Abstract
Leaf venation patterns vary considerably between species and between leaves within a species. A mechanism based on canalization of auxin transport has been suggested as the means by which plastic yet organized venation patterns are generated. This study assessed the plasticity of Arabidopsis thaliana leaf venation in response to ectopic ground or procambial cell divisions and auxin transport inhibition (ATI). Ectopic ground cell divisions resulted in vascular fragments between major veins, whereas ectopic procambial cell divisions resulted in additional, abnormal vessels along major veins, with more severely perturbed lines forming incomplete secondary and higher-order venation. These responses imply limited vascular plasticity in response to unscheduled cell divisions. Surprisingly, a combination of ectopic ground cell divisions and ATI resulted in massive vascular overgrowth. It is hypothesized that the vascular overproduction in auxin transport-inhibited wild-type leaves is limited by simultaneous differentiation of ground cells into mesophyll cells. Ectopic ground cell divisions may negate this effect by providing undifferentiated ground cells that respond to accumulated auxin by differentiation into vascular cells.
Key words: Auxin, BCTV-C4, ectopic cell division, ground tissue, leaf venation, procambium
Introduction
Plant vascular systems consist of a continuous network of interconnected cells throughout the plant, showing organ- and species-specific architectures. While considerable research effort has examined the genetic control of vascular differentiation and several factors are known to affect vascular cell proliferation (reviewed in Mähönen et al., 2000; Dettmer et al., 2009; Caño-Delgado et al., 2010; Ohashi-Ito and Fukuda, 2010), the role of controlled cell divisions in this process is not clear. Procambial cells are derived from patterned cell divisions within the ground tissues, and procambial and ground cell tissue types affect vascular strand formation (Scarpella et al., 2004; Kang et al., 2007). How these two adjacent tissues interact would seem likely, therefore, to affect vascular patterning.
A classic paper published more than 50 years ago proposed that auxin can induce differentiation of vascular cells along paths of active polar auxin transport (PAT; Jacobs, 1952). Numerous subsequent publications have confirmed and expanded on the key role of auxin and PAT in vascular patterning and differentiation (reviewed in Fukuda, 2004). According to the canalization of auxin flow hypothesis (Sachs, 1981), diffuse sources of auxin are gradually channelled into narrow file(s) of cells to induce discrete vascular bundles. The process of auxin transport and its role in vascular differentiation is now being dissected at the molecular genetic level. With respect to leaf venation, the focus of this study, mutant phenotypes range from severely reduced venation in the monopteros (mp) auxin response factor mutant (Hardtke and Berleth, 1998), to increased venation in the pin-formed1 (pin1) auxin efflux carrier mutant (Galweiler et al., 1998; Mattsson et al., 1999). The roles of MP and PIN1 in vein formation are also supported by their expression patterns and the subcellular distribution of the PIN1 auxin carrier proteins (Scarpella et al., 2006; Wenzel et al., 2007). Their expressions undergo a gradual refinement before vein formation (Wenzel et al., 2007), in line with the predictions of the canalization of auxin flow hypothesis (Sachs, 1981). Sachs’ hypothesis also predicts that canalization drains auxin away from surrounding cells, thereby preventing their differentiation into vascular cells. Support for this conclusion comes from incremental pharmacological inhibition of auxin transport, which results in gradual vein overgrowth in the leaf lamina (Mattsson et al., 1999; Sieburth, 1999). The distribution of vessel elements and the accumulated expression of the auxin response marker DR5::GUS in the leaf lamina suggest that the vascular overgrowth is due to auxin accumulation in the leaf lamina (Mattsson et al., 2003).
Although leaf venation patterning shows remarkable plasticity in response to manipulation of auxin transport, other mechanisms are likely to play a role as well. In growing leaf primordia, the pattern and control of cell division activity in the procambial tissue, as well as in the complementary ground cell tissue, are likely to affect the final pattern and density of veins. Support for this notion comes from various studies. In Flaveria bidentis, relatively high vein density was partially explained by an early cessation of mesophyll cell divisions (McKown and Dengler, 2009). Ectopic expression of AINTEGUMENTA, which regulates cell division activity (Mizukami and Fischer, 2000), increases cell proliferation as well as the number of higher-order veins in Arabidopsis leaves (Kang et al., 2007). In contrast, ectopic expression of INHIBITOR OF CYCLIN DEPENDENT KINASE decreases Arabidopsis leaf cell proliferation and the number of higher-order veins (Kang et al., 2007). Leaf mesophyll cell differentiation may also terminate the progressive formation of higher-order veins in Arabidopsis (Scarpella et al., 2004).
To date, there is no study assessing how cell division control, specifically in the procambial tissue or the complementary ground cell tissue, affects the patterning and density of leaf veins. This study addressed this question by inducing ectopic cell divisions, either in the procambial or in the ground tissue domain. To accomplish this, two complementary GAL4-green fluorescent protein (GFP) lines with vascular or ground tissue expression were used to drive GAL4-transactivated expression (Haseloff, 1999) of the C4 protein from beet curly top virus (BCTV) specifically in these tissues. The C4 protein induces ectopic cell divisions in plants (Latham et al., 1997), coupled with induction of the cell-cycle-related genes CYCs, CDKs, PCNA (Park et al. 2010), and RKP (Lai et al., 2009), resulting in activation of the host cell cycle to facilitate viral replication. BCTV C4 protein bound to the AtSKη shaggy-related protein kinase in a yeast two-hybrid assay, indicating an interaction with the brassinosteroid pathway (Piroux et al., 2007), although BCTV C4 expression can affect multiple hormone pathways (Mills-Lujan and Deom, 2010). In Arabidopsis, cross-talk between the brassinosteroid and auxin pathways occurs via AtSK21 interaction with the auxin response factor ARF2 (Vert et al., 2008), and shoot vascular patterning involves brassinosteroid signalling coupled to PAT (Ibañes et al., 2009).
The present study examines the effect of BCTV C4 targeted induction of cell divisions in developing ground or procambial tissues and the correlated effects on auxin response patterns. Major alterations were observed in leaf growth and in vein patterning and density following C4 expression in both procambial and ground tissues. Surprisingly, ectopic cell divisions in the ground cell tissue resulted in fragmentation of higher-order veins. This effect was strongly exacerbated by simultaneous inhibition of auxin transport, resulting in leaf blades almost entirely filled with vascular tissues and widespread expression of the auxin response marker DR5::GUS, suggesting that this synergistic phenotype was associated with increased auxin response or auxin content in both the ground and procambial tissues.
Materials and methods
GAL4-responsive UAS::C4 plasmid construction and plant transformation
The pJITC4 plasmid containing the C4 coding region from BCTV (BCTV-C4) was kindly provided by J. Stanley (JIC Norwich) (Latham et al., 1997). The C4 coding region was used to replace the KNAT3-YFP sequence in a binary vector based on pBI121 (Jefferson et al., 1987) that included five repeats of a modified GAL4-VP16 binding site upstream of the sequence for a KNAT3-YFP fusion protein, with the C4 sequence bordered by a 5' BamHI site and a 3' SacI site (pBINKNAT3-YFP) (K. Siemering and J. Haseloff, unpublished; Haseloff, 1999). C4 DNA was amplified by polymerase chain reaction using Vent DNA polymerase (New England Biolabs, Hitchin, UK) and the following primers: forward primer, 5'-GGCGGATCCAACAATGGGCAACCTCATCTCCAC; and reverse primer, 5'-GGCGAGCTCTTAACGCCTTGGCATATGAG. The forward primer introduced a BamHI restriction site at the 5' end of the C4 sequence and the reverse primer introduced a SacI restriction site at the 3' end of the sequence. The modified C4 DNA fragment was restricted with BamHI and SacI and ligated to BamHI/SacI digested DNA comprising the GAL4-VP16 binding sites from pBINKNAT3-YFP to form pBINC4 (designated UAS::C4). pBINC4 was electroporated into Agrobacterium tumefaciens strain LBA4044 and Arabidopsis thaliana ecotype C24 plants were subsequently transformed by the floral dip method (Clough and Bent, 1998).
Plant material and growth conditions
The GAL4-GFP enhancer trap lines Q0990 and J0571 were previously identified amongst a collection of A. thaliana lines generated in C24 ecotype (Haseloff, 1999). These lines are available from the Nottingham Arabidopsis Stock Centre (http://nasc.nott.ac.uk; Q0990, NASC ID N9217; J0571, NASC ID N9094). Three independent homozygous UAS::C4 lines (T4 generation), encoding C4 under the control of GAL4 upstream activator sequences (UASs), were generated. Each line was crossed to both homozygous GAL4 enhancer trap lines, Q0990 and J0571, and detailed analyses performed on the F1 progeny (designated throughout as Q0990>>C4 or J0571>>C4). The three homozygous UAS::C4 lines conferred different strengths of phenotypic response when crossed to the GAL4 lines, with line C4.1 < C4.2 < C4.3. F1 plants of J0571>>C4.2, Q0990>>C4.1, and Q0990>>C4.2 were also crossed with Col-0 plants containing the auxin-responsive promoter DR5::GUS construct (obtained from Tom Guilfoyle, University of Missouri, Columbia). Progeny containing the GAL4 enhancer trap (Q0990 or J0571), UAS::C4, and DR5::GUS transgenes were examined for DR5::GUS response as described in Mattsson et al. (2003).
Seeds were either surface sterilized by agitation in 2% (v/v) commercial bleach (Domestos) for 15min followed by several rinses in sterile water, or gas sterilized for 3h in an enclosed chamber with 3% (v/v) HCl in commercial bleach. For selection of homozygous GAL4-GFP enhancer trap lines or UAS::C4 lines, seeds were plated in 9cm Petri dishes on sterile A. thaliana salts (ATS) medium (Lincoln et al., 1990) containing either 50mg/l kanamycin or 50mg/l hygromycin, respectively. For seedling growth analyses, plants were grown on either ATS alone or with a final concentration of 10 µM N-naphthylphthalamic acid (NPA; TCI, Tokyo, Japan) to inhibit auxin transport. Seeds were stratified at 4 °C for at least 24h and then grown in a controlled temperature growth chamber at 20 °C under constant fluorescent illumination (~100 µmol m–2 s–1). Plants used for analyses of developing embryos were grown in soil with 16h daylight (~50–80 µmol m–2 s–1) at 20 °C.
Analyses of cellular architecture, GFP, and MP expression
GFP expression patterns of J0571 and Q0990 enhancer trap lines were analysed throughout development. Leaves or dissected embryos were fixed in 4% (w/v) paraformaldehyde in 0.025M phosphate buffer for at least 2h, rinsed in water, mounted in either glycerol/phosphate-buffered saline (1:1 ) or in 70% (v/v) aqueous glycerol and imaged for both GFP expression and background autofluorescence. Vascular patterning was examined in whole-mount 1–3-week-old seedlings or excised leaves, fixed and cleared as in Berleth and Jürgens (1993). In situ mRNA hybridizations of MONOPTEROS (MP) expression were performed on leaf primordia as in Wenzel et al. (2007) to determine the effects of C4 misexpression on procambial strand formation.
Detailed analyses of the cellular architecture in mature embryos was examined in at least 50 F1 embryos of Q0990 or J0571 enhancer trap lines crossed to three independent UAS::C4 lines. These were compared with at least ten of each of the mature control embryos: (i) wild type, C24; (ii) Q0990 or J0571 lines; (iii) UAS::C4; and (iv) F1 embryos of C24>>C4 lines. Cellular architecture in Q0990>>C4 and J0571>>C4 embryos was compared throughout development with wild-type C24 embryos. Embryos were cleared and cell walls stained with propidium iodide using a pseudo-Schiff reaction [described briefly in Haseloff (2003) and Moreno et al. (2006), and in more detail here]. Immature embryos were separated from developing siliques and the seed coat was either punctured (pre-heart stage) or removed (heart stage and older). Mature embryos were obtained by removing the seed coats of imbibed seeds. Embryos were fixed in methanol/acetic acid (5:1) overnight at 4 °C. The embryos were rinsed in distilled water, gently agitated in 1% (w/v) periodic acid for 30min at room temperature, and then rinsed again in distilled water. Embryos were then immersed in aqueous Schiff reagent solution (0.1M sodium metabisulphite, 1.5 µM HCl) containing 10% (v/v) of 1mg ml–1 propidium iodide (Sigma, St. Louis, MO, USA) and gently agitated for 2h at room temperature. The embryos were rinsed thoroughly in distilled water and placed in an aqueous 16M chloral hydrate solution, transferred to a slide (excess chloral hydrate was removed), and mounted in Hoyer’s solution (30g gum arabic, 200g chloral hydrate, 20g glycerol, 50ml water). Embryos were left to clear at least overnight to enable optical sectioning of the entire embryo.
Embryo cellular architecture and GFP images were captured with a Bio-Rad MRC 500 or a Zeiss 510 meta upright laser scanning microscope. For visualization of embryonic 3D cellular architecture, z-series were taken throughout the embryos at 0.1–0.4 µm intervals. A 543nm excitation filter and 560 lp emission filter were used to visualize propidium iodide labelling of cell walls or autofluorescence after fixing in paraformaldehyde. GFP was visualized using 488nm excitation and 505–550nm emission filters. 3D reconstruction and visualization of the confocal z-series was performed with Amira version 2.1 (Visual Concepts, Germany) and ImageJ 1.34s (National Institute of Health, USA) software. Leaf vascular patterns and MP expression were imaged with a Nikon Eclipse E600 microscope and a Canon EOS D30 digital camera.
Results
Q0990 and J0571 lines drive complementary GFP expression in vascular and ground tissues during cotyledon and leaf development
Arabidopsis enhancer trap lines were previously generated through transformation with a T-DNA vector containing a modified GAL4-VP16 transcription activator gene and a GAL4-responsive mGFP5 gene under the control of GAL4 UASs (Haseloff, 1999). Expression of the GAL4-VP16 gene is dependent on the proximity of Arabidopsis genomic enhancer elements, and the GAL4 protein then binds to UAS to drive tissue-specific expression of mGFP5 (Haseloff, 1999). The Q0990 and J0571 enhancer trap lines drive GFP expression in procambial or ground tissue respectively. Ectopic cell divisions were induced through GAL4 driven expression of the BCTV C4 protein in the procambial and ground tissues following genetic crosses between the GAL4 lines Q0990 and J0571, respectively, and the GAL4-responsive UAS C4 lines.
In the Q0990 line, GFP expression was already evident in the procambium and ground tissues of the root and hypocotyl regions in late globular-stage embryos (data not shown). GFP expression became restricted to the vascular and pericycle cells by the early heart stage, and thereafter almost exclusively in the vascular tissues (Fig. 1B) including seedling leaf venation (Fig. 2A–D). A. thaliana leaves have a hierarchical reticulate venation patterning, with primary and secondary lower-order veins and tertiary and quaternary higher-order veins (Mattsson et al., 1999; Kang and Dengler, 2002). In cotyledons and leaves, GFP expression was observed in the developing primary vein, in secondary veins forming loops on either side of the primary vein, and also in higher-order veins (Figs. 1B, 2A–C). Hence GFP expression in Q0990 occurred in preprocambial and procambial cells of embryos, and, as also determined by Sawchuk et al. (2007), in developing procambial cells of leaves (e.g. Figs. 1B, 2A–D). Q0990 GFP expression in leaf was eventually reduced or lost in a basipetal direction as leaf veins matured (e.g. Fig. 2D).
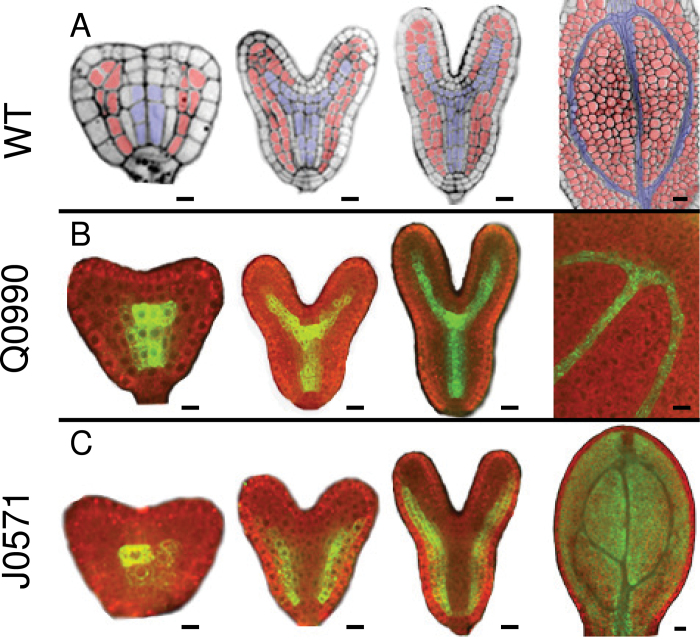
Fig. 1.
GFP expression in vascular (Q0990) and ground (J0571) tissues during embryo development. Confocal images show vascular tissues (blue) and ground tissues (red) in wild-type embryos (A) or GFP expression in, Q0990 (B) and J0571 (C) embryos at different developmental stages (left to right: triangular/early heart, late heart, torpedo, mature embryonic cotyledon). All images are median longitudinal sections except the early heart stage of J0571, which is a tangential longitudinal section taken through the ground tissue showing GFP expression in these cells. Bars, 10 µm.
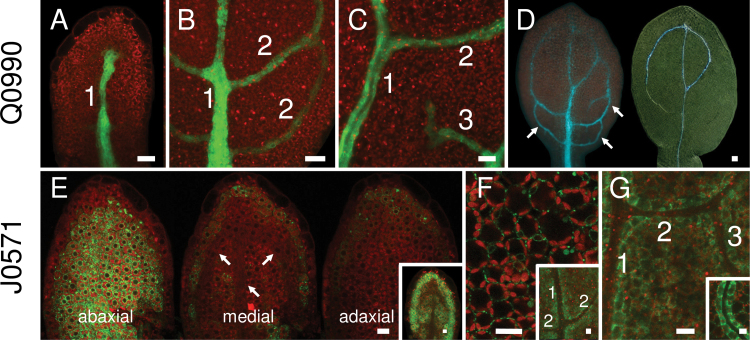
Fig. 2.
GFP expression in vascular (Q0990) or ground (J0571) tissues during leaf development. Images show first rosette leaf primordia at 2 (A), 3 (E), 4 (B, F inset), 5 (C, D, G), 6 (F), and 7 (G inset) days after germination. (A–C) GFP expression in Q0990 leaf primordia was observed progressively in the primary midvein (1), secondary veins (2), and tertiary veins (3). (D) In Q0990, GFP fluorescence (left) and cleared darkfield image of the same leaf showing differentiated xylem (right) indicate that GFP expression was also present in undifferentiated procambial cells (arrows). (E–G) In J0571, GFP expression was initially mostly in abaxial tissue (arrows in medial section show procambial cells) (E) and progressively included more adaxial ground tissues (E inset, F), increasingly delimiting venation (F inset, G), and eventually becoming more prominent in cells directly surrounding the vascular tissue (G inset). Bars, 10 μm (A–C, E–G), 20 µm (D).
In contrast to Q0990, GFP expression in J0571 was restricted predominantly to ground tissues in developing embryos and seedlings. GFP was observed in the developing root and hypocotyl regions from early heart-stage embryos onwards (Fig. 1C). In late heart and torpedo-stage embryos, GFP was also expressed in the abaxial ground tissues of the cotyledons, eventually including medial and adaxial ground cells (data not shown) and clearly outlining vascular tissues in more mature cotyledons (Fig. 1C). In emerging and young leaf primordia (e.g. Fig. 2E, 2–3 days after germination, DAG), GFP was predominantly observed in the abaxial ground tissues and in the ground cell layer underlying the epidermis, although some leaf primordia also had GFP expression in more medial and adaxial tissues (Fig. 2E inset). In more mature leaf primordia (e.g. Fig. 2F inset, G, 4–6 DAG), GFP expression in ground cells eventually delimited the developing primary, secondary, and higher-order veins, and included more adaxial ground tissues. In older J0571 leaves (e.g. Fig. 2G inset, 7 DAG), GFP expression was most abundant in ground cells directly adjacent to the vascular bundles.
In summary, Q0990 and J0571 lines showed procambial and ground tissue GFP expression, respectively, throughout embryonic cotyledon and postembryonic leaf development, and were therefore suitable to drive GAL4-activated C4 expression in these tissues. GFP expression in all leaf procambial cells (Q0990) or the potential procambial domain of tertiary veins (J0571) occurred later than expression of two of the earliest known preprocambial markers (MONOPTEROS mRNA and PINFORMED-1 protein; Wenzel et al., 2007), but approximately concurrently with procambial expression of the auxin response marker DR5::GUS (Mattsson et al., 2003). Thus, the effect of C4 transactivation would only start at the developmental stages corresponding to Q0990 and J0571 GFP expression patterns.
C4-induced ectopic cell divisions
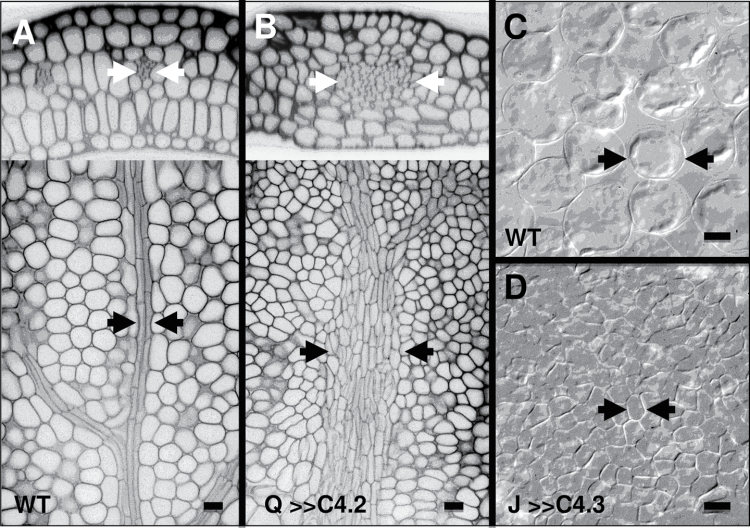
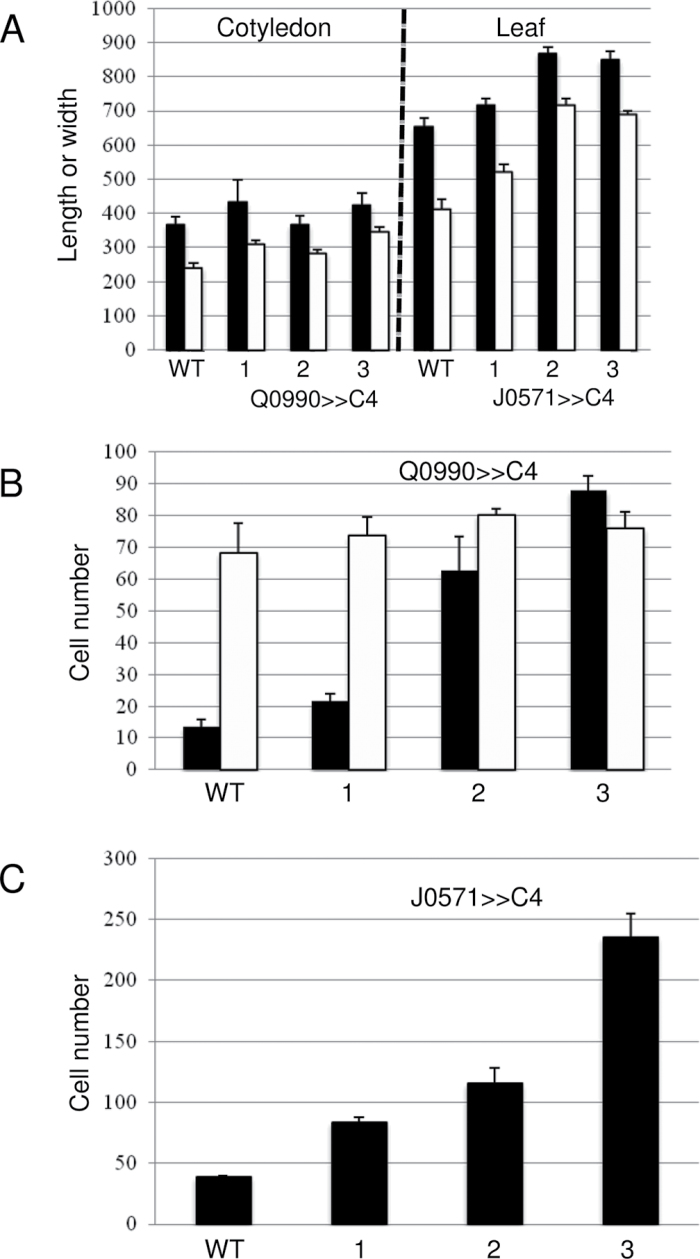
To confirm the assumption that targeted C4 expression would induce ectopic cell divisions, the effects were analysed at the anatomical level. For Q0990>>C4 lines, data were taken from embryonic cotyledons because only one of the three analysed lines, Q0990>>C4.1, consistently produced leaves. Data was also taken for mature leaves of J0571>>C4 lines because the leaves had significant vascular patterning defects. Compared to the controls, C4 expression in developing procambial cells of Q0990>>C4 resulted in ectopic vascular cell production (Fig. 3B compared with A), without concurrent increases in embryonic cotyledon size (Fig. 4A) or mesophyll cell number (i) within a cross-sectional area (Fig. 4B) or (ii) along the length or width of the cotyledon (data not shown). Compared with controls, expression of C4 in developing ground tissues of J0571>>C4 resulted in a larger number of smaller leaf mesophyll cells with small or no intercellular spaces between them (Fig. 3D compared with C, Fig. 4C). J0571>>C4 leaves of 2-week-old plants were slightly larger than controls (Fig. 4A) and had similar leaf vein width and vascular cell widths compared to controls (data not shown), thus showing no apparent ectopic vascular production. Hence, C4 expression did result in ectopic procambial and ground cell divisions in Q0990>>C4 or J0571>>C4 lines, respectively. The extent of induced divisions varied with the different C4 lines, with C4.1 < C4.2 < C4.3 (Fig. 4B, 4C).
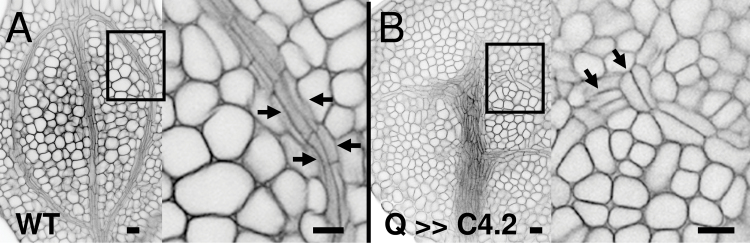
Fig. 3.
Effects of C4 transactivation on cell number. Confocal (A, B) or Nomarski (C, D) images show that C4 increased the number of vascular cells in the embryonic cotyledon midvein of Q0990>>C4.2 (B) compared with wild type (A), as seen in transverse (top) and longitudinal (bottom) sections of the same cotyledons. Leaves of 2-week-old J0571>>C4.1 plants had more numerous, smaller palisade cells with smaller or no intercellular spaces (D) compared with wild type (C). Arrows delimit vascular bundles (A, B) or a typical mesophyll cell (C, D). Q, Q0990; J, J0571. Bars, 10 µm.
Fig. 4.
Effects of C4 transactivation on Q0990>>C4 embryonic cotyledons and 2-week-old J0571>>C4 third leaf sizes (A) and cell numbers (B, C). (A) Compared to WT, Q0990>>C4 (left) had similar sized embryonic cotyledons and J0571>>C4 (right) had slightly larger third leaves in 2-week-old plants; length (black) or width (white) of cotyledons (µm) or leaves (mm x 10–2). (B, C) Compared to WT, Q0990>>C4 embryonic cotyledons had more midvein vascular cells (black) without concurrent increases in mesophyll cell number (white) (B) and J0571>>C4 leaves had more mesophyll cells (C); cell number data are for ~63,000 µm2 of field of view for cross-sections (B) and longitudinal sections (C). Values are mean ± SE for ≥3 leaves/cotyledons. 1, C4.1; 2, C4.2; 3, C4.3.
Expression of C4 in vascular and ground tissues affects seedling morphology
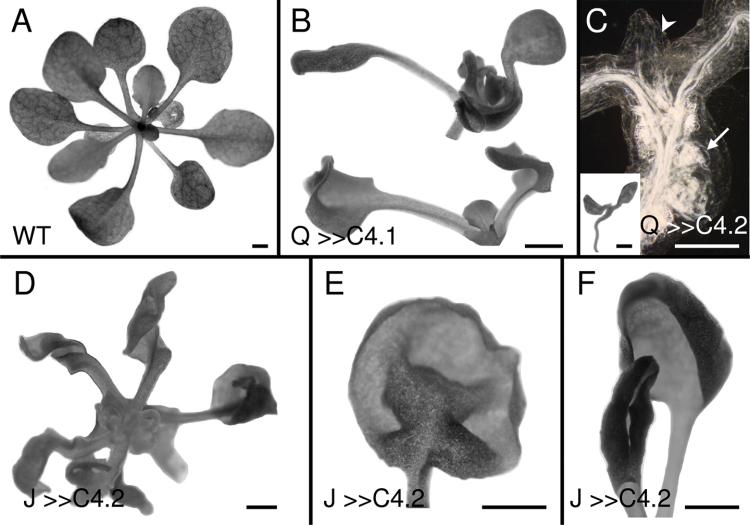
Expression of C4 in vascular (Q0990>>C4) or ground (J0571>>C4) tissues had pleiotropic effects on seedling morphology, with the severity of phenotypic effects varying between different C4 lines (C4.1 < C4.2 < C4.3), correlating with the extent of induced divisions. Q0990>>C4.1 seedlings had weaker phenotypes and were able to produce leaves, which often bent adaxially and/or had twisted petioles that altered leaf positioning (Fig. 5B). For the two strongest Q0990>>C4 lines, leaf primordia were absent or incompletely formed and hypocotyls had tumour-like vascular protrusions (Fig. 5C). Inflorescence stems were stunted (data not shown). All J0571>>C4 seedlings had twisted, curled, or rolled leaves (Fig. 5D–F) and stunted, twisted inflorescence stems (data not shown). Although J0571>>C4 leaves were initially slightly larger than controls (see above), the continuous induction of cell divisions was detrimental and resulted in an overall stunted mature phenotype.
Fig. 5.
Effects of C4 transactivation on 2–3-week-old seedling morphology of wild type (A), Q0990>>C4.1 (B), Q0990>>C4.2 (C), or J0571>>C4.2 (D–F). (B) Q0990>>C4.1 plants had curled leaves and petioles. (C) Q0990>>C4.2 seedlings were stunted and had limited leaf growth (arrowhead and inset) and exhibited tumour-like vascular overgrowth in the hypocotyl (arrow). (D–F) J0571>>C4.2 seedling leaves were twisted (D), buckled (E), or rolled (F). Q, Q0990; J, J0571. Bars, 1mm (A, B, C inset, D–F), 0.5mm (C darkfield image).
C4 expression in procambial cells results in disrupted and ectopic veins
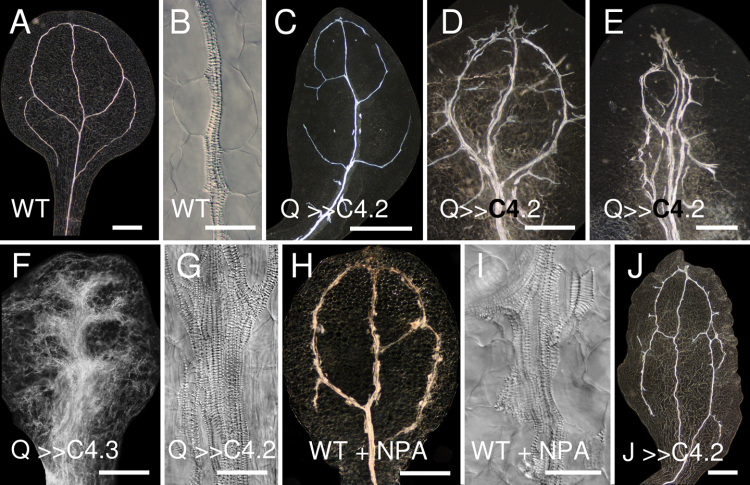
Dark-field microscopy of cleared specimens was used to determine whether ectopic procambial divisions affected cotyledon and leaf venation patterning. In each of the Q0990>>C4 lines, more than 80% of seedlings (n ≥ 30) had defective cotyledon venation (Fig. 6C–G) compared with wild type (Fig. 6A, 6B). In the two weaker lines (Q0990>>C4.1 and Q0990>>C4.2), cotyledons typically had short, discontinuous veins adjacent to or extending from primary and secondary veins, and the secondary vein loops were often discontinuous (e.g. Fig. 6C–E). In more extreme cases, embryonic and seedling cotyledons had multiple ectopic veins in the midvein region and short secondary-like veins only partially extending towards the margin (Fig. 6E–G). In Q0990>>C4.2 and Q0990>>C4.3, leaf growth was absent or severely inhibited, and leaves often had only partial secondary vein formation (Fig. 7D). Q0990>>C4.1 seedlings produced relatively normal sized leaves, but more than 98% of leaves (n ≥ 50) had discontinuous secondary or tertiary veins (Fig. 7B, 7C), and patterning of secondary loops was often aberrant (Fig. 7B compared with A). To determine if aberrant secondary vein formation was correlated with abnormal cell division orientation patterns, cell division orientation was examined in Q0990>>C4 plants. Unlike control plants, Q0990>>C4 secondary procambial veins often had cells with division planes perpendicular or close to perpendicular to the axis of the vein (Fig. 8B compared with A) and partial veins often terminated adjacent to an apparently undivided cell (data not shown). For example, Q0990>>C4.2 embryonic cotyledons (n ≥ 10) had 1.8±0.2 partial secondary veins, whereas the controls only formed looped secondary veins. Hence, ectopic expression of C4 in the procambial domain induced ectopic vascular strands, but in more severe cases it disrupted overall patterning often leading to a reduction of secondary and tertiary venation.
Fig. 6.
Effects of C4 transactivation on cotyledon venation. Images show cleared cotyledons of ~2-week-old plants. Darkfield (A, C–F, H, J) or Nomarski (B, G, I) images of wild-type (A, B, H, I), Q0990>>C4.2 (C–E, G), Q0990>>C4.3 (F), and J0571>>C4.2 (J) cotyledons grown on control ATS media (A–G, J) or ATS media supplemented with 10 µM NPA (H, I). C4 induced ectopic vascular strand formation similar to the effects of N-naphthylphthalamic acid. Q, Q0990; J, J0571. Bars, 500 µm (A, C–F, H, J), 50 µm (B, G, I).
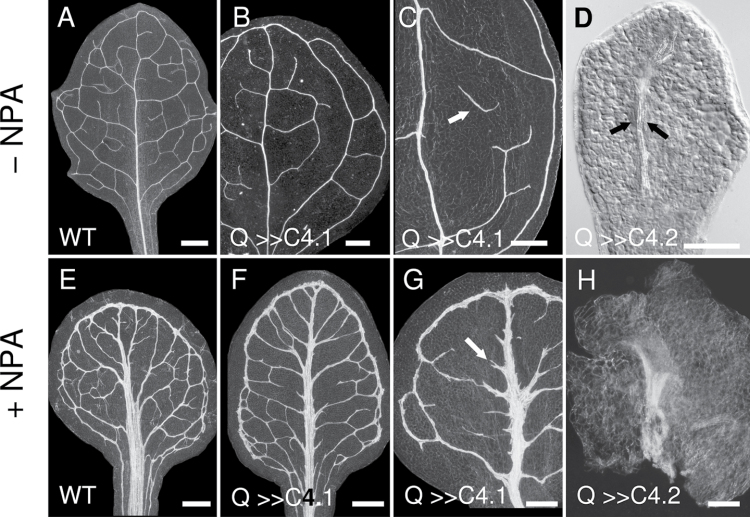
Fig. 7.
Q0990>>C4 leaf venation patterning. Darkfield (A–C, E–H) or Nomarski (D) images of 2-week-old wild-type (A, E), Q0990>>C4.1 (B, C, F, G), or Q0990>>C4.2 (D, H) leaves grown on control media (A–D) or media supplemented with 10 µM N-naphthylphthalamic acid (NPA; E–H). Q0990>>C4.1 leaves had distorted secondary venation patterning and discontinuous secondary and higher-order veins (B, arrow in C), while Q0990>>C4.2 leaves often lacked secondary vein formation (D; arrows delimit midvein). NPA treatment induced vascular overgrowth in wild-type (E) and Q0990>>C4 (F–H) leaves, although vein discontinuities were still evident in some Q0990>>C4.1 leaves (e.g. arrow in G) and in Q0990>>C4.2 (H, veins barely extending from midvein region). Q, Q0990. Bars, 500 µm (A–C, E–G), 100 µm (D, H).
Fig. 8.
Q0990>>C4 procambial cell orientation. Confocal images of embryonic cotyledons of wild type (A) and Q0990>>C4.2 (B), showing higher magnification of boxed regions to the right. Compared with the highly aligned procambial cells of wild-type secondary veins (arrows in A), Q0990>>C4.2 procambial cells were often not in parallel orientations (arrows in B). Q, Q0990. Bars, 10 µm.
Auxin transport inhibition restores vein continuity in Q0990>>C4.1 leaves
The ectopic vessels and vessel elements found in cotyledons of Q0990>>C4.2 plants were similar to those caused by auxin transport inhibition (ATI) through exposure to NPA (Fig. 6H, 6I, Fig. 7E). These effects of NPA treatment have previously been described (Mattsson et al., 1999; Sieburth, 1999). If induced ectopic expression in the procambial domain and auxin transport inhibition contributed independently to the formation of ectopic vessels and vessel elements, a combination of the two treatments should result in a synergistic, exaggerated phenotype. To test this hypothesis, the effect of NPA on vein development was examined in Q0990>>C4.1 seedlings, which are able to form true leaves. Leaves of Q0990>>C4.1 seedlings grown on medium supplemented with NPA (Fig. 7F, 7G) displayed vein overgrowth similar to the effects of NPA alone (Fig. 7E). NPA treatment reduced the frequency of Q0990>>C4.1 leaves with vein continuity defects from ~95 % (control, n ≥ 50) to ~5% (NPA-treated; n ≥ 30) (Fig. 7F). The remaining 5% showed primarily stubby vein protrusions from the midvein (e.g. Fig. 7G arrow), a defect that also occurred in ~20% of wild-type plants exposed to NPA (data not shown). NPA treatment of Q0990>>C4.2 seedlings, which produce small and highly defective leaves, did induce some vascular overgrowth in the midvein region, but did not restore growth of secondary veins (Fig. 7H). In summary, the combination of ectopic procambial cell divisions and ATI did not result in an exaggerated phenotype. Instead, ATI either had little or no effect (the strong Q0990>>C4.2 line) or mostly masked the defects (the weaker Q0990>>C4.1 line), including restoring continuity of secondary veins. From a genetic perspective, this effect is analogous to an epistatic relationship, which would suggest that the two defects affect the same pathway (see also Discussion).
Ectopic cell divisions in ground tissue disrupt higher-order venation
Although leaf procambial cells differentiate from ground meristem cells, the likely response of leaf venation to ectopic ground cell divisions is not obvious and would depend on the timing of cell division induction. If additional ground cells contribute only to mesophyll cells, one would expect larger areoles (areas between major vein loops). If, on the other hand, the ground cells contribute additional cells for vein formation, one might expect higher vein complexity. To test this, C4 transactivation in J0571>>C4 was used to induce ectopic cell divisions in ground tissue. In each of the J0571>>C4 lines, most seedlings (n ≥ 30) had normal cotyledon vascular patterning, but ~30% of cotyledons displayed short ectopic vascular strands and/or vascular strand discontinuities (Fig. 6J). All J0571>>C4 lines had continuous, albeit distorted, primary and secondary veins in leaves, but severely disrupted higher-order venation, particularly in J0571>>C4.2 (Fig. 9B, 9C) and J0571>>C4.3 (data not shown). In summary, induced ground cell divisions disrupted minor but not major veins. In J0571, GFP expression was observed in medial tissues (from which potential procambial cells would be recruited) predominantly from 4 DAG onwards, after the formation of the primary and secondary veins. Hence, the effect of ectopic ground cell divisions on primary and secondary vein formation could not be determined using the J0571 line. Higher-order veins are formed in slightly older leaf primordia (Wenzel et al., 2007), which would coincide with the presumed C4 transactivation in GFP-expressing ground cells of J0571>>C4, thus resulting in predominant disruption of higher-order venation.
Fig. 9.
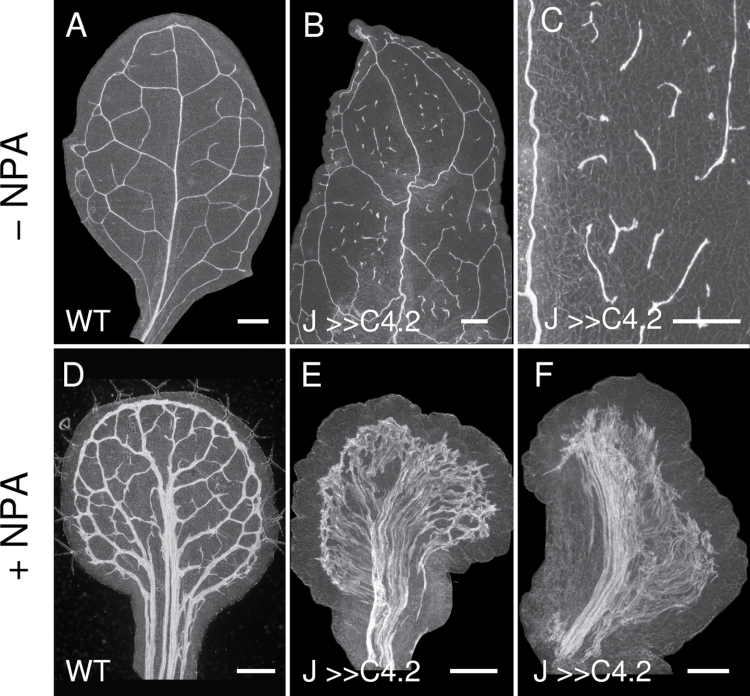
J0571>>C4 leaf venation patterning. Darkfield images of 2-week-old wild-type (A, D) and J0571>>C4.2 (B, C, E, F) leaves grown on control ATS media (A–C) or ATS media supplemented with 10 µM N-naphthylphthalamic acid (NPA; D–F). J0571>>C4.2 leaves had discontinuous tertiary and higher-order veins (B, C), and with NPA treatment had more extensive vascular overgrowth throughout most (E) or predominantly one side (F) of the lamina compared with wild type (D). J, J0571. Bars, 500 µm.
Ectopic ground cell divisions and auxin transport inhibition result in massive vein overgrowth
To determine the combined effects of ectopic ground cell divisions and ATI, J0571>>C4 seeds were germinated and grown in the presence of NPA. Seedlings of J0571>>C4.2 treated with NPA developed leaves with extreme vascular overgrowth (Fig. 9E; n ≥ 30). The vascular strands of NPA-treated leaves extended from near the leaf margin towards the primary vein region and covered almost the entire laminar area, excluding the margins (Fig. 9E). In ~5% of leaves (n > 30), vascular strands were absent on either one or both sides of the central lamina region, correlated with inhibited lateral lamina growth (Fig. 9F). In summary, the combination of ectopic ground cell divisions and ATI resulted in a novel phenotype, comprising a massive vascular overgrowth in leaves (Fig. 9E, 9F), not present in leaves from either of the two single treatments (Fig. 9B–D). From a genetic perspective, this would be analogous to a synergistic effect, indicating that both treatments contributed to the formation of leaf veins independently (see also Discussion).
Vein disruptions were due to defects in vascular differentiation
To understand whether the vein discontinuities observed in Q0990>>C4 and J0571>>C4 leaves were caused during procambial strand formation or subsequent vein differentiation, MONOPTEROS (MP) expression was examined during procambial strand formation in Q0990>>C4.1 and J0571>>C4.2 plants. The expression of MONOPTEROS, an auxin response factor, is one of the earliest known markers of leaf procambial cell fate (Wenzel et al., 2007). In leaves of wild-type C24 seedlings grown in control media, MP was expressed in all developing procambial strands (Fig. 10A). MP expression was similar to that of wild type in leaves of most Q0990>>C4.1 and all J0571>>C4.2 seedlings, indicating formation of continuous procambial strands in these plants (Fig. 10B). Thus, discontinuous veins were due to defects in subsequent vascular differentiation rather than formation of procambial strands, resulting in aberrant differentiation along vein strands (Fig. 10D). MP expression was also examined in more severe Q0990>>C4.2 phenotypes with aberrant secondary vein patterning to determine if procambial strand formation was defective. Q0990>>C4.2 leaves had MP expression in the primary midvein and in short secondary-like strands partially extending from the primary vein (Fig. 10C), indicating that continuous secondary procambial strand loops were not formed. Hence, while vein discontinuities were caused by defects in differentiation, more severely perturbed vascular patterning defects were associated with defective procambial strand formation.
Fig. 10.
MP expression in leaves. Whole-mount in situ hybridizations of MP mRNA transcript in rosette leaves of wild type (A), J0571>>C4.2 (B, D), or Q0990>>C4.2 (C) grown for ~1 week on control media. In wild type and J0571>>C4.2, MP was expressed in procambial cells of all vein types (A, B). MP was only expressed in the primary vein and in short abutting secondary veins in Q0990>>C4.2 (C, arrows). J0571>>C4.2 leaves had normal MP expression (B), but had punctate differentiation along vascular strands (arrows, D and inset). Q, Q0990; J, J0571. Bars 100 µm (A–C), 10 µm (D).
Ectopic cell divisions disrupt formation of discrete auxin response maxima
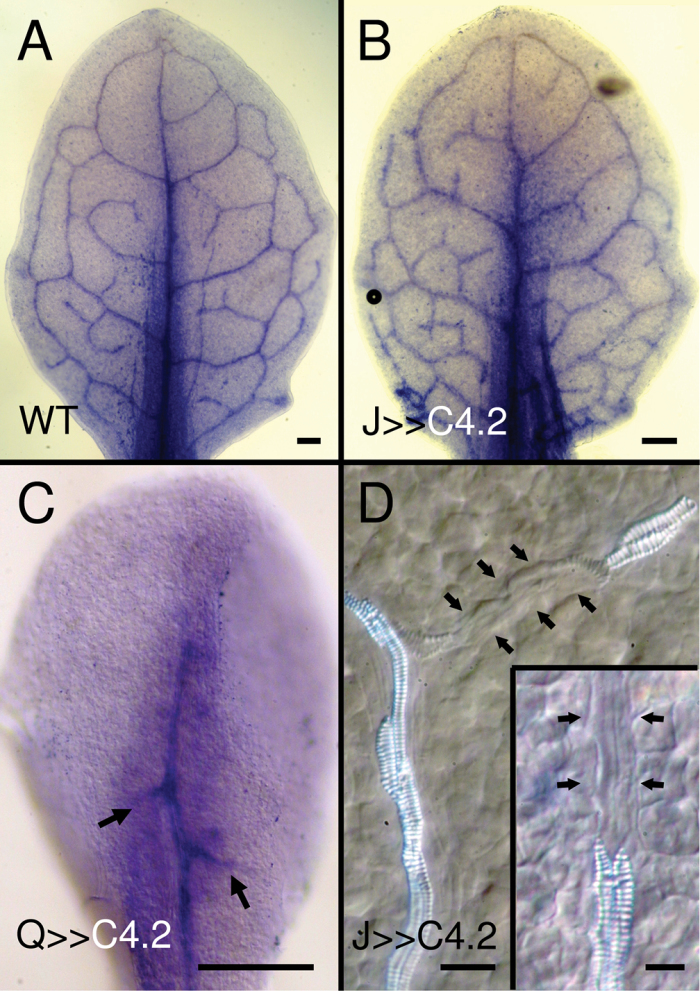
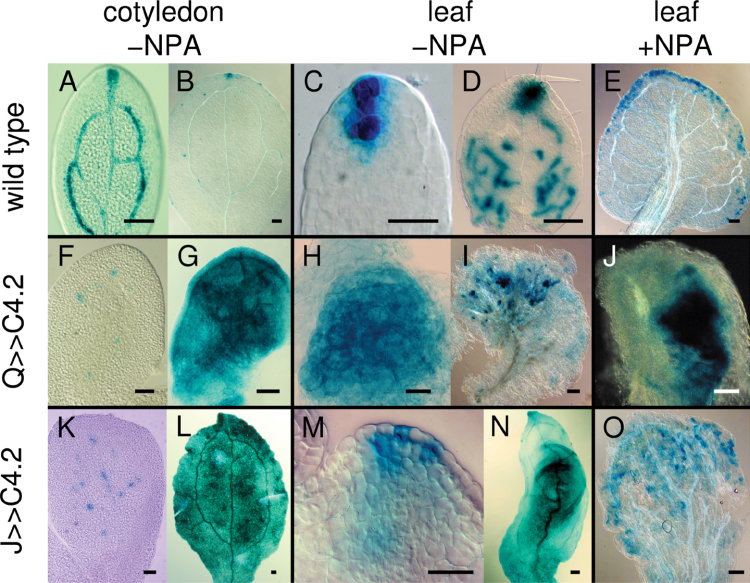
Cotyledon and leaf primordia display auxin response maxima in procambial cells as well as apical and lateral auxin maxima (Aloni et al., 2003; Mattsson et al., 2003). To assess the effect of ectopic cell division activity in the procambial and ground tissues on the distribution of auxin response maxima, expression of the auxin-responsive DR5 promoter fused to GUS was examined. Ectopic divisions in both Q0990>>C4.2 and J0579>>C4.2 disrupted discrete auxin response maxima formation in procambial cells and in the margin convergent sites where auxin presumably would flow from the epidermis towards the developing procambial cells (Scarpella et al., 2006; Wenzel et al., 2007). In wild type, mature embryonic cotyledons had DR5 expression in apical auxin maxima and procambial cells (Fig. 11A), but by 7 DAG, DR5 was predominantly expressed in the apical auxin response maxima (Fig. 11B). In 1–2 DAG wild-type leaf 1 primordia, DR5 was expressed in a few apical cells and also in developing procambial cells up to 7 DAG (Fig. 11C, 11D), after which it became restricted to apical and lateral marginal auxin maxima in the hydathodes (data not shown). Both Q0990>>C4.2 (with severe vascular defects) and J0571>>C4.2 mature cotyledons had sporadic, punctate DR5 expression (Fig. 11F, 11K), with ubiquitous DR5 expression throughout the cotyledon in germinating seedlings up to ~8 DAG (Fig. 11G, 11L), after which it decreased again (data not shown). Both Q0990>>C4.2 (with severe vascular defects; Fig. 11H, 11I) and J0571>>C4.2 (Fig. 11M, 11N) had broad DR5 expression in early leaf primordia and more diffuse expression throughout the lamina of older leaves. This effect was not entirely due to disruption of auxin transport, because NPA-grown leaves had DR5::GUS expression near the leaf margins in wild-type leaves (Fig. 11E), but more sporadically throughout the lamina in Q0990>>C4.2 (Fig. 11J) and J0571>>C4.2 (Fig. 11O) seedlings. Taken together, ectopic cell divisions in procambial or ground cells disrupted formation of auxin response maxima and frequently resulted in DR5::GUS expression throughout most of the leaf lamina.
Fig. 11.
Effects of C4 transactivation on DR5::GUS expression in leaves. DR5::GUS expression in wild-type (A–E), Q0990>>C4.2 (F–J) or J0571>>C4.2 (K–O) mature embryonic cotyledons (A, F, K), 7 days after germination cotyledons (B, G, L), 2–3 days after germination leaf primordia (C, H, M), or 6–8 days after germination leaves (D, E, I, J, N, O) grown in control media (–NPA; A–D, F–I, K–N) or media supplemented with 10 µM N-naphthylphthalamic acid (+NPA; E, J, O). C4 transactivation resulted in ectopic DR5::GUS expression. Q, Q0990; J, J0571. Bars, 100 µm (B, D, E, G, I, L, N, O), 20 µm (A, C, F, H, K, M).
Discussion
The formation of discrete veins with continuous differentiation along strands requires precise control of cell division
Although the venation patterns of dicotyledonous leaves are regular enough to be used as phylogenetic traits (Hickey, 1979; Ellis et al., 2006), leaf venation patterns are not identical, even from leaves of the same individual. This phenotypic plasticity has been attributed to stochastic events during pattern formation, possibly due to a positive feedback mechanism referred to as canalization of auxin flow (Sachs, 1991), with vein patterning and densities dramatically changing upon pharmacological ATI (Mattsson et al., 1999; Sieburth, 1999). Here, the extent to which the plasticity in venation patterning and discrete vein formation respond to ectopic cell divisions was assessed by partly uncoupling cell division activity from its normal regulation, using BCTV-C4 induction of ectopic cell divisions in targeted vascular or ground tissue domains.
Ectopic divisions within the procambial domain had varying effects on cotyledon and leaf vascular patterning. Slight perturbations in procambial cell divisions had little effect on overall vascular patterning but sometimes prevented continuous differentiation along veins, possibly by disturbing regular cell division activity and/or improper alignment of procambial cells, which may have affected auxin transport (see below). More severe perturbations resulted in ectopic veins and vessels, demonstrating the need for controlled procambial divisions in the formation of discrete veins with specific cellular architectures. High rates of ectopic procambial divisions resulted in misaligned procambial cells and discontinuous procambial cell files, impeding secondary and higher-order vein formation and even leaf formation/growth, indicating that both vein patterning and leaf formation/growth collapsed without adequate control of cell division activity within the procambial domain.
Intuitively, ectopic ground cell divisions might be expected to result in enlarged leaves and possibly also more veins, because veins originate from the medial ground tissues. Although ectopic ground cell divisions resulted in slightly larger and non-planar leaves, no obvious increase in venation was observed. Instead, extensive fragmentation of higher-order veins occurred even though the procambial veins were continuous, suggesting that vein differentiation was more sensitive to the unscheduled cell divisions. However, J0571 GFP expression in the medial ground tissue predominantly occurred after procambial formation, suggesting that fewer unscheduled cell divisions may have occurred during procambial vein patterning rather than during vein differentiation. This discontinuous vein phenotype is similar to that of the vascular continuity mutant vascular network3 (van3; Scarpella et al., 2006), which is defective in the subcellular localization of plasma membrane proteins, including PIN-FORMED auxin efflux carriers (Naramoto et al., 2010). Hence, it is possible that ectopic ground cell divisions physically displaced procambial cells in tertiary veins and thereby possibly also inducing unscheduled procambial divisions, disrupting the auxin transport required for differentiation of continuous veins. In addition to auxin, vascular differentiation involves other hormones and genetic regulators (reviewed in Caño-Delgado et al., 2010; Ohashi-Ito and Fukuda, 2010) whose activity or distribution may have also been disrupted as a result of unscheduled ground cell divisions.
Ectopic cell division activity in the procambial domain may also disrupt auxin transport
Ectopic procambial cell divisions resulted in vascular defects reminiscent of the effects of PAT inhibition, particularly in the midvein region of severe Q0990>>C4 cotyledon phenotypes, with wider veins and defective connections between vessel elements. While ATI is likely to result in the selection of wider ground cell domains for procambial differentiation (Mattsson et al., 1999; Sieburth, 1999; Mattsson et al., 2003), ectopic procambial cell divisions may have a similar net effect by triggering additional divisions in existing procambial cells. The imperfect interconnection of vessel elements observed here could be due to disturbances in auxin transport, caused by new unpolarized cells disrupting the intercellular auxin transport that is believed to enhance alignment and interconnection of procambial cells. However, it should be noted that while both ground and procambial ectopic cell divisions affected vein continuity, with similarities to previously described auxin-related phenotypes, the leaf phenotypic defects from ectopic procambial divisions can be much more profound than those resulting from ectopic ground cell divisions (see below).
The C4 protein itself and/or C4-induced disrupted vascular patterning observed in this study are likely to have affected other hormonal pathways. The BCTV C4 protein interacts with AtSKη shaggy-related protein kinase in the brassinosteroid signalling pathway (Piroux et al., 2007), although constitutive expression of BCTV-C4 can affect multiple hormonal pathways in Arabidopsis (Mills-Lujan and Deom, 2010). In Arabidopsis, cross-talk between the brassinosteroid and auxin pathways occurs via AtSK21 action on the auxin response factor ARF2 (Vert et al., 2008), and determination of shoot vascular bundle number involves brassinosteroid signalling coupled to PAT (Ibañes et al., 2009). The brassinosteroid pathway may have also influenced vascular overproduction in Q0990>>C4, as well as factors such as ACAULIS5 (Clay and Nelson, 2005), TORNADO1 (TRN1), and TRN2 (Cnops et al., 2006) that can affect leaf vascular cell divisions and auxin distribution.
Ectopic procambial divisions in leaves of the weaker transactivation line Q0990>>C4.1 also resulted in disruptions in leaf vein continuity. When ectopic cell divisions and ATI were combined, analogous to the construction of a double mutant line, vein continuity was restored and most leaves showed the stronger vascular overproduction phenotype typical of ATI alone. Hence, ATI masked or, if expressed in analogous genetic terms, acted epistatically to ectopic cell divisions, suggesting that both perturbations acted at least broadly on the same pathway, with ATI having the dominant influence on the outcome. This is similar to the situation for FORKED1, which influences PIN1 localization in an auxin dependent manner (Hou et al., 2010). forked1 vein continuity is also restored during ATI, presumably by restoring sufficient auxin concentrations for continuous differentiation along vascular strands (Steynen and Schultz, 2003; Hou et al., 2010).
Stronger C4 effects on procambial activity resulted in reduced lamina expansion, coupled with an almost complete lack of secondary and higher-order veins, collapsing the mechanism that normally governs vein formation. This phenotype is similar to that of the monopteros mutant, which is defective in both PAT (Przemeck et al., 1996) and response to auxin (Mattsson et al., 2003). Other mutants showing incomplete secondary strand formation in cotyledons or leaves also have disrupted auxin response maxima (Cnops et al., 2006; Carland et al., 2010). Hence, severe ectopic procambial cell divisions may have disrupted auxin transport and/or signalling to the extent that vein formation no longer occurred. The fact that these leaves could form a primary vein but secondary vein formation was severely disrupted, and both processes require PIN1-mediated auxin flow inwards from the epidermis (Scarpella et al., 2006; Wenzel et al., 2007), suggests that the feedback loop between secondary strand formation and auxin flow was more susceptible to disruptions in cell division. The parallel1 mutant, which has parallel-like leaf venation running along the distal/proximal axis into the petiole, lacks marginal auxin response domains (Petricka and Nelson, 2007) that are essential for secondary strand loop formation and connection to the midvein (Scarpella et al., 2006; Wenzel et al., 2007). The multiple parallel strands in the midvein region, incomplete secondary strand formation, and disrupted auxin response maxima in severe Q0990>>C4 phenotypes also implicate essential feedback loops between procambial cell divisions and marginal auxin sources.
Massive vascular overgrowth involves independent cell division and ATI pathways
The combination of ectopic ground cell divisions and ATI, again analogous to the construction of a double mutant, resulted in an increased degree of vascular overproduction compared to the single treatments. This strong synergistic interaction implies that ectopic ground cell divisions and ATI interfered with formation of a normal venation pattern via separate pathways.
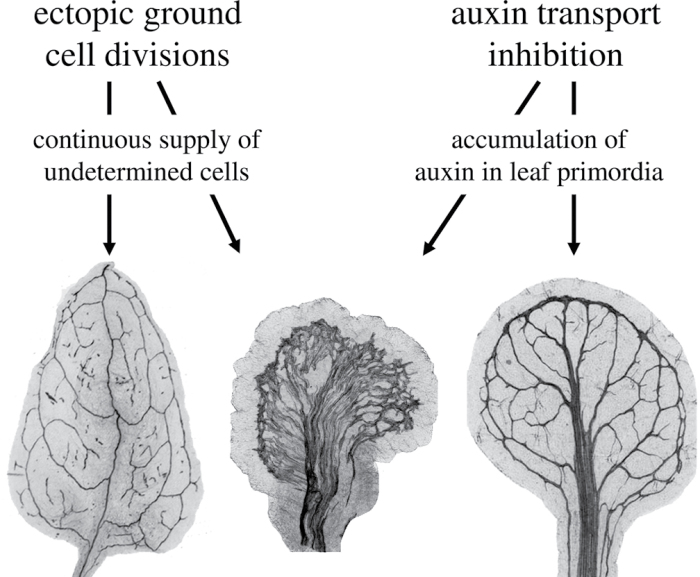
It is possible to explain the above results at the tissue level. ATI results in an increased number of vascular cells (Mattsson et al., 1999). Thus, the similarities and epistatic-like relationship between ectopic procambial cell divisions and ATI can be explained by them acting on the same tissue. Similarly, the synergistic effects of combining ectopic ground cell divisions and ATI can be explained by them acting on both ground and procambial tissues. It appears unlikely though that increasing the number of ground cells alone would result in such a strong synergistic effect on leaf vascularization in combination with ATI. One potential explanation is that ectopic ground cell divisions resulted in enhanced auxin response either by increasing local auxin production or by increasing cellular perception of auxin, which in combination with ATI increased the available auxin and triggered formation of ectopic veins. Indeed, ectopic cell divisions in the ground cell tissues did result in ectopic expression of the DR5::GUS auxin response marker in cotyledons and young leaf primordia (Fig. 10L–O). However, ectopic procambial divisions also resulted in widespread DR5::GUS expression, suggesting that auxin was not the only ground cell factor behind the synergy. It is speculated that ectopic ground cell divisions may continuously provide new cells without fate determination restrictions, allowing a much larger population of cells to respond to the ATI-induced accumulation of auxin in developing leaves (Fig. 12). Regardless of the nature of the factor behind it, the synergistic effect suggests that differences in the regulation of ground cell division activity and auxin production or transport may result in different vein densities observed in plant species.
Fig. 12.
Interpretation of the synergistic effect of combining ectopic ground cell divisions and auxin transport inhibition on leaf vein development. Auxin transport inhibition leads to overproduction of veins in Arabidopsis leaves (right leaf; Mattsson et al., 1999, Sieburth et al., 1999), probably as a consequence of accumulation of auxin in developing leaves as indicated by the DR5::GUS auxin response marker (Mattsson et al., 2003). On its own, ectopic ground cell divisions in developing J0571>>C4 leaves result primarily in fragmentation of higher-order veins (left leaf). When combined, ectopic cell divisions and auxin transport inhibition leads to a phenotype not seen in any of the single treatments: a massive vascularization of the leaf blade (centre leaf). We hypothesize that the vascular overproduction in auxin transport-inhibited leaves is limited by simultaneous differentiation of ground cells into mesophyll cells. Ectopic ground cell divisions may negate this effect by providing undifferentiated ground cells that respond to accumulated auxin by differentiation into vascular cells.
Acknowledgements
The authors thank John Stanley (JIC, Norwich, UK) for kind provision of the C4 construct and Sarah Hodge (LMB, Cambridge, UK) for expert assistance in creating the vectors. They also thank Nick Brown, Joyce Gardner, Anna Lawson, Mengning Liu, Robert Rea, James Snowden (all from University of York, UK) and Sarah Hodge for their help in generating the Arabidopsis transformants. They are grateful to Ottoline Leyser (University of York, presently The Sainsbury Laboratory, Cambridge) for invaluable input throughout the project and to Mathias Schuetz (Simon Fraser University, Burnaby, Canada) for constructive comments and assistance with MP in situ hybridizations. They also thank the reviewers for their helpful comments. This work was supported by a Biotechnology and Biological Sciences Research Council grant G09615 to SMB and JH and an NSERC discovery grant to JM.
References
- Aloni R, Schwalm K, Langhans M, Ulrich CI. 2003. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis Planta 216 841–853 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo Development 118 575–587 [Google Scholar]
- Caño-Delgado A Lee J-Y Demura T 2010. Regulatory mechanisms for specification and patterning of plant vascular tissues Annual Review of Cell and Developmental Biology 26 605–637 [DOI] [PubMed] [Google Scholar]
- Carland F Fujioka S Nelson T 2010. The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products Plant Physiology 153 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK Nelson T 2005. Arabidopsis thickvein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport Plant Physiology 138 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ Bent AF 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana Plant Journal 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cnops G, Neyt P, Raes J, et al. 2006. The TORNADO1 and TORNADO2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana The Plant Cell 18 852–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Elo A, Helariutta Y. 2009. Hormone interactions during vascular development Plant Molecular Biology 69 347–360 [DOI] [PubMed] [Google Scholar]
- Ellis B, Daly D, Hickey LJ, Johnson KR, Mitchell J, Wilf P, Wing SL. Manual of leaf architecture. Ithaca, NY: Cornell University Press; 2006. [Google Scholar]
- Fukuda H. 2004. Signals that control plant vascular cell differentiation Nature Reviews Molecular Cell Biology 5 379–391 [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue Science 282 2226–2230 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development The EMBO Journal 17 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J. 1999. GFP variants for multispectral imaging of living cells Methods in Cell Biology 58 139–151 [DOI] [PubMed] [Google Scholar]
- Haseloff J. 2003. Old botanical techniques for new microscopes Biotechniques 34 1174–1182 [DOI] [PubMed] [Google Scholar]
- Hickey LJ. 1979. A revised classification of the architecture of dicotyledonous leaves Metcalfe CR, Chalk L. Anatomy of the dicotyledons 2ndedition, vol. I Oxford, UK: Clarendon Press; pp 25–39 [Google Scholar]
- Hou H, Erickson J, Meservy J, Schultz EA. 2010. FORKED1 encodes a PH domain protein that is required for PIN1 localization in developing leaf veins The Plant Journal 63 960–973 [DOI] [PubMed] [Google Scholar]
- Ibañes M, Fàbregas N, Chory J, Caño-Delgabo AI. 2009. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot bundles Proceedings of the National Academy of Sciences, USA 106 13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WP. 1952. The role of auxin in differentiation of xylem around a wound American Journal of Botany 39 301–309 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants The EMBO Journal 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Dengler N. 2002. Cell cycling frequency and expression of the homeobox gene ATHB-8 during leaf vein development in Arabidopsis Planta 216 212–219 [DOI] [PubMed] [Google Scholar]
- Kang J, Mizukami Y, Wang H, Fowke L, Dengler NG. 2007. Modification of cell proliferation patterns alters leaf vein architecture in Arabidopsis thaliana Planta 226 1207–1218 [DOI] [PubMed] [Google Scholar]
- Lai J, Chen H, Teng K, Zhao Q, Zhang Z, Li Y, Liang L, Xia R, Wu Y, Guo H. 2009. RKP, a RING finger E3 ligase induced by BSCTV C4 protein, affects geminivirus infection by regulation of the plant cell cycle The Plant Journal 57 905–917 [DOI] [PubMed] [Google Scholar]
- Latham JR, Saunders K, Pinner MS, Stanley J. 1997. Induction of plant cell division by beet curly top virus gene C4 Plant Journal 11 1273–1283 [Google Scholar]
- Lincoln C, Britton JH, Estelle M. 1990. Growth and development of the axr1 mutants of Arabidopsis The Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T. 2003. Auxin signalling in Arabidopsis leaf vascular development Plant Physiology 131 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Renee Sung Z, Berleth T. 1999. Responses of plant vascular systems to auxin transport inhibition Development 126 2979–2991 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. 2000. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes and Development 14 2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. 2009. Shifts in leaf vein density through accelerated vein formation in C4 Flaveria (Asteraceae) Annals of Botany 104 1085–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Lujan K, Deom CM. 2010. Geminivirus C4 protein alters Arabidopsis development Protoplasma 239 95–110 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL. 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis Proceedings of the National Academy of Sciences, USA 97 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno N, Bougourd S, Haseloff J, Feijó JA. 2006. Imaging plant cells Pawley JB. Handbook of biological confocal microscopy 3rdedition. New York, NY: Springer Science and Business Media; pp 769–787 [Google Scholar]
- Naramoto S, Kleine-Vehn J, Robert S, Fujimoto M, Dainobu T, Paciorek T, Ueda T, Nakano A, Van Montagu MCE, Fukuda H, Friml J. 2010. ADP-ribosylation factor machinery mediates endocytosis in plant cells Proceedings of the National Academy of Sciences, USA 107 21890–21895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H. 2010. Transcriptional regulation of vascular cell fates Current Opinion in Plant Biology 13 670–676 [DOI] [PubMed] [Google Scholar]
- Park J, Hwang H-S, Buckley KJ, Park J-B, Auh C-K, Kim D-G, Lee S, Davis KR. 2010. C4 protein of beet severe curly top virus is a pathomorphogenetic factor in Arabidopsis The Plant Cell Reports 29 1377–1389 [DOI] [PubMed] [Google Scholar]
- Petricka JJ, Nelson TM. 2007. Arabidopsis nucleolin affects plant development and patterning Plant Physiology 144 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroux N, Saunders K, Page A, Stanley J. 2007. Geminivirus pathogenicity protein C4 interacts with Arabidopsis thaliana shaggy-related protein kinase AtSKη, a component of the brassinosteroid signaling pathway Virology 362 428–440 [DOI] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. 1996. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization Planta 200 229–237 [DOI] [PubMed] [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues Advances in Botanical Research 9 152–262 [Google Scholar]
- Sachs T. 1991. Cell polarity and tissue patterning in plants Development Supplemental 1 83–93 [Google Scholar]
- Sawchuk MG, Head P, Donner TJ, Scarpella E. 2007. Time-lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation New Phytologist 176 560–571 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Francis P, Berleth T. 2004. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation Development 131 3445–3455 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. 2006. Control of leaf vascular patterning by polar auxin transport Genes and Development 20 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE. 1999. Auxin is required for leaf vein pattern in Arabidopsis Plant Physiology 121 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steynen QJ, Schultz EA. 2003. The FORKED genes are essential for distal vein meeting in Arabidopsis Development 130 4695–4708 [DOI] [PubMed] [Google Scholar]
- Vert G, Walcher CL, Chory J, Nemhauser JL. 2008. Integration of auxin and brassinosteroid pathways by auxin response factor 2 Proceedings of the National Academy of Sciences, USA 105 9829–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J. 2007. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein formation in Arabidopsis thaliana Plant Journal 49 387–393 [DOI] [PubMed] [Google Scholar]