Abstract
In rats, FDA-approved mood stabilizers used for treating bipolar disorder (BD) selectively downregulate brain markers of the arachidonic acid (AA) cascade, which are upregulated in postmortem BD brain. Phase III clinical trials show that gabapentin (GBP) is ineffective in treating BD. We hypothesized that GBP would not alter the rat brain AA cascade. Chronic GBP (10 mg/kg body weight, injected i.p. for 30 days) compared to saline vehicle did not significantly alter brain expression or activity of AA-selective cytosolic phospholipase A2 (cPLA2) IVA or secretory (s) PLA2 IIA, activity of cyclooxygenase-2, or prostaglandin or thromboxane concentrations. Plasma AA concentration was unaffected. These results, taken with evidence of an upregulated AA cascade in the BD brain and that approved mood stabilizers downregulate rat brain AA cascade, support the hypothesis that effective anti-BD drugs act by targeting the AA cascade, and suggest that the rat model might be used for drug screening
Keywords: gabapentin, arachidonic acid, bipolar disorder, mood stabilizer, drug screening
1. Introduction
Bipolar disorder (BD) is characterized by alternating phases of depression and mania. It affects 1% to 5% of the adult population in the United States [1, 2]. BD is treated with the FDA-approved mood stabilizers lithium, carbamazepine, sodium valproate and lamotrigine [3–5]. The atypical antipsychotic olanzapine also is clinically effective and FDA-approved [6]. Multiple signaling pathways have been suggested as targets of these drugs, but the pathophysiology of BD and mechanisms by which these drugs provide therapeutic benefit are not fully understood.
Since BD symptoms often are refractory to monotherapy, off-label drugs often are prescribed as adjunctive therapy. One such drug, the anticonvulsant gabapentin (GBP), was reported to reduce bipolar symptoms in open-label clinical trials. However, in subsequent randomized trials, GBP was shown to be ineffective as a treatment for BD patients with manic, hypomanic, or mixed states, even when taken with lithium or valproate [7, 8].
Mood stabilizers approved for treating BD, as well as the atypical antipsychotic olanzapine, downregulate brain arachidonic acid (AA) metabolic cascade markers when given chronically to rats so as to produce therapeutically relevant plasma concentrations [9–12]. In contrast, topiramate, which was suggested by initial Phase II trials to be effective [13, 14], but later shown in controlled Phase III trials to be ineffective [15], did not change the rat brain AA cascade markers that were downregulated by the mood stabilizers and olanzapine [16, 17]. Together, the data suggest that drugs effective and FDA-approved for BD target the rat brain AA cascade. This conclusion is supported by reports that markers of the AA cascade, as well as markers of neuroinflammation and apoptosis, are elevated in frontal cortex of postmortem BD compared to control brain [10, 18]. Molecular brain AA cascade markers include mRNA and protein levels of AA-selective calcium dependent cytosolic phospholipase A2 (cPLA2) IVA, secretory sPLA2 IIA, cyclooxygenase (COX)-2, and COX-1, and these can be measured in frozen rat or human brain. Markers that are valid only in high-energy microwaved brain, thus brain tissue obtained in rodent studies, are concentrations of unesterified AA, and of its eicosanoid metabolites, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2). Plasma concentrations of unesterified and esterified AA, and of other long chain fatty acids can be measured in both rodents and humans [19].
The brain has limited capacity to synthesize AA from its precursor linoleic acid [20], but derives it from the plasma unesterified pool [21]. This pool is maintained by adipose lipolysis or the secretion of esterified AA (i.e. AA bound to phospholipids, triglycerides or cholesteryl esters) by the liver, and is regulated by diet [22, 23].
As reported, the mood stabilizers when given to rats selectively downregulate brain markers of the AA cascade, sparing turnover of palmitic acid and docosahexaenoic acid (DHA), and expression of calcium independent iPLA2 VIA, which is considered to be comparatively selective for DHA metabolism and is unaffected by the mood stabilizers [24–26]. Based on recent phase III studies indicating that GBP is ineffective as adjunctive or primary therapy in BD, despite reported positive open label trials, we hypothesized that GBP when administered chronically to rats to produce a therapeutically relevant plasma concentration would not target brain AA cascade markers. To the extent that this hypothesis is correct, when combined with evidence that topiramate also does not target rat brain AA cascade markers [16], would support the findings that drugs clinically effective in BD generally target brain AA metabolism, whereas ineffective drugs do not.
To test our hypothesis, in the present study we administered GBP chronically for 30-days i.p., to achieve a therapeutically relevant plasma concentration, and measured brain AA cascade markers and plasma fatty acid concentrations. Rats were maintained on the same low-AA and high-DHA NIH-31 diet (composition reported below) used in our past studies involving the mood stabilizers and topiramate, to allow comparisons with our previous work [27, 24, 28, 16, 17]. It is critical to maintain the same dietary conditions between studies, because plasma and brain fatty acid concentrations and metabolism are dependent on their synthesis-secretion by the liver and availability through the diet [29]. An abstract of part of this work has been published [30].
2. Methods
2.1. Animals and diets
The study was approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and was conducted following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Publication no. 86-23). Male Fischer CDF 344 rats were obtained from Charles River Laboratories (Wilmington, MA, USA) at 2 months of age. Sixteen rats (8 control and 8 GBP treated) were used for brain mRNA, protein and activity and for plasma fatty acid measurements, and 16 rats were used for brain PGE2 and TXB2 measurements. The rats were acclimated for 1 week in an animal facility with regulated temperature, humidity and light cycle, and had free access to food and water. The diet (Rodent NIH-31 auto 18-4 diet, Zeigler Bros, Gardners, PA, USA) contained (as % of total fatty acid) 20.1% saturated, 22.5% monounsaturated, 47.9% linoleic, 5.1% α-linolenic, 0.02% AA, 2.0% eicosapentaenoic, and 2.3% DHA [31], thus was low in AA and high in DHA content.
GBP (RTI International, Research Triangle Park, NC, USA) was dissolved in 0.9% saline (Hospira, Lake Forest, IL, USA) and injected i.p. at a dose of 10 mg/kg, once daily for 30 days. A control group received the same volume of saline (vehicle) under identical conditions. The daily GBP dose was based on an accepted anticonvulsive therapeutic plasma concentration of 2–20 μg/ml in humans and was derived from a published linear relation between dose and plasma concentration in rats [32, 33]. There is no established therapeutic dose or plasma concentration range for BD. The selected dose in rats corresponds to a plasma concentration of 5 μg/ml 2.5 hrs after administration of a single gavage dose, which is within the therapeutic plasma concentration range in humans [32, 33]. On the 30th day, one set of rats (n=16) was injected with saline or GBP, and two hours later, they were euthanized by overdose of CO2 inhalation and decapitated. The brain was excised and blood was collected into EDTA-containing tubes. Plasma was separated by centrifugation (13,000 rpm, 1 min). Another set of animals (n = 18) subjected to the same treatment conditions were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and subjected to head-focused microwave fixation (5.5 kW, 3.4 s, 90% power output (Cober Electronics, Norwalk, CT, USA), to stop brain lipid metabolism [34]. Brain and plasma samples were stored at −80°C until analyzed.
2.2. Total RNA isolation and real time PCR
The brain was dissected sagittally into three parts that were used for Western blotting (WB), RT-PCR and activity measurements. Total RNA was isolated from the frontal cortex using an RNeasy mini kit (Qiagen, Valencia, CA, USA). Complementary DNA was prepared from total RNA using a high-capacity complementary DNA Archive kit (Applied Biosystems, Foster City, CA, USA). mRNA levels of cPLA2 IVA, sPLA2 IIA, iPLA2 VIA, COX-1 and COX-2 were measured by quantitative RT-PCR, using an ABI PRISM 7000 sequence detection system (Applied Biosystems). Specific primers and probes were purchased from TaqManP gene expression assays (Applied Biosystems), and consisted of a 20 X mix of unlabeled PCR primers and Taqman minor groove binder probe (FAM dye-labeled). The fold-change in gene expression was determined by the ΔΔCt method [35]. Data are expressed as fold-change of the target gene (cPLA2 IVA, sPLA2 IIA, iPLA2 VIA, COX-1 and COX-2) normalized to the level of endogenous control (β-2 microglobulin) and relative to the standard (calibrator).
2.3. Western blot analysis
Proteins (50 μg) from the brain cytoplasmic and membrane extracts were separated on 4–20% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA, USA). Following electrophoresis, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad) for immobilization. Protein blots were incubated overnight in Tris-buffered-saline, containing 5% nonfat dried milk and 0.1% Tween-20, with specific primary antibodies (1:200 dilution) for the group IVA cPLA2, group IIA sPLA2, group VIA iPLA2, COX-1 (1:1000), COX-2 (1:500) (Santa Cruz Biotech, Santa Cruz, CA, USA). Protein membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Bio-Rad), washed, treated with chemiluminiscent substrate and exposed to photographic film to detect luminescence (Kodak, Rochester, NY, USA). Optical densities of immunoblot bands were measured using Alpha Innotech Software (Alpha Innotech, San Leandro, CA, USA) and were normalized to β-actin (Sigma-Aldrich, St Louis, MO, USA) to correct for unequal loading. All experiments were carried out with 8 control and 8 GBP treated animals.
2.4. Plasma Fatty Acid concentrations
Total lipids were extracted from frozen plasma samples (150 μl) by the Folch method [36], after adding unesterified heptadecanoic acid as an internal standard. The extracts were separated, alongside authentic standards, by thin layer chromatography on silica gel plates (Whatman, Clifton, NJ, USA) using heptane :diethylether : glacial acetic acid (60:40:2 by volume) as a solvent. The esterified (phospholipid, triglyceride and cholesteryl ester) and unesterified fatty acid bands were scraped and an internal standard (di-17:0 phosphatidylcholine) was added to the esterified lipids. Esterified and unesterified lipids were methylated with 1% methanolic H2SO4 and analyzed using a gas chromatograph (Model 6890N detector; Agilent Technologies, Palo Alto, CA, USA) as described [37].
2.5. PLA2 and COX activities
Brain tissue was homogenized in detergent-free buffer (3 vol, 10 mM HEPES, pH 7.5, with 1 mM EDTA, 0.34 M sucrose, and protease inhibitor cocktail (Roche, Indianapolis, IN, USA) in a glass apparatus and centrifuged at 100,000 g (1 hr, 4°C). The supernatant was separated and used for determining protein content and for cPLA2 IVA, sPLA2 IIA, iPLA2 VIA, COX-1 and COX-2 activity measurements. Protein content was determined by the Bradford assay (Bio-Rad). cPLA2 and iPLA2 activities were measured using a radioisotopic method described elsewhere [38] and sPLA2, COX-1 and COX-2 activities were measured with a commercial kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
2.6. Brain PGE2 and TXB2 concentrations
In a separate experiment and 2 hours after the last of 30-day GBP or vehicle injections, the rats were anesthetized with Nembutal (45 mg/kg, i.p.), and immediately subjected to head-focused microwave irradiation (5.5 kW, 3.8 s (Cober Electronics, Stamford, CT, USA) to stop postmortem brain lipid metabolism [39, 40, 16]. A half-brain was weighed, homogenized with 18 volumes of hexane : isopropanol (3 : 2, by volume) and the homogenates were centrifuged (800 g, 5 min). Tissue residues were rinsed with 3 × 2 volumes of the same solvent and the lipid extracts were pooled and concentrated to dryness under N2. Concentrations of the PGE2 and TXB2 were measured using immunoassay kits (Oxford Biochemical Research, Oxford, MI, USA) with polyclonal antibodies specific to PGE2 and TXB2 according to the manufacturer’s instructions.
2.7. Statistical analysis
Data are presented as means ± SEM. Data were analyzed using Student’s unpaired t-test employing statistics software (Prism version 4.0b, GraphPad Software, San Diego, CA, USA and Excel, Microsoft, Redmond, WA, USA). Statistical significance was taken at p < 0.05. P values for all measured markers are shown in the
3. Results
3.1. mRNA, protein and activity of cPLA2, sPLA2 and iPLA2
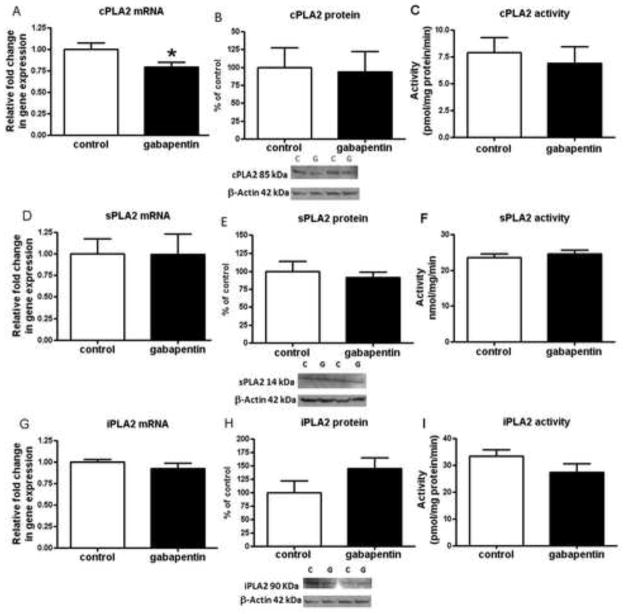
There was no significant change in the cPLA2 IVA, sPLA2 IIA and iPLA2 VIA protein or activity levels in GBP treated rats compared to controls (Fig. 1b, c, e, f, h, i). There was a significant 20% decrease in cPLA2 IVA mRNA (Fig. 1a) but no significant difference from control in the mRNA level for sPLA2 IIA or iPLA2 VIA in the GBP treated animals (Fig. 1d, g).
Fig. 1.
PLA2 mRNA, protein and activity levels in brains of gabapentin treated and control rats. Mean mRNA for cPLA2 (p = 0.02) (a); sPLA2 (p = 0.98) (d); and iPLA2 (p = 0.33) (g) in control (open bars) and gabapentin (filled bars) treated rat brain (n = 6 in control and gabapentin groups), measured using RT-PCR. mRNA levels in brain are normalized to the endogenous control (β2 microglobulin) and relative to control level (calibrator), using the ΔΔCT method. Mean brain protein levels with representative immunoblots for cPLA2 IVA (n = 8 per group; p = 0.88) (b); sPLA2 IIA (n = 8 per group; p = 0.60) (e); and iPLA2 VIA (h) (n = 7 per group, gabapentin due to one outlier in each group that was removed; p = 0.15) from control and gabapentin treated rats. Data are ratios of optical densities of cPLA2, sPLA2, or iPLA2 expressed as percentage of control. Mean activity of cPLA2 (n = 8, control and n = 7, gabapentin, one sample omitted due to activity level below blank; p = 0.38) (c); sPLA2 (n = 8 per group; p = 0.49) (f); and iPLA2 (n = 8 per group; p = 0.14) (i) in control and gabapentin treated rat brain. Values are mean ± SEM, *p< 0.05
mRNA, protein and activity of COX-1 and COX-2
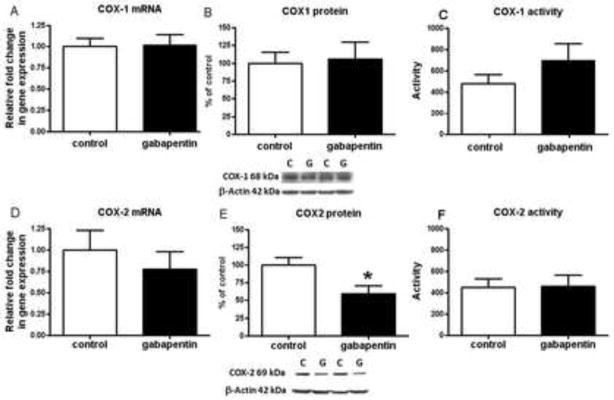
There was no change in the mRNA, protein, or activity level of COX-1 in GBP treated rats (Fig. 2 a–c). COX-2 protein was reduced significantly, whereas mRNA and activity levels were not altered significantly (Fig. 2d–f).
Fig. 2.
Mean COX mRNA, protein and activity in brain of gabapentin treated and control rats. Mean mRNA levels for COX-1 (p = 0.90) (a) and COX-2 (p = 0.46) (d) in control (open bars) and gabapentin (filled bars) treated rat brain (n = 6 per group), measured using RT-PCR. Data are mRNA level normalized to the endogenous control (β2 microglobulin) and relative to control level (calibrator), using the ΔΔCT method. Mean protein levels with representative immunoblots for COX-1(p = 0.83) (b) and COX-2 (p = 0.049) (e) in control and gabapentin treated rat brain (n = 8 per group). Data are ratios of optical densities of COX-1 or COX-2 expressed as percentage of control. Mean activity of COX-1 (n=6, control (one sample omitted due to activity below level of blank and one outlier removed) and n=8, gabapentin; p = 0.41) (C) and COX-2 (n=7, control (one sample omitted due to activity below level of blank) and n=8, gabapentin; p = 0.91) (f) in control and gabapentin treated rat brain. Values are mean ± SEM, *p< 0.05
Concentrations of PGE2 and TXB2
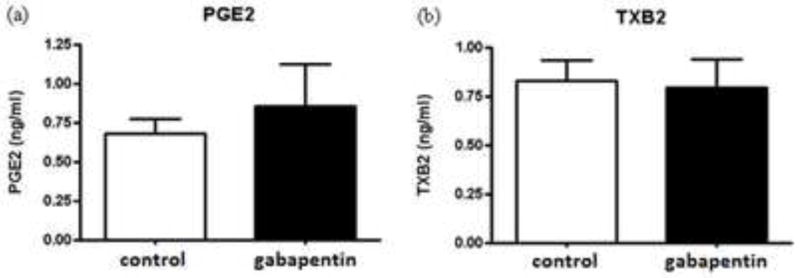
There was no significant change in brain PGE2 or TXB2 concentration in GBP treated rats compared to controls (Fig. 3).
Fig. 3.
PGE2 and TXB2 concentrations in brain of gabapentin treated and control rats. Mean concentrations (ng/ml) of PGE2 (n = 8, control and n = 9 gabapentin (one control sample omitted because its concentration was 6-fold greater than the mean, suggesting inadequate microwave fixation; p = 0.85)(a) and TXB2 (b) in control (open bars) and gabapentin (filled bars) treated rat brain (n = 9 per group; p = 0.85), determined by an ELISA assay. Values are mean ± SEM.
3.2. Plasma fatty acid concentrations
Of plasma unesterified fatty acids, the concentrations in nmol/ml for palmitate (16:0), stearate (18:0) and α-linolenate (18:3 n-3) were significantly increased in GBP treated compared with control rats (Table 1). Of esterified fatty acids within triglycerides, phospholipids and cholesteryl esters, only stearate (18:0), γ-linolenate (18:3 n-3) and behenate (22:0) concentrations were increased significantly in cholesteryl esters of GBP-treated rats (Table 1). Plasma unesterified and esterified concentrations of AA (20:4 n-6), as well as eicosapentaenoic acid (EPA) (20:5 n-3) and docosahexaenoic acid (DHA) (22:6 n-3), did not differ significantly between the two groups.
Table 1.
Plasma fatty acid concentrations (nmol/ml) in unesterified and esterified fatty acids of vehicle and GBP treated rats
| Unesterified fatty acids | Esterified fatty acids
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Triglyceride | Phospholipid | Cholesteryl ester | ||||||
|
| ||||||||
| (nmol/ml plasma) | (nmol/ml plasma) | (nmol/ml plasma) | (nmol/ml plasma) | |||||
| 14:0 | ND | ND | 79.8 ± 9.7 | 86.8 ± 6.9 | 6.3 ± 0.6 | 6.1 ± 0.7 | 13.6 ± 0.5 | 15.8 ± 1.7 |
| 14:1 n-9 | ND | ND | 2.6 ± 0.5 | 2.5 ± 0.5 | ND | ND | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 16:0 | 273.3 ± 15.0 | 391.4 ± 51.0* | 1467.4 ± 156.7 | 1618.8 ± 126.2 | 636.5 ± 23.5 | 590.3 ± 60.4 | 109.5 ± 5.3 | 116.1 ± 1.0 |
| 16:1 n-7 | 33.7 ± 4.6 | 60.2 ± 13.3 | 210.8 ± 35.9 | 271.4 ± 45.4 | 18.4 ± 1.7 | 17.9 ± 2.5 | 29.3 ± 3.6 | 33.0 ± 2.8 |
| 18:0 | 92.5 ± 3.9 | 115.5 ± 9.0* | 105.5 ± 8.1 | 108.5 ± 6.2 | 525.8 ± 23.4 | 558.4 ± 60.0 | 10.8 ± 0.4 | 19.8 ± 1.3*** |
| 18:1 n-9 | 110.8 ± 7.2 | 161.8 ± 26.0 | 908.9 ± 101.6 | 961.7 ± 78.0 | 74.7 ± 3.3 | 67.8 ± 7.1 | 43.0 ± 1.5 | 41.5 ± 0.7 |
| 18:1 n-7 | 25.0 ± 1.7 | 31.1 ± 3.5 | 150.3 ± 14.2 | 144.5 ± 10.8 | 71.8 ± 3.8 | 60.0 ± 6.2 | 11.6 ± 0.7 | 10.6 ± 0.2 |
| 18:2 n-6 | 198.5 ± 11.4 | 217.4 ± 19.9 | 1511.6 ± 151.8 | 1407.0 ± 72.6 | 505.2 ± 21.0 | 438.9 ± 44.9 | 267.0 ± 14.6 | 257.2 ± 5.1 |
| 18:3 n-6 | ND | ND | 9.7 ± 0.9 | 11.7 ± 0.7 | 1.4 ± 0.1 | 1.7 ± 0.2 | 6.0 ± 0.5 | 8.8 ± 0.6** |
| 20:0 | ND | ND | 109.5 ± 12.7 | 101.2 ± 5.5 | 2.4 ± 0.1 | 6.6 ± 1.4 | 3.6 ± 0.3 | 7.1 ± 0.7** |
| 18:3 n-3 | 14.2 ± 2.6 | 25.2 ± 1.9** | 12.2 ± 1.2 | 12.8 ± 0.7 | 2.3 ± 0.1 | 1.9 ± 0.2 | 0.9 ± 0.1 | 1.3 ± 0.3 |
| 20:3 n-6 | ND | ND | 15.2 ± 1.6 | 17.4 ± 1.4 | 19.1 ± 1.6 | 21.0 ± 2.6 | 6.8 ± 0.5 | 8.3 ± 1.3 |
| 20:4 n-6 AA | 35.0 ± 2.1 | 37.9 ± 2.8 | 86.2 ± 5.8 | 92.3 ± 5.9 | 357.6 ± 14.6 | 367.1 ± 40.1 | 464.6 ± 32.7 | 488.1 ± 15.7 |
| 22:1 n-9 | ND | ND | 2.9 ± 0.1 | 2.9 ± 0.1 | ND | ND | 1.0 ± 0.4 | 0.2 ± 0.2 |
| 20:5 n-3 EPA | 6.4 ± 0.5 | 7.2 ± 0.4 | 80.3 ± 8.3 | 88.9 ± 5.3 | 19.2 ± 1.8 | 20.4 ± 2.9 | 35.6 ± 3.9 | 42.6 ± 3.3 |
| 22:4 n-6 DTA n-6 | 33.4 ± 0.8 | 37.3 ± 1.3* | 22.0 ± 1.3 | 24.6 ± 1.4 | 3.8 ± 0.3 | 3.4 ± 0.4 | ND | ND |
| 22:5n-3 DPA n-3 | 4.8 ± 0.4 | 6.1 ± 0.5 | 68.6 ± 8.1 | 82.4 ± 6.8 | 15.1 ± 1.1 | 15.8 ± 1.8 | 0.4 ± 0.1 | 0.5 ± 0.0 |
| 22:6 n-3 DHA | 13.9 ± 0.9 | 16.1 ± 1.3 | 152.5 ± 15.0 | 172.7 ± 11.5 | 88.7 ± 5.5 | 91.5 ± 10.3 | 18.5 ± 1.4 | 20.2 ± 0.5 |
| Total SFAs | 365.8 ± 18.4 | 507.0 ± 59.7* | 1762.3 ± 186.0 | 1915.3 ± 142.7 | 1170.9 ± 45.9 | 1161.4 ± 120.9 | 137.5 ± 5.6 | 158.9 ± 2.3** |
| Total MUFAs | 169.5 ± 13.3 | 253.2 ± 42.6 | 1272.6 ± 150.8 | 1380.1 ± 130.4 | 164.9 ± 8.5 | 145.7 ± 15.5 | 84.5 ± 5.4 | 85.7 ± 3.6 |
| Total n-6 PUFAs | 266.9 ± 13.1 | 293.6 ± 22.1 | 1636.7 ± 158.2 | 1543.2 ± 78.1 | 870.6 ± 28.8 | 813.8 ± 84.2 | 737.6 ± 43.8 | 754.1 ± 16.7 |
| Total n-3 PUFAs | 32.9 ± 3.4 | 47.4 ± 3.5* | 233.4 ± 24.2 | 267.8 ± 18.3 | 107.0 ± 6.7 | 109.6 ± 12.3 | 19.9 ± 1.5 | 22.0 ± 0.6 |
| Total fatty acids | 841.5 ± 46.8 | 1108.3 ± 124.5 | 5003.4 ± 522.6 | 5215.7 ± 361.1 | 2349.1 ± 91.2 | 2269.2 ± 235.8 | 1022.9 ± 57.0 | 1071.8 ± 18.1 |
Values are mean ± SEM of n= 8/group. ND, not detectable. Data were analyzed with an unpaired t-test.
p < 0.05,
p < 0.01,
p < 0.001
4. Discussion
GBP, an anticonvulsant that was reported in Phase III trials to lack clinical efficacy in BD mania [7, 8], although suggested by open label trials to be effective, did not significantly change most brain AA cascade markers (with the exception of reduced COX-2 protein and cPLA2 IVA mRNA levels), or plasma AA concentration, when given chronically to rats to produce a therapeutically relevant plasma concentration. Our results lend indirect support to the hypothesis that clinically effective drugs in BD target the brain AA cascade, whereas clinically ineffective drugs such as GBP do not.
In contrast to these minimal effects of GBP in the rat, drugs that have shown efficacy in BD, and have been approved by the FDA for its treatment (e.g. the mood stabilizers lithium, carbamazepine, sodium valproate, and lamotrigine, and the atypical antipsychotic olanzapine), downregulate or inhibit multiple markers of the brain AA cascade when given chronically to adult rats (mRNA, protein and activity of cPLA2 IVA by lithium and carbamazepine, inhibition of acyl-CoA synthetase-4 activity by valproate, and reduced COX-2 activity or protein and the PGE2 concentration by each of the four mood stabilizers and olanzapine), AA turnover in brain phospholipids (lithium, carbamazepine, valproate, olanzapine), and plasma unesterified fatty acid including AA concentrations (olanzapine)) [41, 9, 42, 43]. None of the effective drugs or GBP altered expression of DHA-selective iPLA2 or, when tested, DHA turnover in rat brain phospholipids [24, 28]. Finally, topiramate, another drug that had been reported based on Phase II trials to be effective in BD [13, 14] but later shown ineffective in controlled Phase III clinical trials [44], like GBP, did not alter brain AA cascade markers or plasma AA concentration when given chronically to rats at a relevant anticonvulsant dose [16, 17].
Taken together, the present study and the published literature indicate that drugs that have been proven clinically effective against BD downregulate multiple aspects of the rat brain AA cascade, whereas two drugs suggested initially to work but later shown in Phase III trials to be ineffective (GBP and topiramate) do not. Since the five effective drugs that target rat brain AA metabolism have very different chemical structures, these results suggest that drugs are effective against BD because they commonly downregulate important aspects of the brain AA cascade, and that our rat model for assessing drug efficacy may be useful for screening potential new anti-BD drugs [45].
Supporting the idea that targeting the cascade by the FDA-approved drugs contributes to their clinical efficacy are data showing that markers of the cascade are upregulated in the postmortem BD compared with control brain. These markers include mRNA and protein levels of cPLA2 IVA, COX-2, sPLA2 IIA, and membrane prostaglandin E synthase (mPGES). In contrast, mRNA and protein levels of iPLA2 VIA, 5-, 12-, and 15-lipoxygenase, thromboxane synthase and cytochrome p450 epoxygenase were not significantly different, and levels of COX-1 and cytosolic PGES (cPGES) were reduced [10]. The changes in the postmortem BD brain are accompanied by molecular evidence of neuroinflammation, synaptic loss and apoptosis [46]. These findings support the AA hypothesis of BD and extend it to GBP by further demonstrating that drugs ineffective in the treatment of BD have no effect on the AA cascade enzymes.
The statistically significant reduction in COX-2 protein was not accompanied by a significant change in COX-2 mRNA or activity, and the decline in cPLA2 IVA mRNA did not correlate with a change in its protein or activity. This is consistent with the lack of change in brain PGE2 and TXB2. Thus, unlike the mood-stabilizers, GBP did not reduce PGE2 concentration or COX activity [47, 48, 42], which correlates with its lack of clinical efficacy in BD [7, 8]. Since we did not quantify GBP concentration in plasma, the fact that cPLA2 mRNA and COX-2 protein were reduced, suggests that the drug was properly delivered and that it entered the brain.
It is possible that other pathways that are targets of anti-BD drugs are also not regulated by GBP. One suggested effect of the mood stabilizers in addition to the AA cascade is their ability to cause neuronal cone growth spreading [49]. GBP or topiramate have not been tested with regard to this growth cone effect, as far as we know, but it would be of interest to do so.
Evidence indicates that the effective mood stabilizers, lithium, valproate, carbamazepine and lamotrigine, when given chronically to rats, exert their effects on the brain AA cascade directly, by mechanisms including blocking of glutamatergic N-methyl-D-aspartate and dopaminergic D2-like receptors coupled to the activation of cPLA2 [50–54]. On the other hand, olanzapine’s effect seems to occur by its reducing the unesterified plasma AA concentration by a peripheral mechanism, thereby limiting plasma AA availability to the brain [9]. The present study indicates that GBP did not affect brain AA metabolism by either a direct or indirect (via reducing unesterified plasma availability to brain) effect. The measured concentrations of plasma esterified and unesterified fatty acids in this study are similar to concentrations reported in other publications for unanesthetized rats maintained on similar diets [25, 17].
In conclusion, GBP, when given chronically to rats, did not generally change the plasma unesterified AA concentration or brain AA cascade metabolizing enzyme or activity levels. Our negative findings are consistent evidence that GBP and topiramate, another anticonvulsant, were no more effective than placebo in treating bipolar mania in double-blind, randomized, placebo-controlled trials [7, 8, 44]. These results support the hypothesis that effective anti-BD drugs downregulate brain AA metabolism and that measuring AA metabolic markers in rats could be useful to screen prospective anti-BD drugs.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Statement of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59(Suppl 1):S5–S30. doi: 10.1016/s0165-0327(00)00203-2. [DOI] [PubMed] [Google Scholar]
- 2.Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese JR, Bowden CL, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- 5.Nasrallah HA, Ketter TA, Kalali AH. Carbamazepine and valproate for the treatment of bipolar disorder: a review of the literature. J Affect Disord. 2006;95:69–78. doi: 10.1016/j.jad.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry. 2003;160:1263–1271. doi: 10.1176/appi.ajp.160.7.1263. [DOI] [PubMed] [Google Scholar]
- 7.Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord. 2000;2:249–255. doi: 10.1034/j.1399-5618.2000.20305.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheon Y, Park JY, Modi HR, et al. Chronic olanzapine treatment decreases arachidonic acid turnover and prostaglandin E concentration in rat brain. J Neurochem. 2011;119:364–376. doi: 10.1111/j.1471-4159.2011.07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2009;16:419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese JR, Keck PE, Jr, McElroy SL, Shelton MD. A pilot study of topiramate as monotherapy in the treatment of acute mania. J Clin Psychopharmacol. 2001;21:340–342. doi: 10.1097/00004714-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol. 2002;22:431–435. doi: 10.1097/00004714-200208000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double-blind placebo-controlled trials. Bipolar Disord. 2006;8:15–27. doi: 10.1111/j.1399-5618.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghelardoni S, Bazinet RP, Rapoport SI, Bosetti F. Topiramate does not alter expression in rat brain of enzymes of arachidonic acid metabolism. Psychopharmacology (Berl) 2005;180:523–529. doi: 10.1007/s00213-005-2189-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Ghelardoni S, Chang L, Bosetti F, Rapoport SI, Bazinet RP. Topiramate does not alter the kinetics of arachidonic or docosahexaenoic acid in brain phospholipids of the unanesthetized rat. Neurochem Res. 2005;30:677–683. doi: 10.1007/s11064-005-2756-3. [DOI] [PubMed] [Google Scholar]
- 18.Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMar JC, Jr, Lee HJ, Ma K, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochimica et biophysica acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Green JT, Liu Z, Bazinet RP. Brain phospholipid arachidonic acid half-lives are not altered following 15 weeks of N-3 polyunsaturated fatty acid adequate or deprived diet. J Lipid Res. 2010;51:535–543. doi: 10.1194/jlr.M000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bairras C, Mauriege P, Bukowiecki L, Atgie C. Regulation of lypolysis in white adipose tissues of lean and obese Zucker rats. J Physiol Biochem. 2007;63:287–296. doi: 10.1007/BF03165760. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Kiesewetter D, Chang L, Rapoport SI, Igarashi M. Quantifying conversion of linoleic to arachidonic and other n-6 polyunsaturated fatty acids in unanesthetized rats. Journal of lipid research. 2010;51:2940–2946. doi: 10.1194/jlr.M005595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- 27.Basselin M, Kim HW, Chen M, et al. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. J Lipid Res. 2010;51:1049–1056. doi: 10.1194/jlr.M002469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2005;182:180–185. doi: 10.1007/s00213-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport SI, Igarashi M, Gao F. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot Essent Fatty Acids. 2010;82:273–276. doi: 10.1016/j.plefa.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RJ, Reese EA, Cheon Y, Rapoport SI, et al. Society for Neuroscience. Washington, D.C: 2011. Effects of gabapentin on plasma and brain markers of arachidonic acid metabolism. [Google Scholar]
- 31.DeMar JC, Jr, Lee HJ, Ma K, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier D, Gupta R. Determination of gabapentin in plasma by liquid chromatography with fluorescence detection after solid-phase extraction with a C18 column. Clin Chem. 2002;48:2259–2261. [PubMed] [Google Scholar]
- 33.Hurley RW, Chatterjea D, Rose Feng M, Taylor CP, Hammond DL. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97:1263–1273. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 34.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 37.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- 39.Anton RF, Wallis C, Randall CL. In vivo regional levels of PGE and thromboxane in mouse brain: effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 1983;26:421–429. doi: 10.1016/0090-6980(83)90177-6. [DOI] [PubMed] [Google Scholar]
- 40.Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem Res. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- 41.Bazinet RP, Weis MT, Rapoport SI, Rosenberger TA. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berl) 2006;184:122–129. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- 42.Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry. 2004;56:248–254. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SG, Powers PS, Kushner SF, Wang D, et al. Topiramate in adults with acute bipolar I mania: pooled results; 157th Annual Meeting of the American Psychiatric Association; New York, NY. 2004. [Google Scholar]
- 45.Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 46.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosetti F, Rintala J, Seemann R, et al. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry. 2002;7:845–850. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- 48.Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–696. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 49.Shimshoni JA, Dalton EC, Watson P, Boris Y, Bialer M, Harwood AJ. Evaluation of the effects of propylisopropylacetic acid (PIA) on neuronal growth cone morphology. Neuropharmacology. 2009;56:831–837. doi: 10.1016/j.neuropharm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic administration of valproic acid reduces brain NMDA signaling via arachidonic acid in unanesthetized rats. Neurochem Res. 2008;33:2229–2240. doi: 10.1007/s11064-008-9700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res. 2008;33:1373–1383. doi: 10.1007/s11064-008-9595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- 53.Rintala J, Seemann R, Chandrasekaran K, et al. 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport. 1999;10:3887–3890. doi: 10.1097/00001756-199912160-00030. [DOI] [PubMed] [Google Scholar]
- 54.Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]