irrigated once daily with 50% of daily ETc, distributed evenly between the two containers; and (iv) PRD50, one container irrigated once daily with 50% of daily ETc, the other receiving no irrigation. Daily irrigation of all treatments occurred in the evening. Irrigation of PRD treatments was alternated between wet and dry root compartments on day of year (DOY) 208, 215, and 223 when soil moisture extraction was observed to have levelled off in the dry compartment [~35% of field capacity (FC)]. Unless otherwise noted, trees were watered by hand once per day (at dusk) using watering cans filled with the exact percentage of their daily ETc. In the case of C and PRD100, all replications received an additional 20% ETc to account for losses due to drainage. Semi-transparent, polyethylene covers were fitted across the surface area of all containers following irrigation to eliminate the evaporative component of ET. The experimental period was from 19 July to 18 August, 2004 (DOY 200–230). Following completion of two PRD drying cycles, deficit treatments were terminated and full irrigation was provided, but the distribution of water was unchanged (i.e. PRD50 became PRD100, and DI50 became C). The switch to full irrigation was intended to evaluate the recovery time of gas exchange and water use of the former deficit treatments.
The medium was a native soil:organic mixture (~1:1 v/v). The organic constituents, and their relative proportions within the organic faction, had not been experimentally determined. The experimentally derived bulk density (Bd) of the medium (0.75g cm–3) and known Bds of various organic components were used to calibrate θv measurement by time-domain reflectometry (TDR) (Tektronix Model 1502C, Beaverton, OR, USA), based on the equations of Topp et al. (1980). Stainless steel waveguides, 6mm in diameter, 300mm in length, were inserted into each container of the split-rooted units for all replications. Waveguides captured the entire depth of the container, spanning from the bottom of containers, and protruding ~2.5cm from the medium surface to enable measurements. Throughout the first 12 d of the experimental period, θv was measured in the morning, after drainage from the preceding night, and again in the evening, prior to irrigation. Daily water use was calculated as the difference between the morning and evening measurements. Thereafter, water content was measured once daily, in the morning. For the first 7 d of the experiment, gravimetric measurements were made using a transportable 4 m high wooden tripod utilizing three S-type load cells (34kg load capacity per cell with an accuracy ±0.01% of applied load), and wired to a digital display for manual recording. Gravimetric data from C trees were used to calculate daily replacement irrigation (ETc), and correlate with θv.
Daily water use and water transport dynamics of the approach-grafted units were determined from measurements of sap flow using the Tmax heat pulse method (Cohen et al., 1981; Green et al., 2003). Miniature stem flow sensors were installed into parallel holes drilled radially into the stems and root shanks of two replications of C, PRD100, and PRD50 trees. Heat pulse probes, each comprised of a line heater and two temperature sensors (Tranzflow, Palmerston North, New Zealand), were inserted into each root shank (below the approach graft union but above the original rootstock–scion graft) and in the stem above the approach graft union. Sap velocity was measured at two radial depths (5mm and 10mm) in the stem and one depth (5mm) in the root shanks. A 0.7 s heat pulse was applied every 30min and data were collected via a data logger (Campbell Scientific, Logan, UT, USA). Heat pulse velocity was converted to sap flow based on the trunk cross-sectional area (TCA) at the sites of insertion. Correction factors for wounding were derived according to Green et al. (2003).
Gas exchange [A, photosynthesis (µmol m–2 s–1); g s, stomatal conductance (mmol m–2 s–1); E, transpiration (mmol m–2 s–1); WUEA/gs, water use efficiency] was quantified using a PP systems Ciras-2 gas analyser (PP systems, Amherst, MA, USA) on three replications per treatment (two leaves per replication). Measurements were taken on the first fully mature sunlit leaves of extension shoots, between 12:00h and 14:00h on days with fairly similar climatic conditions. In addition, hourly measurements were made on several dates to capture diurnal changes in gas exchange. All measurements were made under natural saturating photosynthetically active radiation (PAR) for apple leaves (>1000 µmol m–2 s–1),unless otherwise stated.
Pre-dawn leaf water potential (Ψpd) was determined on three replications (two leaves per replication) using a pressure chamber (model 600, PMS, Corvallis, OR, USA) according to the Scholander method (Scholander et al., 1965).
Leaf area (LA) was measured at the conclusion of the experimental period on a 10% sample (w/w) per tree with a calibrated leaf area meter (Licor LA 3100, Lincoln, NE, USA).
Whole leaves were collected for ABA analysis from three replications (two leaves per replication), immediately dipped in liquid N, and held at –80 ºC for several weeks prior to lyophilization. Procedures for ABA extraction and purification of apple tissue were previously developed for enzyme-linked immunosorbent assay (ELISA) (Soejima et al., 1990). The water fraction collected during separatory phases containing conjugated ABA was not examined.
Quantification of 2-cis-(S)-ABA was by a competitive ELISA (Agdia, Elkhart, IN, USA), using a spectrophotometer (Spectramax 384 Plus, Molecular Devices Corp., Sunnyvale, CA, USA) equipped with a 96-well microtitre plate reader and temperature-controlled incubation chamber. Procedures were outlined by Agdia Inc. (Elkhart, IN, USA). Internal standards [2-cis-(S)-ABA; Sigma-Aldrich, St. Louis, MO, USA] were added to sample tissue prior to the extraction protocol for calculation of recovery rates. In all samples, ABA recovery rates were between 75% and 85%. Standard curves were prepared with each plate and linearized using log logit transformation. Samples always fell within the linear range of the standard curves. Samples were excluded from further interpretation if the coefficient of variability for duplicate values was >0.10.
Statistical analyses were performed using the SAS system software (SAS Institute, Cary, NC, USA). Treatment means were compared using analysis of variance (ANOVA; PROC GLM) and significance was tested at P ≤ 0.05. Mean separation was determined by Fisher’s Protected LSD. PROC REG was used for regression analyses. In the case of the relationship between relative TCA and sap flow of individual root shanks of a split-rooted unit, percentages were arcsine transformed prior to analysis.
Results
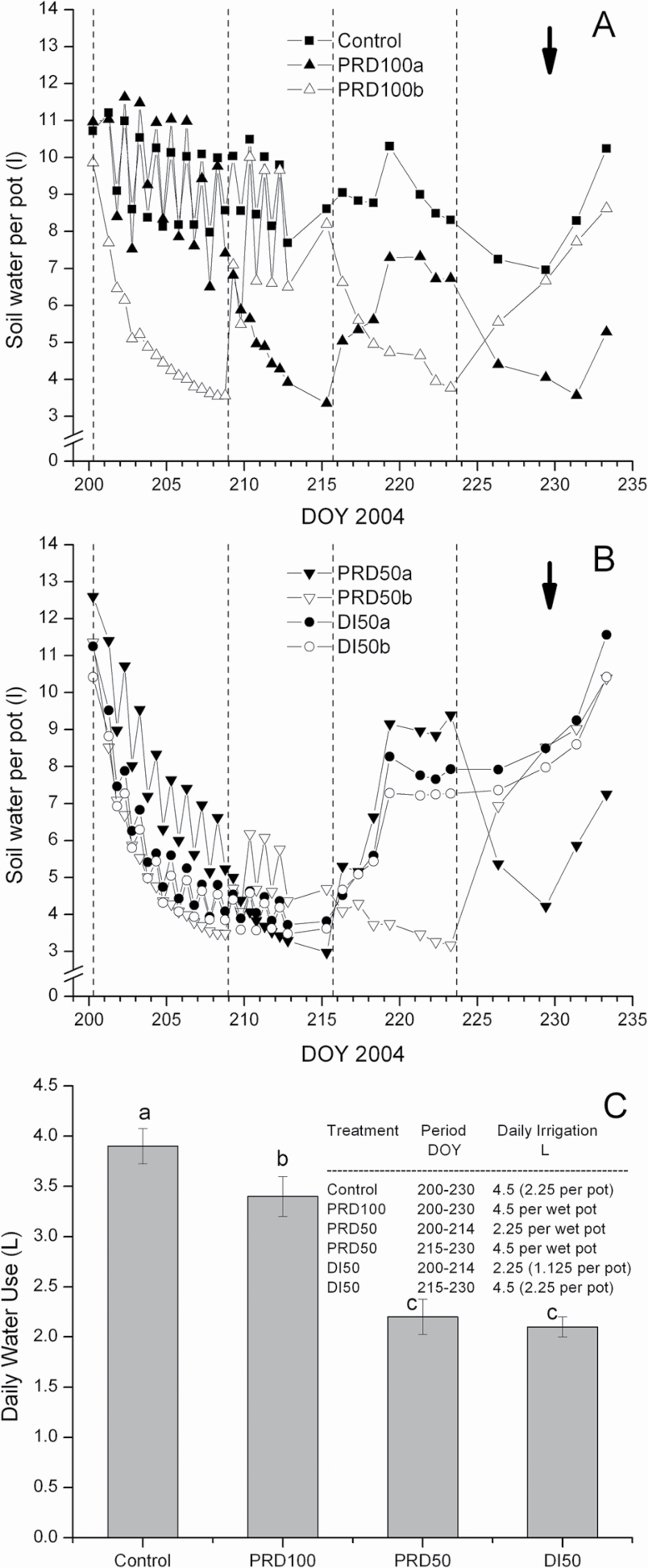
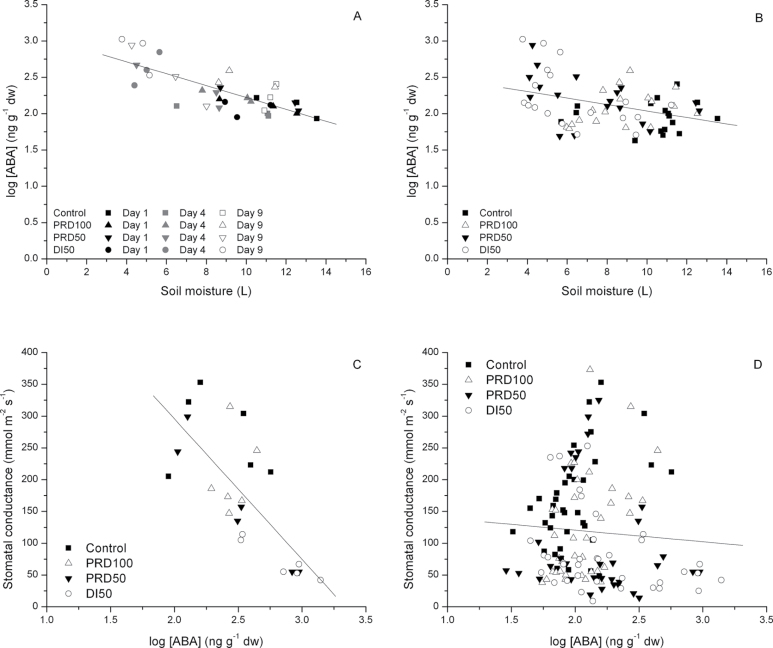
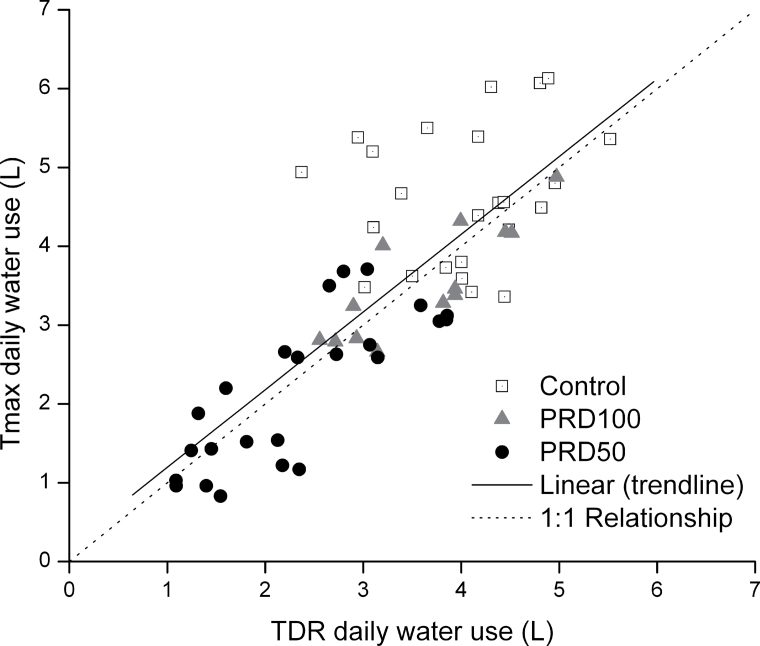
To determine whether TDR accurately estimated θv, θv data were regressed against gravimetric soil moisture content (θwt). The relationship between θwt and θv was highly significant (P < 0.0001) [θv (L) = 1.028 θwt (kg), r2 = 0.85, (n = 167)]. There was also generally good agreement between Tmax and θv; data fit the 1:1 relationship (Fig. 1), though variability existed in the relationship (r2 = 0.63), especially at higher flows (i.e., Controls). Variance between the two measured parameters is likely attributed to several assumptions inherent in each of the techniques. The θv of the irrigated root compartment of PRD100 plants was comparable with θv of C containers from DOY 200 to DOY 214, while the PRD100 non-irrigated container was progressively depleted to a minimum total water content of 3.5 litres on DOY 207, following 8 d of drying (Fig. 2A). PRD100 irrigation was then alternated between sides on DOY 208. Subsequent switching of irrigation between wet and dry compartments was conducted on DOY 215 and 223 when ~3.5 litres of total soil water remained in the ‘dry’ profile, which constituted drying periods of 9 d and 7 d, respectively. The irrigated compartment of the PRD100 treatment only recovered to 70% of C θv between the second and third switch (DOY 215–222). The PRD50 ‘dry’ compartment levelled off at 3.5 litres on DOY 207 (similar to the PRD100 ‘dry’ compartment on that date). The irrigated compartment of PRD50, however, steadily declined over the first deficit cycle, reaching 35% of C θv on DOY 207. On DOY 215, PRD50 was changed to PRD100, and the irrigated compartment reached and sustained θv of C by DOY 219. The average θv of both containers of the PRD50 treatment remained ~15% higher than that of DI50 throughout the first drying cycle, but attained similar levels on DOY 214. The mean θv of DI50 declined to 3.6 litres on DOY 214, after which it was returned to a C treatment (Fig. 2B). The ensuing daily application of >100% ETc to DI50 required an additional 7 d for θv to recover to C levels. Total daily water use was reduced for PRD100 relative to C despite receiving a similar volume of water, but differences in total daily water use between PRD50 and DI50 were not observed (Fig. 2C)
Fig. 2.
The effects of irrigation treatments on average soil water content of split-rooted, 2-year-old ‘Gale Gala’ apple trees; C and PRD100 (A), and DI50 and PRD50 (B). Total daily water use of C, PRD100, PRD50, and DI50 calculated from the difference between morning and evening time-domain reflectometry measurements on successive days from DOY 200 to 211 (C). Data are means of five replications. Mean separation by Fisher’s Protected LSD test at P < 0.05. The inset in C provides the daily irrigation volume for individual treatments throughout the entire experimental period. Vertical dashed lines signify switches in irrigation between PRD ‘wet’ and ‘dry’ compartments (A, B). The bold arrow at the top indicates termination of the treatment period. On DOY 215, PRD50 and DI50 were changed to PRD100 and Control treatments, respectively.
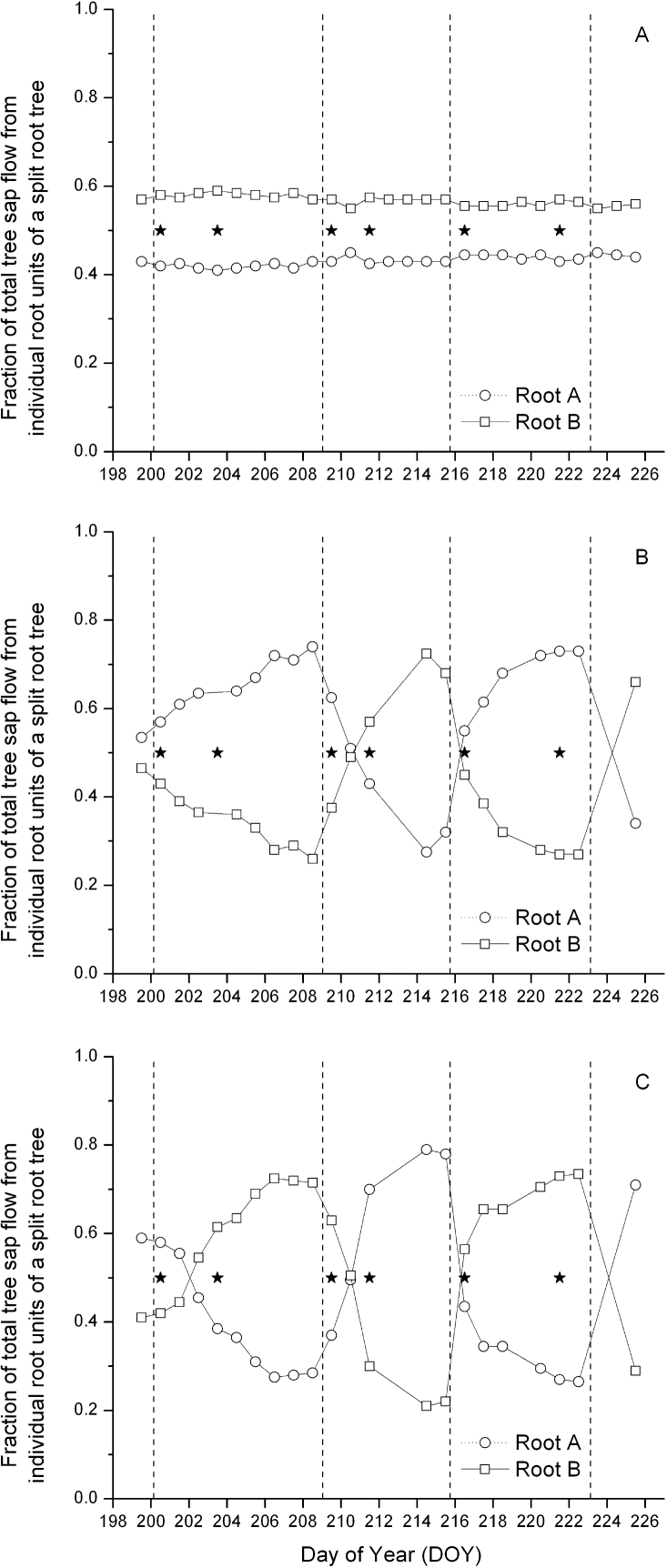
Prior to the start of the experiment, θv in all containers was brought up to pot capacity. A highly significant, positive relationship was observed between relative sap flow of roots comprising individual split-rooted units and their TCA (sap flow=2.19×root TCA–0.59, r 2=0.51, P=0.009, n=12; also observed in Fig. 3A). The dependency of sap flow on TCA indicated that the approach graft union did not markedly alter the hydraulic properties of the split-rooted units, irrespective of the origin of the root system (i.e. whether the sap flow was measured in the root of the tree whose canopy had been removed above the approach graft, or the root of the intact tree). Further, the sum of sap flow of the two root systems below the approach graft unions comprised 100.2% (SD 17.8%) of the sap flow measured above the approach graft union (mean of the six split-rooted units fitted with heat pulse sensors). Individual root sap flow of split-rooted C units maintained consistent levels throughout the entire experimental period (Fig. 3A). Root activity of individual compartments of all PRD split-rooted replications responded consistently and rapidly to wetting events by increasing sap flow, and daily water use, from the irrigated side, while reducing sap flow from the drying side (Fig. 3B, 3C). Throughout this biphasic process, with the exception of the day immediately succeeding the irrigation switch between PRD containers, total daily water use of PRD100 trees remained fairly consistent during the experimental period (Fig. 2A, 2C).
Fig. 3.
Daily sap flow (Tmax method) of individual root systems (root A and B) as the fraction of total tree sap flow of split-rooted, 2-year-old control (A), PRD100 (B), and PRD50 (C) ‘Gale Gala’ apple trees. Asterisks in the centre signify sampling dates for [ABAleaf] presented in Table 1. DOY 200 was day 1 of the experiment. On DOY 215, PRD50 and DI50 were changed to PRD100 and control treatments, respectively. Vertical dashed lines signify switches in irrigation between PRD ‘wet’ and ‘dry’ compartments (B, C). Sap flow data are means of two replications.
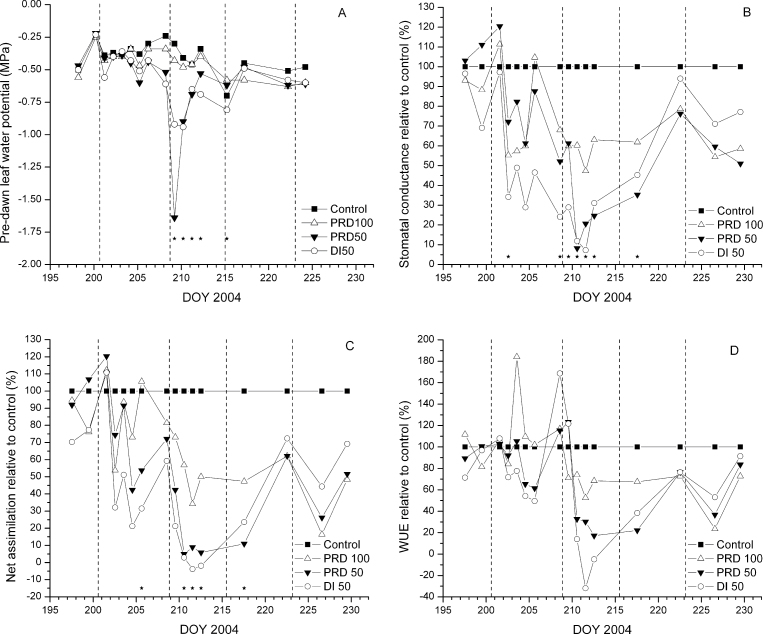
The Ψpd of PRD100 was not significantly different from that of C throughout the entire experimental period, although PRD100 values were slightly lower than those of C on several occasions (Fig. 4A). Both deficit treatments had lower Ψpd than C and PRD100 beginning on the fifth day into the drying cycle (DOY 204) and, with the exception of DOY 209, Ψpd of PRD50 and DI50 units was similar (Fig. 4A). Both treatments recovered to C and PRD100 levels following DOY 214, when the deficit period was terminated.
Fig. 4.
The effects of irrigation treatments on 2-year-old, split-root containerized ‘Gale Gala’ apple leaf pre-dawn water potential (A), stomatal conductance (B), net assimilation (C), and water use efficiency (D). Data are expressed as a percentage of control (C). On DOY 215, PRD50 and DI50 were changed to PRD100 and Control treatments, respectively. Vertical dashed lines signify switches in irrigation between PRD ‘wet’ and ‘dry’ compartments. Asterisks on the bottom represent significance at P < 0.05. Each point is the mean of three replications (n=2).
Mid-day g s, A, and WUE of PRD100, PRD50, and DI50 trees were progressively reduced during the first 10 d of the experimental period, relative to C (Fig. 4B–D). The percentage reduction of g s relative to C during the 15 d deficit period was 30, 45, and 54% for the PRD100, PRD50, and DI50, respectively. Similar reductions were observed for E and A (Fig. 4C). Gas exchange of PRD50 recovered to PRD100 levels within 1 week from its change to a PRD100 regime; however, DI50 never reached C values for g s or A after receiving full irrigation, despite maintaining an equivalent soil moisture status to that of C.
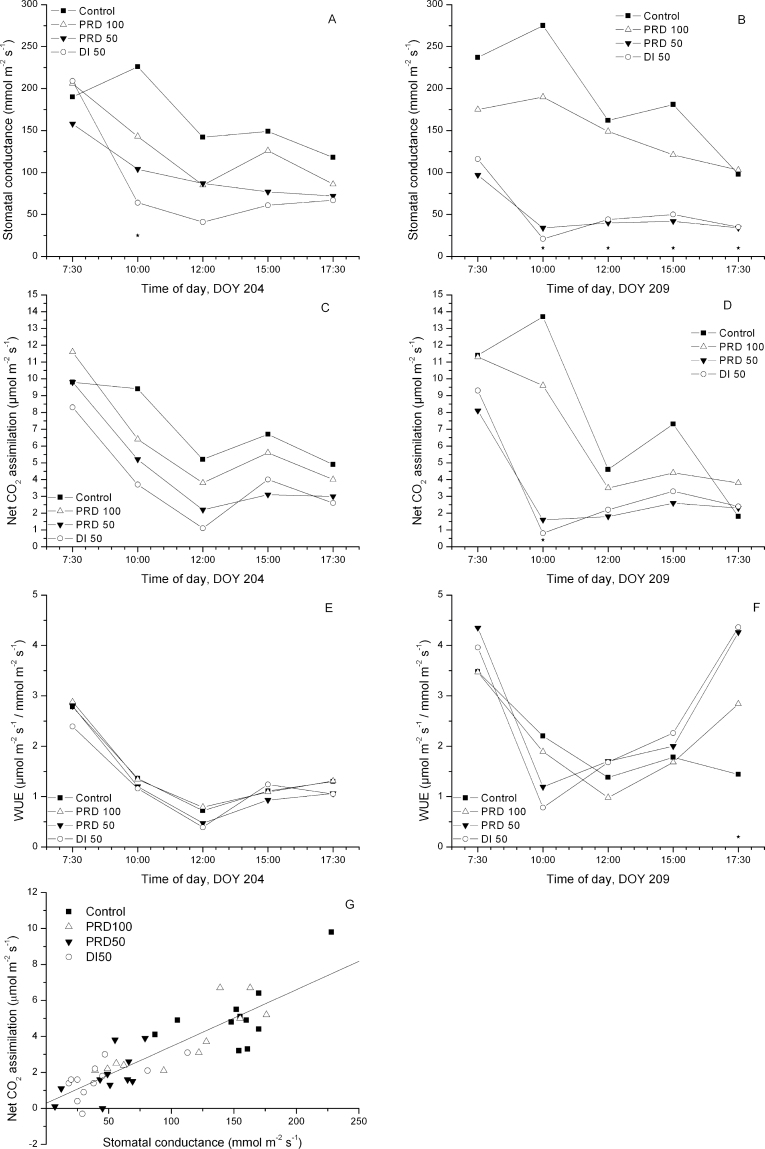
Examples of the diurnal patterns of gas exchange are presented for DOY 203 and DOY 208 (4 d and 9 d into the experimental period) (Fig. 5A–F). Irrespective of soil moisture status (Fig. 2), all treatments experienced a diurnal fluctuation in gas exchange, though the magnitude varied with treatment. On DOY 203, g s of both PRD50 and DI50 was significantly different from that of C by mid-morning, and approached significance for solar noon (P=0.07) and late afternoon (P=0.06) (Fig. 5A). Although DI50 values were lower than PRD50, the differences were not significant. The average mid-day E for PRD100, PRD50, and DI50 expressed as a percentage of C was 79, 68, and 55%, respectively. Substantial soil water deficits had accrued by DOY 208 (Fig. 2), resulting in marked and significant reductions of g s for deficit treatments (Fig. 5B). Average daily single-leaf transpiration of PRD100 was ~87% of that of C, while those of PRD50 and DI50 were reduced to 33% and 37% of C, respectively. Whole-plant transpiration was similarly reduced as determined by TDR (86, 42, and 49% of C for PRD100, PRD50, and DI50 trees, respectively; Fig. 2C), or for trees instrumented with Tmax (PRD100 and PRD50 were 77% and 42% of C, respectively). On both dates, diurnal patterns of A were similar to those of g s, though significant differences among treatments were not detected at P < 0.05 (Fig. 5C, 5D). Cloud cover at solar noon on DOY 208 markedly reduced the PAR (from 1600 µmol m–2 s–1 at 10:00h to 200 µmol m–2 s–1 at 12:00h) and thus limited A of C and PRD100 treatments (Fig. 5D). Because of the strong linear relationship between A and g s (r 2 =0.78, P=0.0001; Fig. 5G), WUE was not improved by deficit irrigation treatments (Fig. 5E, 5F).
Fig. 5.
The effects of irrigation treatments on single-leaf gas exchange of split-rooted, 2-year-old containerized ‘Gale Gala’apple trees, on DOY 203 (A, C, E) and DOY 208 (B, D, F), 2004. Stomatal conductance (A and B), net assimilation (C and D), and WUE (E and F). Asterisks on the bottom represent significance at P < 0.05. Each point is the mean of three replications (n=2). (G) The relationship between photosynthesis and stomatal conductance recorded at solar noon. Data were combined for both dates due to non-significant differences between slopes for individual dates. The regression line was fitted through the data: y=0.032x+0.286, (r 2=0.78, P < 0.0001).
Bulk [ABAleaf] (ng g–1 dw) for PRD100 and C did not significantly differ throughout the experimental period, although PRD100 generally had slightly higher [ABAleaf] than C (Table 1). In contrast, [ABAleaf] of deficit treatments increased sharply by DOY 203 and remained significantly elevated over C until DOY 221, which was 1 week following the return of these treatments to their >100% ETc counterparts (Table 1). On DOY 203 and 208 [ABAleaf] of DI50 was markedly higher than that of PRD50, though not significantly. Low treatment sampling (three replications; n=2), high variability associated with [ABAleaf] content, concomitant with observed heterogeneity within treatment θv limited the statistical separation between treatments. On DOY 211, 3 d after PRD irrigation was alternated between wet and dry compartments, PRD50 had significantly higher [ABAleaf] than its deficit counterpart (Table 1). The relative effects of soil drying can be observed when the data are expressed as a percentage of C to account for fluctuations in the absolute [ABAleaf] of C trees (Table 1). [ABAleaf] increased with decreasing soil moisture through the first drying cycle in all treatments relative to C (Table 1, Fig 2A, 2B), irrespective of irrigation placement (i.e. applied to one container or two).
Table 1.
The effect of irrigation treatment on leaf abscisic acid concentration (ng g–1 dw) of split-rooted, 2-year-old containerized ‘Gale Gala’ apple trees on selected dates [day of year (DOY)].
DOY 200 was day 1 of the experiment. Irrigation was alternated between the wet and dry containers of PRD treatments on the evenings of DOY 208, 215, and 223. On DOY 215, PRD50 and DI50 were changed to PRD100 and control treatments, respectively.
| Treatment | DOY 200 | DOY 203 | DOY 208 | DOY 211 | DOY 216 | DOY 221 |
|---|---|---|---|---|---|---|
| Control | 97 | 107 b | 253 b | 73 b | 58 | 106 |
| PRD100 | 110 (114) | 157 b (147) | 296 b (117) | 88 b (120) | 76 (132) | 92 (87) |
| PRD50 | 107 (110) | 261 a,b (244) | 438 a,b (173) | 221 a (302) | 100 (173) | 93 (87) |
| DI50 | 108 (111) | 438 a (409) | 774 a (306) | 139 b (190) | 82 (141) | 93 (87) |
Data are means of three replications (n=2).
Means within columns for each date followed by different letters are significantly different at P < 0.05.
Values in parentheses are treatment ABA as a percentage of control.
[ABAleaf] was significantly, negatively correlated with θv of the wet compartment during the first 8 d of the drying cycle (r 2=0.61, P < 0.0001) (Fig. 6A). A similar relationship was observed between total θv (sum of both containers) and [ABAleaf] (r 2=0.6, P < 0.0001), but the relationship was markedly weakened when θv of the dryer container was regressed against [ABAleaf] (r 2=0.39, P < 0.0001). [ABAleaf] versus θv of the wet compartment remained highly significant when the entire data set was analysed (P < 0.0005); however, the variability was noticeably higher (r 2=0.16, Fig. 6B), as was the case when the relationships between [ABAleaf] and either total θv or minimum container θv were analysed. Stomatal conductance and [ABAleaf] were negatively correlated (P < 0.0001) following 7 d of soil drying (Fig. 6C), but the relationship was substantially weakened when all data points were analysed (Fig. 6D).
Fig. 6.
The relationships between soil moisture content of the container having the higher soil moisture content of split-rooted apple trees and leaf abscisic acid concentration [ABAleaf] for days 1, 4, and 9 [day of year (DOY) 200, 203, and 208] of the first drying cycle (A), and for the entire experimental period (B), and stomatal conductance and [ABAleaf] on day 9 (DOY 208) of the first drying cycle (C), and throughout the experimental period (D). Each point for (A) and (B) represents one split-rooted unit and is the mean of two leaves for ABA concentration. Regression lines were fitted through the data: y= –0.083x+3.044 (r 2=0.61, P < 0.0001) (A); y= –0.045x+2.482 (r 2=0.16, P=0.0005) (B). Each point for (C) and (D) is a single leaf. Regression lines were fitted through the data: y= –204.6x+685.4 (r 2=0.58, P < 0.0001) (C); y= –25.3x+169.1 (r 2=0.01, P=0.227) (D).
Discussion
For the 30 d experimental period, apple trees receiving >100% ETc to one container of a PRD100, split-rooted system, while the other was allowed to dry, exhibited a 30% and 28% reduction in mid-day single-leaf gas exchange (Fig. 4B, 4C), and whole plant transpiration (determined by sap flow), respectively, relative to well-watered C plants. Drainage from the irrigated compartment of the PRD100 containers was not measured, though the net increase of soil moisture in the irrigated container following irrigation and drainage was roughly equivalent to the previous day’s water use (determined via TDR measurements).
Reduced gas exchange could not be attributed to a difference in [ABAleaf] between PRD100 and C. Although [ABAleaf] of PRD100 increased, albeit non-significantly, to 147% of C levels on DOY 203 (Table 1), it was only slightly higher than C on all but the last sampling date, when it was in fact lower than C. Because prevailing xylem sap [ABA] has been more sensitively related to g s than [ABAleaf] (Jia and Zhang, 1999), and intraleaf redistribution of ABA to the guard cell apoplast can elicit stomatal control without changing [ABAleaf] (Zhang and Outlaw, 2001), it is not surprising that, at best, only 58% of the variability of g s could be explained by [ABAleaf] (Fig. 6C), though examples of stronger relationships between [ABAleaf] and g s have been reported in other taxa (Stoll et al., 2000; Aasamaa et al., 2002; Lovisolo et al., 2002). Several modulators increase guard cell sensitivity to ABA during soil drying [i.e. increased alkalinity of xylem sap (Gollan et al., 1992; Wilkinson and Davies, 1997; Wilkinson et al., 1998; Sobeih et al., 2004; Wang et al., 2012), high VPD (Tardieu and Davies, 1992; Soar et al., 2006), and declining Ψ (Behl and Hartung, 1984; Tardieu and Davies, 1993)]. It is therefore plausible that a minimal shift in one or several of these factors may have amplified the PRD100 signal; however, these mechanisms were not investigated in the present study.
That total θv of both containers, or of the container possessing the higher θv, was better correlated with [ABAleaf] than θv of the non-irrigated container of a PRD unit, or the container with the lower θv for C or DI50 units, indicates that drying roots in the ‘wet’ compartment probably contributed additional ABA to the leaves, or modified signal strength. Liu et al. (2008) observed a strong relationship between average xylem [ABA] of the wet and dry roots and xylem [ABA] of potato plants. Measured differences in [ABAleaf] between PRD100 and PRD50, and nearly identical rates of sap flow and θv depletion of dry containers prior to re-wetting of these treatments (Figs 2, 3B, C), suggest that [ABAleaf] depends strongly on the θv of the wet container, as modelled by Dodd et al. (2008a) and observed experimentally (Romero et al., 2012). If [ABAleaf] production and transport were wholly dependent upon the status of the dry roots, PRD100 and PRD50 would have had similar [ABAleaf]. Additionally, a plausible explanation for why [ABAleaf] was highest in DI treatments early in the experimental period (Table 1, Fig. 2) is that more roots were in contact with an ‘optimal’ range of θv for both root ABA synthesis and its transport to the shoot, as hypothesized by Dodd et al. (2008b). The higher [ABAleaf] of PRD50 on DOY 211, following re-irrigation of drying roots on DOY 208, might have derived from liberated pools of ABA from previously drying roots (Dodd et al., 2006); however, this was not observed for the DI50 treatment when returned to full irrigation on DOY 215, or PRD100 following switches (Table 1, Fig. 3B). The frequency of the sampling interval used was too long to identify a threshold θv for the irrigated root system of a PRD unit to optimize [ABAleaf], but the data generally support modelled predictions of Dodd et al. (2008a), and underscore the need to account for θv of both root systems in order to sustain [ABAleaf] when soil moisture heterogeneity exists (Wang et al., 2012).
Similar reductions in gas exchange for PRD100 relative to well-watered controls have been reported for tomato (Mingo et al., 2004) and olive (Olea europaea) (Wahbi et al., 2005). The reduction in the present study was not strongly associated with reduced LA; LA of split-rooted trees was 1.22, 1.11, 1.1, and 1.23 m2 (P=0.49) for the C, PRD100, PRD50, and DI50 treatments, respectively. Although significant interactions were not detected between irrigation volume (100% and 50% ETc) and placement (PRD and DI) (P=0.88), two-way ANOVA showed a slight, albeit non-significant, PRD effect (P=0.09) on reducing LA. On an LA basis, apple water use of C trees was 3.3 l m–2 d–1, and is comparable with published values for apple growing in arid climates (Angelocci and Valancogne, 1993). Despite lower whole plant transpiration of PRD100 trees (70% of C), sap flow from the irrigated side exceeded maximum C sap flow by ~30% when averaged across the 30 d experimental period. Apple roots have previously been shown to increase sap flow rates rapidly in response to wetting events (Figs 2, 3; Green et al., 1997), although the mechanism controlling this response in apple is not clear. Higher sap flow rates have been similarly observed from well-watered roots of split-rooted treatments, relative to root sap flow from fully irrigated rootzones for other species(Kang et al., 2003; Dodd et al., 2008b; Liu et al., 2008). Further, the data show a rapid recovery in soil moisture uptake of roots in previously dry root compartments, as similarly documented by Liu et al. (2008). The high evaporative demand (VPDs typically exceeded 4 kPa at mid-day), large container volume (i.e. 11 litres of water per container after drainage of free water), and the high proportion of extractable water in the potting media (i.e. 7.5 litres per container) would render a relatively low soil–root resistance and permit the higher sap flow rates observed up-stream from the irrigated PRD100 roots (also observed by Kang et al., 2003; Dodd et al., 2008b).
Results for PRD50 and DI50, however, were quite different. On DOY 203, under conditions of mild stress after only 3.5 d of pot drying, [ABAleaf] of PRD50 and DI50 was 2.6- and 4.4-fold higher than that of C, and g s was reduced by 32% and 61% of C, respectively. Concomitantly, Ψpd of both treatments was sustained at C levels. In contrast, on DOY 208, the afternoon preceding the first switch for PRD regimes, Ψpd of both deficit treatments was significantly lower than that of C, and g s was reduced by ~74% (Fig. 4A, 4B), indicating severe stress, despite increased levels of endogenous [ABAleaf]. The marked increase observed in [ABAleaf] for both of these treatments, and particularly for DI50, may have been the result of leaf ABA synthesis. Soar et al. (2004) have documented synthesis of ABA in grape leaves when relative water content fell to 85%. Stoll et al. (2000) observed a 5-fold increase in [ABAleaf] of DI-treated grapevines compared with PRD, and this difference was related to the reduced leaf water status of the DI compared with the PRD treatment. Others, however, have shown that leaf synthesis of ABA does not occur until turgor approaches zero (Pierce and Raschke, 1980). The lower Ψ recorded for PRD50 plants is at odds with tight stomatal regulation of leaf water status, although, in our case, Ψpd reflected the energy status of the soil, assuming equilibrium between the plant and the soil was reached. Further, apple leaves remained visually turgid at mid-day on all measurement dates, irrespective of irrigation treatment.
In two separate field studies with apple in the temperate climate of New Zealand and the semi-arid climate of Washington State, USA, it has been shown that irrigation volumes of 50% relative to a control were insufficient to maintain a wet profile with PRD (Leib et al., 2006), as was also confirmed in the present container study (Fig. 2B). In fact, the cumulative development of soil water deficit in the wet profile of PRD treatments irrigated at 0.5 ETc is evident in studies with grapevine (Santos et al., 2005), tomato (Zegbe-Dominguez et al., 2003; Kirda et al., 2004; Zegbe et al., 2006), hot pepper (Capsicum annum L. ‘Ancho St. Luis’) (Dorji et al., 2005), potato (Liu et al., 2006), maize (Kirda et al., 2005), common bean (Wakrim et al., 2005), and olive (Wahbi et al., 2005). Subsequently, in most of the afore-mentioned studies, leaf Ψ of PRD plants was reduced relative to the well-watered control. In the present study, 50% ETc applied via PRD had an equally deleterious effect to DI50, because progressive drying of the ‘wet’ soil profile led to similar degrees of plant water stress (Figs 4, 5). In contrast, studies which maintained soil moisture near FC in the irrigated side of PRD (such as in PRD100) did not detect a significant decline in plant water potential, but observed a significant reduction in g s (Dry and Loveys, 1999; Toit et al., 2003; Mingo et al., 2004; Sobeih et al., 2004).
The difficulty of reconciling a chemical signal to physiological effects under controlled environments (cf. Fig. 6C, 6D) underscores the challenges associated with experimental and commercial application of PRD in field situations; slow drying of heavy soils, heterogeneity of physical and chemical properties of soil and moisture availability within a profile, low root length densities (e.g. perennial crops), atmospheric factors, and, in apple, strong active osmotic regulation (Lakso, 1979) do not favour agricultural management of such a highly transient, and rapid signal. Non-stressed apple WUE is quite high (Lakso, 2003) and, significant stomatal closure of apple leaves, whether due to hydraulic or chemical signals, will adversely affect carbon supply (Fig. 5G), and ultimately dry matter accumulation in fruit. One tenet of PRD is the increase in WUE when water supply is limited, though in the climate studied here, intrinsic WUE of apple was not increased for any deficit treatments when gas exchange was reduced (Fig. 5), contrary to previous studies of PRD-treated perennial crops (Egea et al., 2011; Romero et al., 2012). While intrinsic WUE is determined from point measurements, the lack of a consistently elevated ABA signal (Table 1), similar water use (Fig 2C), and non-significant differences in canopy leaf area in PRD plants compared with their equally watered counterparts suggest that long-term WUE would probably behave similarly. However, it should be noted that in the climate in the present experiments apple shoot growth is largely complete by mid to late July (i.e. coinciding with the beginning of the experimental period in the current study). Therefore, in other climates, or during periods of rapid vegetative growth, reductions in gas exchange similar to those that were observed here would probably have marked impacts on leaf area, and subsequently alter long-term WUE. PRD-treated lemon [Citrus limon (L.) Burm. fil.] trees had increased long-term WUE compared with DI, despite both treatments possessing similar intrinsic WUE (Pérez-Pérez et al., 2012).
Split-rooted, well-developed apple trees were capable of producing chemical signals during soil drying when irrigation was limited to 50% ETc, irrespective of water placement (e.g. DI or PRD), but differences in mid-day stomatal regulation between the two treatments were not significant (Fig. 4) (Wang et al., 2011). However, when provided with >100% ETc, PRD100 [ABAleaf] was not significantly elevated above C levels to link it unequivocally to the lower daily water use and gas exchange of PRD100 compared with C (Figs 2C, 4 B–D). The fact that PRD50 had higher [ABAleaf] than PRD100 despite possessing equivalent dry container θv indicates that the soil moisture status of the irrigated rootzone of PRD-treated plants significantly modulates canopy gas exchange (as previously modelled; Dodd et al., 2008a, b; and observed experimentally; Romero et al., 2012). The relatively low soil moisture availability of PRD50 wet and dry root compartments did not result in a consistent, optimal ABA supply (Table 1), or maintain high plant water relations (Figs 4, 5) (Wang et al., 2012). Further investigations are required to define the threshold soil moisture status and its relationship with ABA signalling of the drying root system of PRD apple for alternating irrigation between wet and dry containers. In the present experiment, soil water depletion of the dry root system of PRD100 may have been too severe prior to the switch in irrigation on DOY 208 (Fig. 2A) to elicit augmented [ABAleaf] (Table 1). Insignificant changes in PRD100 gas exchange immediately succeeding irrigation switches (Fig. 4B, 4C) and only slight, non-significant differences in [ABAleaf] between PRD100 and C 3 d following the first switch (DOY 211) and the day immediately succeeding the second switch (DOY 216) (Table 1), do not concur with previously published results showing liberated ABA pools following re-watering of dry PRD roots (Dodd et al., 2006). In general, the data indicate that apple water use and [ABAleaf] are associated with the overall soil moisture environment. Future characterization of [ABA] immediately following irrigation alternation and its dependency on a threshold sap flow from roots in drying soil (Dodd et al., 2008a, b) would elucidate the physiological potential, and inform both the experimental and agronomic utility of PRD.
Fig. 1.
The relationship between sap flow (Tmax method) and time-domain reflectometry (TDR) estimates of daily water use of split-rooted, 2-year-old ‘Gale Gala’ apple trees. Each symbol represents one split-rooted unit instrumented with sap flow sensors and TDR probes. The regression line was fitted through the data: y=1.07x+0.64 (r 2=0.63, P < 0.0001).
Acknowledgements
We would like to thank the Washington Tree Fruit Research Commission for support. TCE would like to thank Dr. Greg Litus for manufacturing the tripod system used for gravimetric measurement, Dr. Cecil Stushnoff for his analytical guidance during development of ABA protocols, and Dr. Ian Dodd for his review and insightful comments on the manuscript.
References
- Aasamaa K, Sõbr KA, Hartung W, Niinemets Ü. Rate of stomatal opening, shoot hydraulic conductance and photosynthetic characteristics in relation to leaf abscisic acid concentration in six temperate deciduous trees. Tree Physiology. 2002;22:267–276. doi: 10.1093/treephys/22.4.267. [DOI] [PubMed] [Google Scholar]
- Angelocci LR, Valancogne A. Leaf area and water flux in apple trees. Journal of Horticultural Science. 1993;68:299–307. [Google Scholar]
- Behl R, Hartung W. Transport and compartmentation of abscisic acid in roots of Hordeum distichon under osmotic stress. Journal of Experimental Botany. 1984;35:1433–1440. [Google Scholar]
- Bingham GE. 1972. Stomatal response of field corn (Zea mays L.) and apple (Malus sylvestris). PhD thesis, Cornell University; USA: [Google Scholar]
- Blackman PG, Davies WJ. Root to shoot communication in maize plants of the effects of soil drying. Journal of Experimental Botany. 1985;36:39–48. [Google Scholar]
- Cohen Y, Fuchs M, Green GC. Improvement of the heat pulse method for determining sap flow in trees. Plant, Cell and Environment. 1981;4:391–397. [Google Scholar]
- Comstock JP. Hydraulic and chemical signaling in the control of stomatal conductance and transpiration. Journal of Experimental Botany. 2002;53:195–200. doi: 10.1093/jexbot/53.367.195. [DOI] [PubMed] [Google Scholar]
- Davies FS, Lakso AN. Water relations in apple seedlings: changes in water potential components, abscisic acid levels and stomatal conductances under irrigated and non-irrigated conditions. Journal of the American Society for Horticultural Science. 1978;103:310–313. [Google Scholar]
- Davies WJ, Zhang J. Chemical regulation of growth and physiology in drying soil: the case for abscisic acid. Current Topics in Plant Biochemistry. 1989;8:100–109. [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:55–76. [Google Scholar]
- Dodd IC. Hormonal interactions and stomatal responses. Journal of Plant Growth Regulation. 2003;22:32–46. [Google Scholar]
- Dodd IC. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signaling of abscisic acid. Functional Plant Biology. 2007;34:439–448. doi: 10.1071/FP07009. [DOI] [PubMed] [Google Scholar]
- Dodd IC. Rhizosphere manipulations to maximize ‘crop per drop’ during deficit irrigation. Journal of Experimental Botany. 2009;60:2454–2459. doi: 10.1093/jxb/erp192. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. Accounting for sap flow from different parts of the root system improves the prediction of xylem ABA concentration in plants grown with heterogeneous soil moisture. Journal of Experimental Botany. 2008a;59:4083–4093. doi: 10.1093/jxb/ern246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. Abscisic acid signaling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant, Cell and Environment. 2008b;31:1263–1274. doi: 10.1111/j.1365-3040.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Theobald JC, Bacon MA, Davies WJ. Alternation of wet and dry parts during partial rootzone drying irrigation alters root-to-shoot signaling of abscisic acid. Functional Plant Biology. 2006;33:1081–1089. doi: 10.1071/FP06203. [DOI] [PubMed] [Google Scholar]
- Dorji K, Behboudian MH, Zegbe-Dominguez JA. Water relations, growth, yield, and fruit quality of hot pepper under deficit irrigation and partial rootzone drying. Scientia Horticulturae. 2005;104:137–149. [Google Scholar]
- Dry PR, Loveys BR. Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis. 1999;38:151–156. [Google Scholar]
- Dry PR, Loveys BR, Botting D, Düring H. 1996. Effects of partial rootzone drying on grapevine vigour, yield, composition of fruit and use of water. In:Stockley CS, Sas AN, Johnstone RS, Lee TH, eds. Proceedings of the 9th Australian Wine Industry Technical Conference Adelaide, Australia: Winetitles; 126 131 [Google Scholar]
- Dry PR, Loveys BR, Düring H. Partial drying of the rootzone of grape. I. Transient changes in shoot growth and gas exchange. Vitis. 2000a;39:3–7. [Google Scholar]
- Dry PR, Loveys BR, Düring H. Partial drying of the rootzone of grape. II. Changes in the pattern of root development. Vitis. 2000b;39:9–12. [Google Scholar]
- Egea G, Dodd IC, Gonzalez-Real MM, Domingo R, Baille A. Partial rootzone drying improves almond tree leaf-level water use efficiency and afternoon water status compared with regulated deficit irrigation. Functional Plant Biology. 2011;38:372–385. doi: 10.1071/FP10247. [DOI] [PubMed] [Google Scholar]
- Gollan T, Schurr U, Schulze ED. Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. I. The concentration of cations, anions, amino acids in, and pH of, the xylem sap. Plant, Cell and Environment. 1992;15:551–559. [Google Scholar]
- Gowing DJG, Davies WJ, Jones HG. A positive root-sourced signal as an indicator of soil drying in apple, Malus×domestica Borkh . Journal of Experimental Botany. 1990;41:1535–1540. [Google Scholar]
- Green SR, Clothier BE, Jardine B. Theory and practical application of heat pulse to measure sap flow. Agronomy Journal. 2003;95:1371––1379. [Google Scholar]
- Green SR, Clothier BE, McLeod DJ. The response of sap flow in apple roots to localised irrigation. Agricultural Water Management. 1997;33:63–78. [Google Scholar]
- Gu S, Guoqiang D, Zoldoske D, Hakim A, Cochran R, Fugelsang K, Jorgensen G. Effects of irrigation amount on water relations, vegetative growth, yield and fruit composition of Sauvignon blanc grapevines under partial rootzone drying and conventional irrigation in the San Joaquin Valley of California, USA. Journal of Horticulture Science and Biotechnology. 2004;79:26–33. [Google Scholar]
- Hartung W, Sauter A, Hose L. Abscisic acid in the xylem: where does it come from, where does it go? Journal of Experimental Botany. 2002;53:27–32. [PubMed] [Google Scholar]
- Jia W, Zhang J. Stomatal closure is induced rather by prevailing xylem abscisic acid than by accumulated amount of xylem-derived abscisic acid. Physiologia Plantarum. 1999;106:268–275. [Google Scholar]
- Kang S, Hu T, Jerie P, Zhang J. The effects of partial rootzone drying on root, trunk sap flow and water balance in an irrigated pear (Pyrus communis L.) orchard. Journal of Hydrology. 2003;280:192–206. [Google Scholar]
- Kirda C, Cetin M, Dasgan Y, Topcu S, Kaman H, Ekici B, Derici MR, Ozguven AI. Yield response of greenhouse grown tomato to partial root drying and conventional deficit irrigation. Agricultural Water Management. 2004;69:191–201. [Google Scholar]
- Kirda C, Topcu S, Kaman H, Ulger AC, Yazici A, Cetin M, Derici MR. Grain yield response and N-fertiliser recovery of maize under deficit irrigation. Field Crops Research. 2005;93:132–141. [Google Scholar]
- Lakso AN. Seasonal changes in stomatal response to leaf water potential in apple. Journal of the American Society for Horticultural Science. 1979;104:58–60. [Google Scholar]
- Lakso AN. 2003. Water relations of apples. In: Ferree DC, Warrington IJ, eds Apples: botany, production and uses Wallingford, UK: CAB International; 167 194 [Google Scholar]
- Leib BG, Caspari HW, Redulla CA, Andrews PK, Jabro J. Partial rootzone drying and deficit irrigation of ‘Fuji’ apples in a semi-arid climate. Irrigation Science. 2006;24:85–99. [Google Scholar]
- Liu F, Song R, Zhang X, Shahnazari A, Andersen MN, Plauborg F, Jacobsen SE, Jensen CR. Measurement and modeling of ABA signaling in potato (Solanum tuberosum L.) during partial rootzone drying. Environmental and Experimental Botany. 2008;63:385–391. [Google Scholar]
- Liu F, Shahnazari A, Andersen MN, Jacobsen SE, Jensen CR. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Scientia Horticulturae. 2006;109:113–117. [Google Scholar]
- Loveys B. Diurnal changes in water relations and abscisic acid in field grown Vitis vinifera cultivars. III. The influence of xylem derived abscisic acid on leaf gas exchange. New Phytologist. 1984;98:563–573. [Google Scholar]
- Loveys BR, Robinson SP, Downton WJS. Seasonal and diurnal changes in abscisic acid and water relations of apricot leaves (Prunus armeniaca L.) New Phytologist. 1987;107:15–27. [Google Scholar]
- Lovisolo C, Hartung W, Schubert A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Functional Plant Biology. 2002;29:1349–1356. doi: 10.1071/FP02079. [DOI] [PubMed] [Google Scholar]
- Mingo DM, Theobald JC, Bacon MA, Davies WJ, Dodd IC. Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial rootzone drying: enhancement of root growth. Functional Plant Biology. 2004;31:971–978. doi: 10.1071/FP04020. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez JG, Dodd IC, Botía P. Partial rootzone drying increases water-use-efficiency of lemon Fino 49 trees independently of root-to-shoot ABA signaling. Functional Plant Biology. 2012;39:366–378. doi: 10.1071/FP11269. [DOI] [PubMed] [Google Scholar]
- Pierce M, Raschke K. Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta. 1980;148:174–182. doi: 10.1007/BF00386419. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Santos TP, Rodrigues AP, de Souza CR, Lopes CM, Maroco JP, Pereira JS, Chaves MM. Hydraulic and chemical signaling in the regulation of stomatal conductance and plant water use in field grapevines growing under deficit irrigation. Functional Plant Biology. 2008;35:565–579. doi: 10.1071/FP08004. [DOI] [PubMed] [Google Scholar]
- Romero P, Dodd IC, Martinez-Cutillas A. Contrasting physiological effects of partial root zone drying in field-grown grapevine (Vitis vinifera L. cv. Monastrell) according to total soil water availability. Journal of Experimental Botany. 2012;63:4071–4083. doi: 10.1093/jxb/ers088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos TP, Lopes CM, Rodrigues ML, de Souza CR, Ricardo-Da-Silva JM, Maroco JP, Pereira JS, Chaves MM. Effects of partial root-zone drying irrigation on cluster microclimate and fruit composition of field-grown Castelao grapevines. Vitis. 2005;44:117–125. [Google Scholar]
- Sauter A, Davies WJ, Hartung W. The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. Journal of Experimental Botany. 2001;52:1991–1997. doi: 10.1093/jexbot/52.363.1991. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Soar CJ, Spiers J, Maffei SM, Loveys BR. Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Functional Plant Biology. 2004;31:659–669. doi: 10.1071/FP03238. [DOI] [PubMed] [Google Scholar]
- Soar CJ, Spiers J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research. 2006;12:2–12. [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial rootzone drying. Journal of Experimental Botany. 2004;55:2353–2363. doi: 10.1093/jxb/erh204. [DOI] [PubMed] [Google Scholar]
- Soejima J, Watanabe M, Moriguchi T, Yamaki S. Good correlation between enzyme-linked immunosorbent assay and gas chromatographic analysis of abscisic acid in apple organs. Journal of the Japanese Society for Horticultural Science. 1990;58:819–826. [Google Scholar]
- Stoll M, Loveys BR, Dry P. Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany. 2000;51:1627–1634. doi: 10.1093/jexbot/51.350.1627. [DOI] [PubMed] [Google Scholar]
- Tan CS, Buttery BR. The effect of soil moisture stress to various fractions of the root system on transpiration, photosynthesis, and internal water relations of peach seedlings. Journal of the American Society for Horticultural Science. 1982;107:845–849. [Google Scholar]
- Tardieu F, Davies WJ. Stomatal response to abscisicacid is a function of current plant water status. Plant Physiology. 1992;98:540–545. doi: 10.1104/pp.98.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Davies WJ. Integration of hydraulic and chemical signaling in the control of stomatal conductance and water status of droughted plants. Plant, Cell and Environment. 1993;16:341–349. [Google Scholar]
- Toit PG, Dry PR, Loveys BR. A preliminary investigation on partial rootzone drying (PRD) effects on grapevine performance, nitrogen assimilation and berry composition. South African Journal of Enology and Viticulture. 2003;24:43–54. [Google Scholar]
- Topp GC, Davis JL, Annan AP. Electromagnetic determination of soil water content: measurements in coaxial transmission lines. Water Resources Research. 1980;16:574–582. [Google Scholar]
- Wahbi S, Wakrim R, Aganchich B, Tahi H, Serraj R. Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate. I. Physiological and agronomic responses. Agriculture, Ecosystems and Environment. 2005;106:289–301. [Google Scholar]
- Wakrim R, Wahbi S, Tahi H, Aganchich B, Serraj R. Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agriculture, Ecosystems and Environment. 2005;106:275–287. [Google Scholar]
- Wang Y, Liu F, Jensen CR. Comparative effects of deficit irrigation and alternate partial root-zone irrigation on xylem pH, ABA and ionic concentrations in tomatoes. Journal of Experimental Botany. 2012;63:1907–1917. doi: 10.1093/jxb/err370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Corlett JE, Oger L, Davies WJ. Effects of xylem pH on transpiration from wild-type and flacca tomato leaves: a vital role for abscisic acid in preventing excessive water loss even from well-watered plants. Plant Physiology. 1998;117:703–709. doi: 10.1104/pp.117.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiology. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegbe JA, Behboudian MH, Clothier BE. Responses of ‘Petopride’ processing tomato to partial rootzone drying at different phenological stages. Irrigation Science. 2006;24:203–210. [Google Scholar]
- Zegbe-Dominguez JA, Behboudian MH, Lang A, Clothier BE. Deficit irrigation and partial rootzone drying maintain fruit dry mass and enhance fruit quality in ‘Petopride’ processing tomato (Lycopersicon esculentum, Mill.) Scientia Horticulturae. 2003;98:505–510. [Google Scholar]
- Zhang J, Schurr U, Davies WJ. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. Journal of Experimental Botany. 1987;38:1174–1181. [Google Scholar]
- Zhang SQ, Outlaw WH., Jr Abscisic acid introduced into the transpiration stream accumulates in the guard-cell apoplast and causes stomatal closure. Plant, Cell and Environment. 2001;24:1045–1054. [Google Scholar]