Abstract
An oxyma derivative, (2,2-dimethyl-1,3-dioxolan-4-yl)methyl 2-cyano-2-(hydroxyimino)acetate (2) displayed remarkable physico-chemical properties as a peptide-coupling additive for peptide-forming reactions in water. Short-to oligo-peptides could be synthesized by using 2, EDCI, and NaHCO3 in water without measurable racemization. Significantly, a simple basic and acidic aqueous work-up procedure can remove all reagents utilized in the reactions to afford only coupling products in consistently excellent yields.
The solid-phase peptide synthetic method has significantly contributed to basic and applied life sciences.1 In parallel, peptide bond-forming reactions in the solution phase remain an important C-N bond-forming processes in organic, inorganic, medicinal, and bioorganic chemistries.2 To date, a large number of peptide coupling reagents and coupling additives have been developed, and a wide variety of peptide coupling reagents are now commercially available. In amide-forming reactions with α-amino acids, the use of a coupling additive such as benzotriazole derivatives (e.g. 1-hydroxy-7-azabenzotriazole (HOAt) and hydroxybenzotriazole (HOBt)) is essential to suppress racemization and improve the efficacy of peptide synthesis.3 Recently, thorough evaluations of ethyl 2-cyano-2-(hydroxyimino)acetate, Oxyma (1 in Table 1), for peptide synthesis have been reported by several research groups.4 Oxyma is considered as an excellent non-explosive replacement for HOBt and HOAt. Due to the fact that active esters of peptide-coupling additives are often the intermediates in peptide bond-forming reactions, the pKa value of the peptide-coupling additive is one of the key factors that determine the reactivity of the additives and the stability of the active esters. The pKa values of HOBt, HOAt, and Oxyma are 4.60, 3.28, and 4.60, respectively. Thus, effectiveness in suppressing racemization and increasing the reaction rate of peptide-forming reactions using Oxyma are believed to be similar to those of HOBt.5 Suirós-Funosas and co-workers reported that dipeptide formation with Z-L-Phg-OH and H-L-Pro-NH2 using the conditions of Oxyma (3 equiv) and N,N′-diisopropylcarbodiimide (DIC, 3 equiv) in DMF provided the desired peptide Z-L-Phg-L-Pro-NH2 in 89.9% together with the racemized product (1.1%).4c On the other hand, the same reaction with HOBt and DIC furnished the desired product in 81% with the racemized product, Z-D-Phg-L-Pro-NH2 (9.3%).6 These experimental results indicated that the degree of racemization in peptide-forming reactions with Oxyma can be minimized using non-basic conditions with salt-free amino acids or weakly basic conditions. To date, several attractive features of Oxyma in peptide chemistry have been reported using a conventional organic solvent such as DMF. Although peptide bond-forming reactions can often be performed in water containing organic solvents, no practical peptide coupling additive has been developed for the synthesis of oligopeptides in water.7 Herein, we report the development of non-racemizable peptide-forming reactions of α-amino acids with glyceroacetonide-Oxyma derivative 2 in water.
Table 1.
Yield and diastereomeric excess (de) of Z-L-Phg-L-Pro-NH2 isolated from the reactions in water with or without the additives 1, 2, 3.a
| ||||
|---|---|---|---|---|
| entry | additive | time (h) | yield (%) | de (%)c |
| 1 | 1 | 2 | 25 | >99 |
| 2 | 2 | 2 | 95 | >99 |
| 3 | 3 | 2 | 5 | >99 |
| 4 | 3 | 12 | <10 | >99 |
| 5 | -b | 2 | 45 | 75 |
7 (1.5 equiv), 8 (1.0 eqiv), additive (1.2 equiv), EDCI (1.2 equiv), NaHCO3 (3 equiv) in H2O (0.2 M concentrations);
no additive was added; de was determined via HPLC and 1H-NMR analyses.
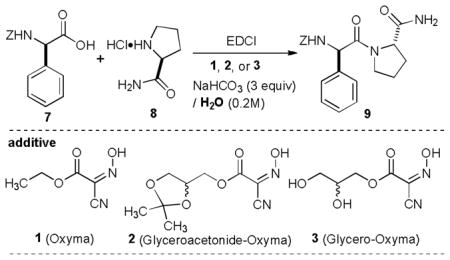
Several Oxyma derivatives that increase water solubility were designed and synthesized. As summarized in Scheme 1, 2,3-dihydroxypropyl 2-cyano-2-(hydroxyimino)acetate (3, Glycero-Oxyma) could be synthesized in three steps from 2-cyanoacetic acid (4) with an excellent overall yield. The ester 6, which was synthesized in a single step with N,N,-dimethyl phosphoramidodichloridate,8 was subjected to the hydroxyimination reaction using NaNO2 and AcOH to furnish (2,2-dimethyl-1,3-dioxolan-4-yl)methyl 2-cyano-2-(hydroxyimino)acetate (Glyceroacetonide-oxyma, 2) in 95% yield.9 Hydrolysis of the acetonide group of 2 yielded 3 in greater than 97% yield. Several attempts at synthesizing other types of water-soluble Oxyma derivatives could not be achieved due to the fact that many water solubilizing groups such as (p-hydroxyphenyl)dimethylsulfonium group and dimethylaminoalcohols were not amenable to the hydroxyimination reactions (6→2 in Scheme 1). Nonetheless, analyses of water solubility of 2 and 3 revealed that the glycerol and glyceroacetonide groups improved the water solubility of 2.1 and >5 times greater than that of Oxyma in 0.2 M NaHCO3 water solution (pH 8.3).10 Thus, new Oxyma derivatives 2 and 3 are completely solubilized even at 0.1 M concentrations in water at pH value of 8.3. Effectiveness of the coupling yields and degree of racemization of Oxyma derivatives 2 and 3 were examined by an established model study using Z-L-Phg-OH and HCl•H-L-Pro-NH2.4c In these studies we performed the reactions with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI)11 and NaHCO3 (3 equiv) in water solution. Yields and diastereomeric excess (de) of Z-L-Phg-L-Pro-NH2 obtained with 1, 2, and 3 are summarized in Table 1.
Scheme 1.
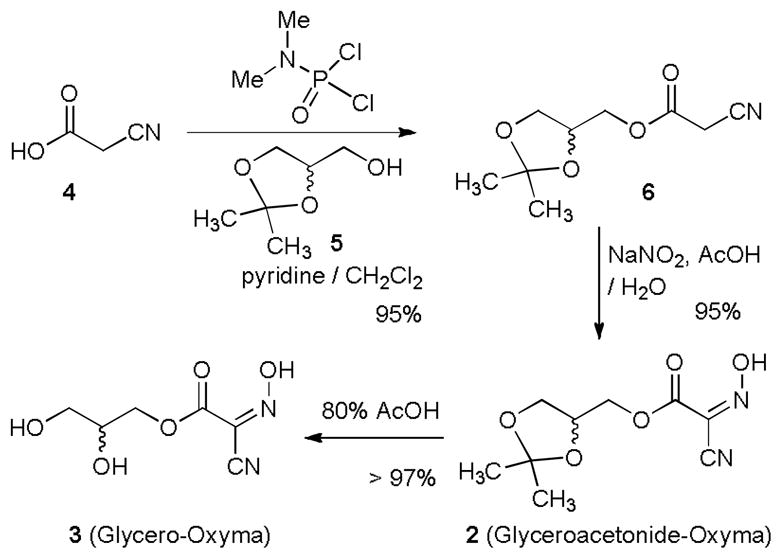
Syntheses of the Oxyma derivatives, 2 and 3.
Scheme 2.
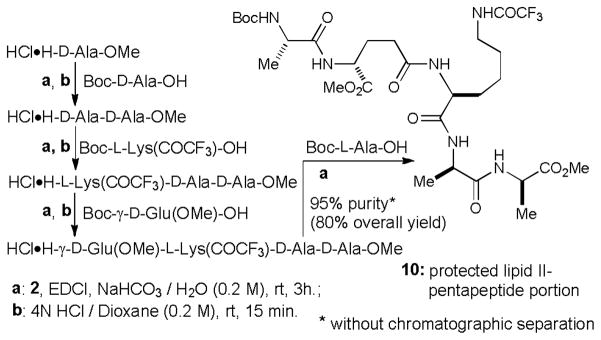
Efficient synthesis of 10 in water.
The reaction with Oxyma 1 furnished the desired product 9 in 25% yield in 2h with >99% de (entry 1).12 Although an excellent de was attained, the product yield with 1 could not be improved even after extended reaction times. Interestingly, the same reaction with glyceroacetonide-Oxyma 2 provided 9 in 95% yield with greater than 99% de (entry 2). On the other hand, the reaction with glycero-Oxyma 3 did not provide a useful level of the product yield, but the de of the product showed greater than 99% (entry 3). The product yield of 9 with 3 was not noticeably improved even after 12h (entry 4). Due to the fact that the coupling reaction of 7 and 8 without the coupling additive (1, 2, or 3) furnished 9 in 45% with only 75% de (entry 5), thus, high des observed in entries 1–4 were attributed to the formation of the less or non-racemizable oxyme esters of 1, 2, or 3 in water media at the pH value of less than 8.3. However, the hydrophilic-hydrophobic balance of the coupling additive seems to be very important to achieve high yielding amide coupling reaction between 7 and 8 in water.
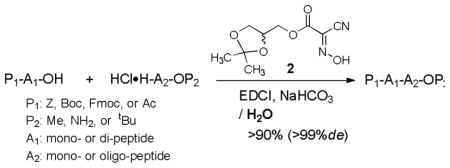
In order to understand the scope and limitations of the peptide-forming reactions with EDCI, glyceroacetonide-Oxyme 2, and NaHCO3 in water, these conditions were applied to the syntheses of a wide variety of dipeptides and tripeptides with partially protected α-amino acids. Selected examples are summarized in Table 2. N-Protected α-amino acids with commonly utilized carbamate protecting groups such as Cbz (Z), Boc, and Fmoc could be applied to the water-mediated amide-forming reactions; all reactions tested with methyl esters and primary amides of α-amino acids provided the desired dipeptides in greater than 90% yield without detectable diastereomers (analyzed by 1H-NMR or HPLC) (entries 1–5).13 The pH value of the water-mediated peptide coupling reactions established in Table 1 is below 8.3, thus, the formation of 9-methylene-9H-fluorene in the coupling reactions with Fmoc-L-Val-OH was not observed even at extended reaction times (entry 6). The dipetide-forming reactions of a hydrophilic α-amino acid, Boc-L-Lys(COCF3)-OH with a hydrophilic or a hydrophobic C-protected amino acid provided the corresponding products in greater than 92% yield with >92% de (determined by 1H-NMR) within 2h (entries 7 and 8). We have investigated the feasibility of forming hydrophobic dipeptides in water media (entries 9–13). The coupling reaction between Boc-L-Val-OH and HCl•H-Gly-OMe in water yielded Boc-L-Val-Gly-OMe in 95% with >92% de in 2h. Significantly, the reaction of Z-L-Phg-OH with HCl•H-L-Phe-OMe underwent in water to furnish Z-L-Phg-L-Phe-OMe in 92% yield without detectable diastereomers in 1H-NMR after 4h. On the other hand, the reaction of Z-L-Phg-OH with HCl•H-L-Phe-OtBu yielded the coupling product in only 40% yield even after 12h, but the de of the product exhibited > 99% (entry 11). The low yield reaction of Z-L-Phg-OH with HCl•H-L-Phe-OtBu in water was dramatically improved by the addition of a phase-transfer catalyst, octyltrimethylammonium bromide (entry 12) or by using a mixture of H2O and DMF (2:1).14 The hydroxy group of Boc-L-Thr-OH did not affect the coupling reactions in water (entry 14). The nucleophilicity of secondary amines of α-amino acids in peptide-forming reactions in water was also investigated (entries 15–18). HCl•N-Me-Gly-OMe could form the amide bond with Boc-L-Tyr-OH in 2h to afford Boc-L-Tyr-N-Me-L-Ala-OMe in 90% yield with 99% de. The reaction rate of the coupling reaction of HCl•N-Me-L-Ala-OMe with Boc-L-Tyr-OH was slower than that of HCl•N-Me-Gly-OMe in water, but Boc-L-Tyr-N-Me-L-Ala-OMe was isolated in 92% yield after 6h. As observed in Table 1, an excellent nucleophilicity of HCl•H-L-Pro-OMe in water could be confirmed by the reaction with Boc-L-Tyr-OH (entry 18).
Table 2.
Syntheses of di- and tri-peptides using EDCI, glyceroacetonide-Oxyme 2, and NaHCO3 in water-based solvent system.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | N-protected α-amino acid | C-protected α-amino acid | conditionsb | time (h) | product | yield (%) | de (%) |
| 1 | Z-L-Tyr-OH | HCl•H-L-Ala-OMe | A | 2 | Z-L-Tyr-L-Ala-OMe | 93 | >99c |
| 2 | Boc-L-Tyr-OH | HCl•H-L-Ala-OMe | A | 2 | Boc-L-Tyr-L-Ala-OMe | 95 | >99c |
| 3 | Boc-L-Val-OH | HCl•H-L-Pro-NH2 | A | 2 | Boc-L-Val-L-Pro-NH2 | 94 | >92d |
| 4 | Fmoc-L-Tyr-OH | HCl•H-L-Ala-OMe | A | 2 | Fmoc-L-Val-L-Pro-NH2 | 95 | >99c |
| 5 | Fmoc-L-Val-OH | HCl•H-L-Pro-NH2 | A | 2 | Fmoc-L-Val-L-Pro-NH2 | 94 | >99c |
| 6 | Fmoc-L-Val-OH | HCl•H-L-Pro-NH2 | A | 12 | Fmoc-L-Val-L-Pro-NH2 | 98 | >99c |
| 7 | Boc-L-Lys(COCF3)-OH | HCl•H-L-Pro-NH2 | A | 2 | Boc-L-Lys(COCF3)-L-Pro-NH2 | 93 | >92d |
| 8 | Boc-L-Lys(COCF3)-OH | HCl•H-L-Ala-NH2 | A | 2 | Boc-L-Lys(COCF3)-L-Ala-NH2 | 92 | >92d |
| 9 | Boc-L-Val-OH | HCl•H-Gly-OMe | A | 2 | Boc-L-Val-Gly-OMe | 95 | >92d |
| 10 | Z-L-Phg-OH | HCl•H-L-Phe-OMe | A | 4 | Z-L-Phg-L-Phe-OMe | 92 | >92d |
| 11 | Z-L-Phg-OH | HCl•H-L-Phe-OtBu | A | 12 | Z-L-Phg-L-Phe-OtBu | 40 | >99c |
| 12 | Z-L-Phg-OH | HCl•H-L-Phe-OtBu | B | 4 | Z-L-Phg-L-Phe-OtBu | 90 | >99c |
| 13 | Z-L-Phg-OH | HCl•H-L-Val-OtBu | C | 4 | Z-L-Phg-L-Val-OtBu | 90 | >99c |
| 14 | Boc-L-Thr-OH | HCl•H-L-Ala-OMe | A | 2 | Boc-L-Thr-L-Ala-OMe | 92 | >92d |
| 15 | Boc-L-Tyr-OH | HCl•N-Me-Gly-OMeg | A | 2 | Boc-L-Tyr-N-Me-L-Ala-OMe | 90 | 98c |
| 16 | Boc-L-Tyr-OH | HCl•N-Me-L-Ala-OMe | A | 2 | Boc-L-Tyr-N-Me-L-Ala-OMe | 70 | >99c |
| 17 | Boc-L-Tyr-OH | HCl•N-Me-L-Ala-OMe | A | 6 | Boc-L-Tyr-N-Me-L-Ala-OMe | 92 | >99c |
| 18 | Boc-L-Tyr-OH | HCl•H-L-Pro-OMe | A | 2 | Boc-L-Tyr-L-Pro-OMe | 95 | >99c |
| 19 | Ac-L-Ala-OHe | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Ala-L-Ala-OMe | 95 | >92d |
| 20 | Ac-L-Phe-OHe | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Phe-L-Ala-OMe | 93 | 98c |
| 21 | Ac-L-Tyr-OHe | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Tyr-L-Ala-OMe | 93 | >99c |
| 22 | Boc-L-Ala-D-(4-OH)Phg-OHf | HCl•H-L-Ala-OMe | A | 2 | Boc-L-Ala-D-(4-OH)Phg-L-Ala-OMe | 95 | >92d |
| 23 | Boc-L-Ala-L-Phe-OH | HCl•H-D-Val-OMe | A | 2 | Boc-L-Ala-L-Phe-D-Val-OMe | 95 | >99c |
| 24 | Boc-L-Ala-L-Phe-OH | HCl•H-D-Ala-OMe | A | 2 | Boc-L-Ala-L-Phe-D-Ala-OMe | 95 | >99c |
N-protected α-amino acid (1.5 equiv), C-protected α-amino acid (1.0 eqiv), 2 (1.2 equiv), EDCI (1.2 equiv), NaHCO3 (3 equiv);
A: in H2O (0.2 M), B: H2O : DMF = 2 : 1 (0.2 M).; C: Octyltrimethylammonium bromide (2 equiv) in water (0.2 M);
de was determined by HPLC;
de was determined by 1H-NMR analysis;
3 equv. of acetylated α-amino acid was used;
(4-OH)Phg = 4-hydroxyphenylglycine.;
N-Methylglycine methyl ester hydrochloride (Sar-OMe).
One of the challenges in peptide syntheses of α-amino acids is to achieve high-yielding reactions with N-acyl protected α-amino acids without racemization.15 Several acetylated α-amino acids were examined for the coupling reactions with HCl•H-L-Ala-OMe in water (entries 19–21). Ac-L-Ala-OH could be coupled with HCl•H-L-Ala-OMe to afford Ac-L-Ala-L-Ala-OMe in 95% yield with >92% de (entry 19). The same reaction with a standard condition (HOAt, EDCI, NMM in DMF) furnished Ac-L-Ala-L-Ala-OMe in less than 5% yield with 75% de in 2h. Similar to the result observed in entry 19, Ac-L-Phe-OH and Ac-L-Tyr-OH were reacted with HCl•H-L-Ala-OMe to yield the corresponding acetylated dipeptides in high yields without noticeable diastereomers (determined by HPLC analyses) (entries 20 and 21). Encouraged by the feasibility of peptide-forming reactions with N-acetylated α-amino acids, syntheses of tripeptides using the N-protected dipeptides were demonstrated via the water-mediated peptide-forming reactions (entries 22–24). Boc-L-Ala-D-(4-OH)Phg-OH was coupled with HCl•H-L-Ala-OMe to form Boc-L-Ala-D-(4-OH)Phg-L-Ala-OMe in 95% yield with >92% or >99 de (determined by 1H-NMR or HPLC).
In glyceroacetonide-Oxyma 2/EDCI mediated coupling reactions in water, 2 can be removed by basic aqueous-workup or by hydrolysis of the acetonide of 2. Excess starting materials (N-protected and C-protected amino acids), EDCI, and the urea by-product are readily washed out by a standard aqueous work-up procedure. Thus, protected oligopeptides synthesized via the conditions utilized in Table 2 can be isolated with high purity by basic and acidic aqueous work-ups (Figure 1). To date, we have demonstrated the syntheses of 4- to 18-mers of linear oligopeptides in water without chromatographic purifications. In most cases, syntheses of oligopeptides could be achieved in greater than 90% overall yield using the Boc strategy. For example, over one gram quantity of a hydrophobic penptapeptide Boc-L-Ala-γ-D-Glu(OMe)-L-Lys(COCF3)-D-Ala-D-Ala-OMe (10), the peptide portion of lipid II,16 was synthesized in water solution without chromatographic purifications (Scheme 2). In this synthesis the Boc groups of the intermediates were conveniently deprotected with 4N HCl in dioxane. LC-MS, 1H- and 13C-NMR analyses of the crude product 10 revealed that purity of the product was concluded to be greater than 95% (Figure 2).
Figure 1.
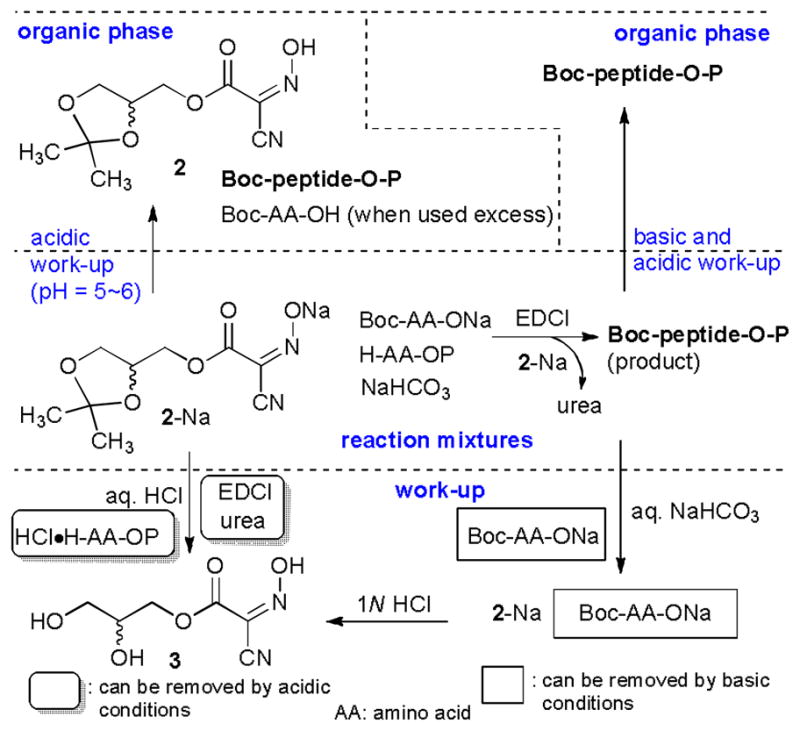
General work-up procedures to extract coupling products from water media.
In conclusion, we have developed a new Oxyma derivative, (2,2-dimethyl-1,3-dioxolan-4-yl)methyl 2-cyano-2-(hydroxyimino)acetate (2) which displays remarkable physico-chemical properties as a peptide-coupling additive in water media. Short-to oligo-peptides could be synthesized by using 2, EDCI, and NaHCO3 in water without measurable racemization products. Significantly, simple basic and acidic aqueous work-up procedures can remove all reagents utilized in the reactions to afford coupling products in high yield with excellent purity.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIAID grant AI AI084411) and University of Tennessee for generous financial support.
Footnotes
Supporting Information Available Experimental procedures and copies of NMRs. This is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Merrifield RB. Pure Appl Chem. 1971;50:643–653. [Google Scholar]
- 2.(a) El-Faham A, Albericio F. Chem Rev. 2011;9:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]; (b) Bray BL. Nature Rev. 2003;2:587–593. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 3.Han S-Y, Kim Y-A. Tetrahedron. 2004;60:2447–2467. and references therein. [Google Scholar]
- 4.(a) Khattab SN. Bull Chem Soc Jpn. 2010;83:1374–1379. [Google Scholar]; (b) El-Faham, Subirós-Funosas R, Albericio F. Chem Eur J. 2010;19:3641–3649. doi: 10.1039/c003719b. [DOI] [PubMed] [Google Scholar]; (c) Subirós-Funosas R, Prohens R, Barbas R, El-Faham A, Albericio F. Chem Eur J. 2009;15:9394–9403. doi: 10.1002/chem.200900614. [DOI] [PubMed] [Google Scholar]
- 5.Itoh M. Bull Chem Soc Jap. 1973;46:2219–2221. [Google Scholar]
- 6.The same reaction with EDCI, HOAt, NMM in DMF afforded Z-L-Phg-L-Pro-NH2 in 95% yield with the racemized product (9.5%).
- 7.Galanis AS, Albericio F, Grøtli M. Org Lett. 2009;11:4488–4491. doi: 10.1021/ol901893p.Hojo K, Maeda M, Tanakamaru N, Kawasaki K. Protein Pept Lett. 2006;13:189–192. doi: 10.2174/092986606775101607.Kouge K, Koizumi T, Okai H, Kato T. Bull Chem Soc Japan. 1987;60:2409–2418.Kunz H. Angew Chem Int Ed Engl. 1978;17:67–68.and references therein.
- 8.Amancha PK, Liu HJ, Wei T, Shia KS. Eur J Org Chem. 2010;18:3473–3480. [Google Scholar]
- 9.Cheng LJ, Lightner DA. Synthesis. 1999;1:46–48. [Google Scholar]
- 10.Water solubility of 1, 2, and 3 was measured via the shake-flask method; 1 (14.90 mg/mL), 2 (31.5 mg/mL), and 3 (80 mg/mL), respectively.
- 11.Kurzer F, Douraghi-Zadeh Chem Rev. 1967;67:107–152. doi: 10.1021/cr60246a001. [DOI] [PubMed] [Google Scholar]
- 12.The diastereomer was not detected by HPLC analysis (see Supporting Information).
- 13.The corresponding diastereomers were synthesized with N-protected D-α-amino acids for the 1H-NMR studies. In all 1H-NMR analyses of the products (entries 1–24), we did not observe the diatereomers. However, because of the detection limit of 1H-NMR spectroscopy, de was tentatively determined >92% in Table 2.
- 14.All reactions summarzied in Table 2 could also be performed using 2, EDCI, and NaHCO3 in H2O-DMF (2/1).
- 15.Bodanszky M, Conklin LE. Chem Commun. 1967:773–774. [Google Scholar]
- 16.Lipid II is a membrane-anchored bacterial cell-wall precursor. The structure of M. tuberculosis lipid II represents GlcNAc-MurNAc-(L-Ala-γ-D-Glu(OMe)-L-Lys(COCF3)-D-Ala-D-Ala)-diphosphoryl-decaprenol.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.