Abstract
The aim of this study was to investigate the potential of diffuse optical spectroscopy for monitoring of patients with locally advanced breast cancer (LABC) undergoing neoadjuvant chemotherapy. Fifteen women receiving treatment for LABC had the affected breast scanned before; 1 week, 4 weeks, and 8 weeks after treatment initiation; and before surgery. Optical properties related to tissue microstructure and biochemical composition were obtained. Clinical and pathologic tumor response was evaluated using whole-mount pathology after mastectomy. Patients who responded to treatment demonstrated an initial increase followed by a drop in optical parameters measured in the whole breast, whereas nonresponding patients demonstrated only a drop in the same parameters 1 week after treatment initiation. Responding patients demonstrated a significant increase of 17% ± 7%, 8% ± 8%, 10% ± 7%, 11% ± 11%, and 16% ± 15% in deoxygenated hemoglobin, oxygenated hemoglobin, total hemoglobin concentrations, water percentage, and tissue optical index, 1 week after treatment initiation, respectively. In contrast, nonresponding patients had a decrease of 14% ± 9%, 18% ± 7%, 17% ± 7%, 29% ± 7%, and 32% ± 9% in their corresponding optical parameters. Deoxygenated hemoglobin concentration (with 100% sensitivity, 83% specificity) and water percentage (with 75% sensitivity, 100% specificity) were found to be the best predictors of treatment response at 1 week after starting treatment. The results of this study suggest that optical parameters can be potentially used to predict and monitor patients' responses to neoadjuvant chemotherapy and can form a basis for the customization of treatments in which inefficacious treatments can be switched to more efficacious therapies.
Introduction
Breast cancer is the most common malignancy for women in North America. Approximately 5% to 15% of the estimated 200,000 new cases diagnosed each year will present with locally advanced breast cancer (LABC) [1,2]. Women with LABC have a very poor outcome in both local and systemic recurrence (5-year survival rate of ∼50%) [3]. Standard treatment for these patients is now usually neoadjuvant systemic treatment (chemotherapy or, less frequently, endocrine therapy) followed by surgery and radiotherapy [4]. However, LABC treatment remains controversial because determining the optimal treatment paradigm is fraught with uncertainties, both in terms of the best treatment regimen to administer and its duration [2,5,6]. While complete pathologic response to neoadjuvant chemotherapy has been demonstrated to correlate strongly with patient survival [7], conventional clinical surrogates based on anatomic information such as physical assessment, mammography, and standard clinical imaging such as ultrasound suffer from an inability to objectively assess treatment response early during the course of treatment [8].
To assess response to treatment, it is important to know the extent of initial disease. In this regard, magnetic resonance imaging (MRI) has shown promise as a staging tool that can accurately determine the extent of cancer when compared with pathology specimens [9]. In particular, contrast-enhanced MRI has been proven to consistently detect residual disease [10,11]. Another functional imaging technique, positron emission tomography (PET), has been reported to identify tumor changes as early as 8 days after treatment [12]. However, both these approaches can be costly. Furthermore, PET and MRI require the use of exogenous contrast agents and use lengthy scan times that are often unfeasible for ongoing monitoring of patients with late-stage cancer. Hence, an alternative modality that can provide rapid and quantitative functional information about the extent of disease without the requirement of exogenous contrast agents would be of considerable value for evaluating responses to neoadjuvant therapy. It is important to know if tumors and, by inference, dispersed cancer cells are responding to neoadjuvant chemotherapy because, if it is ineffective, it should be changed to an effective therapy.
The need for a noninvasive and inexpensive imaging modality to monitor treatment response has led to renewed interest in the potential of optical imaging. Diffuse optical spectroscopy (DOS) and diffuse optical tomography (DOT) are noninvasive, nonionizing techniques that use near-infrared light to rapidly provide quantitative spectral information (in tens of seconds) regarding the absorption and scattering properties of tissue [13,14]. Typically, DOS uses a large spectral bandwidth with a sparse number of spatial measurements, whereas DOT is used to produce three-dimensional optical property maps at 1- to 3-mm resolution with lower spectral bandwidth. This relationship is similar to that of magnetic resonance (MR) spectroscopy and MRI. Measured optical properties can be converted to parameters related to tissue microstructure and biochemical composition such as oxygenated hemoglobin ([HbO2]), deoxygenated hemoglobin ([Hb]), total hemoglobin concentration ([HbT] = [Hb] + [HbO2]), relative oxygen saturation (StO2 = [HbO2] / [HbT]), relative oxygen desaturation (St = [Hb] / [HbT]), water percentage (%H2O), lipid percentage (%Li), scattering power (SP), scattering amplitude (SA), and tissue optical index (TOI = [Hb](%H2O) / (%Li)). The TOI combines both functional ([Hb] and %H2O) and structural (%Li and %H2O) information for enhanced contrast, and this was used by previous investigators [15] for DOS. This functional information is not readily available through conventional structural imaging techniques. Because optical contrast comes from intrinsic tissue components, the technique does not require exogenous contrast agents, making it ideal for frequent, repeat monitoring. Furthermore, DOS technology is relatively inexpensive compared with MRI and PET and provides functional information as a potential complement to traditional structural imaging techniques such as MRI [16] and ultrasound [17]. In normal breast tissue, optical information can be used to evaluate underlying physiological differences as a result of age, body mass index, menopausal status, and fluctuations in the menstrual cycle [18]. DOS has been used previously as a means of differentiating between normal and malignant breast tissues [19]. Hypoxia, cellular proliferation, angiogenesis, and extracellular matrix breakdown are biologic factors that have all been linked with cancer and shown to directly influence the concentration of optical parameters such as [HbT], %H2O, and %Li [19,20]. Because hypoxia and blood flow are also correlated with chemotherapeutic resistance and degree of response, both oxygen saturation and hemoglobin concentration may be good indicators of tumor response [21,22].
Previous studies have suggested that DOS may provide clinically useful information on whether a particular treatment regimen is successful [15,23–26]. In a recent study, Soliman et al. [25] investigated the potential of both DOS and DOT for treatment monitoring in a study of 10 patients undergoing neoadjuvant chemotherapy. They demonstrated that volume-weighted optical information such as Hb, HbO2, and SP could provide an indication of response within 4 weeks in patients undergoing various LABC neoadjuvant treatments. In the work here, we present a different whole-breast approach to predict the clinical response to neoadjuvant chemotherapy by monitoring changes in optical parameters and relating them to clinical and postsurgical pathologic outcome. We report the results of multiperiod study on 15 patients who received a variety of neoadjuvant treatment regimens. We investigated the changes in 10 optical parameters (HbO2, Hb, HbT, StO2, St, H2O, Li, SP, SA, and TOI) during the treatment of patients who responded to the treatment and who did not. We also compared results to a volume-weighted method indicating that the whole-breast method can potentially detect changes earlier. The long-term goal of this work was to use this method to customize chemotherapies to adapt to the patient's response profile.
Materials and Methods
Study Protocol
The study included 15 patients with LABC treated at the Odette Cancer Centre of the Sunnybrook Health Sciences Centre in accordance with research ethics approval. These included the 10 patients from the previous study using the volume-weighted method [25] in addition to five new patients. Before the start of the study, signed informed consent was obtained from all patients whose affected breasts were scanned five times: before treatment; at 1 week, 4 weeks, and 8 weeks after initiation of treatment; and before surgery. Clinical examinations were also carried out before each imaging session in addition to regular patient visits. In accordance with institutional LABC care policies, a 1.0-T MRI study (GE Healthcare, Waukesha, WI) using a dedicated radiofrequency coil was performed only at baseline and immediately before surgery as a measure of tumor size. Pathology was examined after mastectomy on whole-mount [27] 5 x 7-in pathology slides digitized using a confocal scanner (TISSUEscope; Huron Technologies, Waterloo, Ontario, Canada) at 2-µm resolution. Data on grade, histologic subtype, size, and tumor response were recorded. Patients were categorized as having either a good pathologic response or a minimal response. Good pathologic response was defined as before [25] and by others [28] as having a more than 50% reduction in the tumor size in comparison to pretreatment size along with a remarkable decrease in cellularity (<10% of cells appear to be viable and invasive). Minimal pathologic response meant a small change in the tumor size (<50% reduction or enlargement) compared to the pretreatment tumor size.
DOS/DOT Instrument
DOS images were collected using a commercial optical system (SoftScan; ART, Inc, Montreal, Quebec, Canada) composed of four individual pulsed semiconductor diode lasers (LDH-P; PicoQuant, Berlin, Germany) operating at 690, 730, 780, and 830 nm, with a pulse duration at full-width at half-maximum less than 150 picoseconds, an average output of 0.5 mW when driven at 20 MHz (PDL 808; PicoQuant), with an oscillator module to synchronize drivers. Photons were collected by a mobile detector in a 1 cm-X constellation composed of five optical fibers and detected by a photomultiplier (H7422P-50; Hamamatsu, Bridgewater, NJ). A router processed the electrical pulse from the photomultiplier before sending it to a time-correlated single-photon counting board (SPC-130; Becker & Hickl, Berlin, Germany). The count was time correlated with the synchronization signal provided by the laser system driver.
Because the spectral range of the system does not extend to regions where water and lipid concentrations are significant contributors to absorption, estimation of the water (H2O) and lipid (Li) percentages was based on the empirical linear relationship with the estimated scattering power b, where b comes from µ′s = Aλ-b. The scattering amplitude A (arbitrary units) and the scattering power b (dimensionless) are estimated from the relationship that approximates Mie scattering in tissue in the near-infrared range, where µ′s is the scattering coefficient (cm-1) and λ is the light's wavelength (nm) [29]. The results of non-clinical studies show that the sensitivity of the system allows detection and quantification of absorption changes equivalent to less than 1 µmol of blood [30].
Patients were scanned in a prone position and positioned into the breast aperture under the guidance of a clinical research coordinator. The scanning area encompassed the whole breast. To ensure consistency, stabilizing plates were used to secure the breast in place and all imaging parameters (i.e., angle, height, distance etc) at baseline were recorded and used for all subsequent scans. A liquid optical compensation medium (an oil-in-water emulsion that mimics average optical properties of the human breast, with an average absorption coefficient of 0.04 cm-1 and an average effective scattering coefficient of 10 cm-1 at the four wavelengths used) was used in the breast aperture to minimize light reflections at the breast interface that can degrade image quality. The acquired data were reconstructed using commercially available software associated with the SoftScan device, and three-dimensional tomographic images were created from the optical parameters with a 3-mm3 voxel size.
Data Analysis and Statistics
Mean measured values of [HbO2], [Hb], [HbT], StO2, St, % H2O, %Li, SP, SA, and TOI in the entire breast were obtained and integrated over the entire breast volume to calculate the integrated optical index for each parameter. Volume-weighted parameters were also calculated as before [25], as follows: The tumor was identified in the pretreatment DOS images for each optical parameter. A volume of interest (VOI) was created by choosing a threshold value that was adjusted such that the VOI corresponded in size and location to the tumor as known through other imaging and clinical examinations. The threshold value was kept consistent at each time point for the same optical parameter for each patient. Mean measured values in the VOI were obtained and multiplied by the VOI volume to obtain the volume-weighted parameters. These were limited to Hb, HbO2, H2O, and SP as only those provided a volume easily identified and consistent with tumor location. Percentage changes in the values from baseline between clinical/pathologic responders and nonresponders at each time for each of the optical parameters were compared independently. Normality violations for each parameter were checked using the Shapiro-Wilk test (GraphPad Prism 4; GraphPad Software, Inc, La Jolla, CA). Depending on whether the changes were normally distributed or not, statistical analysis using either a t test or a Mann-Whitney test (two-sided, 95% confidence) was carried out to assess if patients showing statistically significant changes in optical parameters at weeks 1 and 4 correlated to the patient population demonstrating treatment response as defined by clinical criteria. In addition, a linear mixed model with repeated-measures analysis was performed to determine the statistical significance of each optical parameter.
Discriminant analysis (PASW Statistics 18; SPSS, Inc, Chicago, IL) was used to determine which optical parameters discriminate between responders and nonresponders at weeks 1 and 4. The changes in the values of HbO2, Hb, HbT, StO2, St, H2O, Li, SP, SA, and TOI were used as predictors in the analysis that aimed at providing the best separation between the two groups. Sensitivity and specificity were calculated to measure the performance of the classification test. Statistics were also calculated on the volume-weighted data and compared with whole-breast results.
Results
Patient and Tumor Characteristics
Patients' physical, tumor, and treatment characteristics are summarized in Table 1. Ten patients had all five scans as planned. The mean patient age was 49 years (SD = 9 years, range = 36–64 years). The average maximum tumor size was 7.9 cm (SD = 2.6 cm, range = 3.2–12 cm). Of the 15 subjects, 2 had lobular carcinoma, 10 were ER/PR.positive, 7 were Her-2-neu-positive, and 9 were menstruating. Two patients had tumor grade 1, one patient had tumor grade 3, and the rest had tumor grade 2.
Table 1.
Patients' Characteristics.
| Patient No. | Age (years) | Menopausal Status | MR Tumor Dimensions (AP x ML x SI; cm) | Histologic Diagnosis | Grade | ER/PR | Her-2-neu | Residual In Situ Size (%) | Neoadjuvant Treatment |
| 1 | 47 | Premenopausal | 5 x 4.5 x 4.5 | Lobular | 2 | + | - | 70 | Chemoradiotherapy |
| 2 | 38 | Premenopausal | 11.2 x 7.3 x 8.3 | Ductal | 3 | + | + | 0 | Sunitinib, trastuzumab → docetaxel, trastuzumab, pamidronate |
| 3 | 54 | Postmenopausal | 6.1 x 7.8 x 3.3 | Ductal | 2 | - | - | 5–10 | Epirubicin, docetaxel |
| 4 | 61 | Postmenopausal | 10 x 6 | Ductal | 2 | - | - | 30 | Chemoradiotherapy |
| 5 | 56 | Postmenopausal | 11 x 6.3 x 5.5 | Ductal | 2 | + | - | 5–10 | Epirubicin, docetaxel |
| 6 | 48 | Premenopausal | 7.8 x 4.6 x 5.5 | Ductal | 2 | - | + | >80 | AC + paclitaxel, trastuzumab |
| 7 | 64 | Postmenopausal | 7.5 x 6.5 x 5.5 | Ductal | 2 | + | - | 5–10 | AC + paclitaxel, trastuzumab |
| 8 | 45 | Premenopausal | 5.1 x 5.5 x 4.5 | Ductal | 2 | + | + | >90 | Chemoradiotherapy |
| 9 | 47 | Premenopausal | 4.8 x 3.1 x 5.5 | Ductal | 2 | + | + | 60 | Docetaxel, carboplatinum, trastuzumab |
| 10 | 57 | Postmenopausal | 10.2 x 7.2 x 6.8 | Lobular | 1 | + | + | 5–10 | FEC + docetaxel |
| 11 | 43 | Premenopausal | 6.6 x 3.7 x 7.9 | Ductal | 2 | + | - | 85 | FEC + docetaxel |
| 12 | 56 | Postmenopausal | 2.4 x 2.7 x 3.2 | Ductal | 2 | - | + | 0 | AC + paclitaxel, trastuzumab |
| 13 | 49 | Premenopausal | 2.4 x 2.8 x 1.4 and 1.4 x 2.8 x 1.3 | Ductal | 2 | - | + | >90 | AC + paclitaxel, trastuzumab |
| 14 | 38 | Premenopausal | 9 x 6.6 x 6 | Ductal | 2 | + | - | 60 | AC + paclitaxel |
| 15 | 36 | Premenopausal | 12 x 11.6 x 8.9 | Ductal | 1 | + | - | 20 | AC + paclitaxel |
AC indicates adriamycin and cytoxan; FEC, fluorouracil (5-FU), epirubicin and cyclophosphamide.
Most of the tumors had in situ components occupying between 5% and more than 90% of the overall tumor size. The remaining portion of the tumor was invasive. Subjects had a variety of neoadjuvant treatment plans. Seventy-five percent of patients had combined anthracycline and taxane-based chemotherapy. Three patients had combined chemoradiotherapy and one patient had sunitinib and trastuzumab chemotherapy during the first three cycles. This was later changed to docetaxel, trastuzumab, and pamidronate.
Clinical and Pathologic Assessment of Tumor Response
All studies were examined in the same manner by a board certified pathologist (J.Z.) who interpreted all LABC pathology and determined tumor response. Pathologic response results of all patients are presented in Table 2. Patients 4, 10, and 12 had a significant reduction in the tumor size, and they were categorized as good responders. Patients 6, 8, 9, 11, and 13 were classified as good responders owing to the minimally invasive disease left. Although the remaining pathologic tumor size was large in some cases, the appearance of decreased tumor cellularity and rare groups of residual cells indicated a good response. Patients 1, 3, 5, 7, 14, and 15 had a poor response. Their pathologic tumor size slightly changed compared with their pretreatment MR tumor size. Patient 2 was an exceptional case. Pretreatment MR showed an invasive ductal carcinoma measuring 11.2 (AP) x 7.3 (ML) x 8.3 (SI) cm. She was started on sunitinib/trastuzumab chemotherapy. Three cycles later, her clinical condition worsened, with axillary nodes increasing in size. Her chemotherapy was changed to docetaxel/pamidronate/trastuzumab to which she responded with tumor decreasing to 3 (ML) x 3 (SI) cm in size. She also developed bone metastases and did not have a mastectomy.
Table 2.
Patients' Pathologic Response Results.
| Patient No. | Pathologic Tumor Dimensions (AP x ML x SI) | Notes | Pathologic Response |
| 1 | 1.8 x 4 x 4.5 | Some response; very high nuclear grade with bizarre nuclei | Poor |
| 2 | N/A | Clinically good response with change in chemotherapy | Initially poor then good |
| 3 | 5.2 x 7 x 1.8 | Large volume of tumor remaining | Poor |
| 4 | 1 x 1 x 0.7 | Very small volume of tumor remaining | Good |
| 5 | 4.5 x 4 x 9 | Large volume of residual invasive duct carcinoma | Poor |
| 6 | 2 x 7 x 3 | Scattered foci of invasion; minimal tumor cellularity | Good |
| 7 | 8.9 x 7.7 x 3 | High tumor cellularity | Poor |
| 8 | 2.5 x 4.8 x 3.6 | Numerous small foci of invasive disease | Good |
| 9 | 2 x 6 x 3 | Very small nests of cells remaining | Good |
| 10 | 1 x 0.9 x 0.7 | Small volume of low-grade invasive duct carcinoma remaining | Good |
| 11 | 1.7 x 6 x 4.5 | Rare groups of invasive cells remaining | Good |
| 12 | 0.2 x 0.2 | Probable or definite response to presurgical therapy | Good |
| 13 | 3.5 x 2.3 x 1.3 | Negligible invasive tumor remaining | Good |
| 14 | 2 x 2.9 x 1.5 and 2 x 1.1 x 1 | Multifocal invasive duct carcinoma | Poor |
| 15 | 8 x 7.5 x 6 | Large volume of low-grade invasive duct carcinoma remaining | Poor |
Notes were taken from pathology reports.
DOS Assessment of Tumor Response
Three-dimensional functional tomographic images were created for each patient. In Figure 1, representative DOS images and whole-mount pathologic slides for a nonresponder and a responder acquired at baseline, at week 1, at week 4, at week 8, and before surgery are presented. A comparison of the previously used method (volume-weighted) and the whole-breast method for HbO2 is presented in Figure 2. Figure 3 demonstrates changes in four optical parameters using the volume-weighted method. Table 3, A and B, presents values as a percentage of the pretreatment scan for each of the optical parameters at 1 and 4 weeks of time, respectively. Volume-weighted parameters averaged for nonresponders and responders were significantly different at 4 weeks for Hb (P = .005), H2O (P = .04), and SP (P = .046). None of the four parameters studied were significantly different at week 1 (P > .05). A repeated-measures analysis was also done using a linear mixed model statistical analysis. The mean percent change was -74.1% (SE = 4.7%) for Hb in responders versus -18.7% (SE = 7.4) for Hb in nonresponders, exhibiting a statistically significant difference (P < .001). The mean percent change was -65.7% (SE = 7.3) for HbO2 in responders versus -29.2% (SE = 9.1) for HbO2 in nonresponders. This difference was also statistically significant (P = .037). Both SP and H2O were found not to be statistically significant (P > .05).
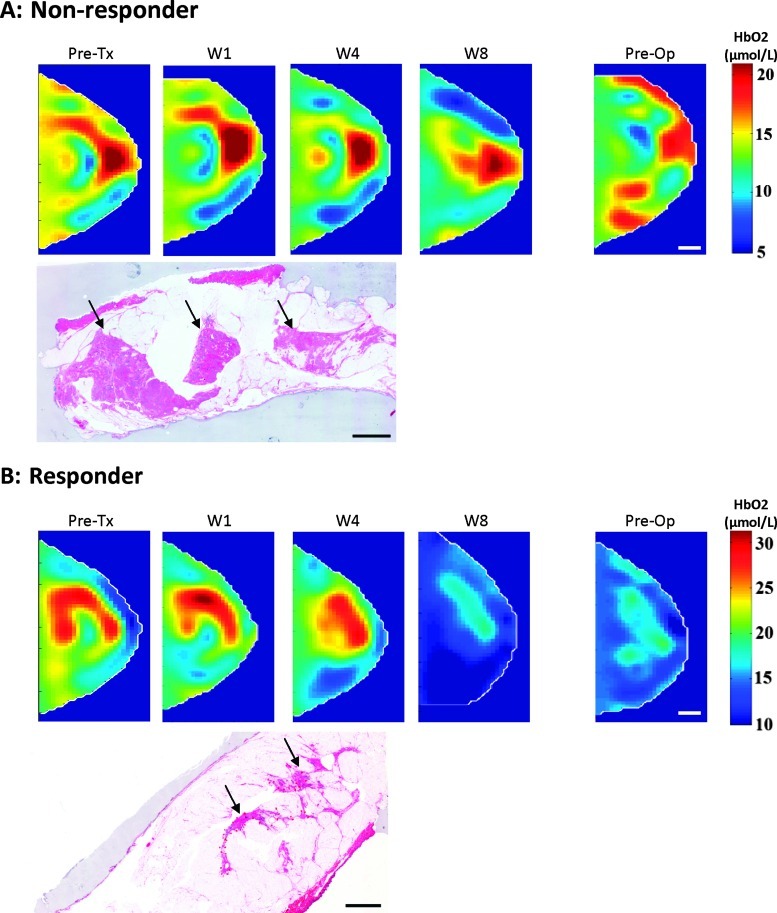
Figure 1.
Transverse DOS images of the breast in a craniocaudal direction and whole-mount pathology. A representative nonresponder is shown in A and a responder in B. The cross section for each time point was chosen at the center of the tumor. The color bar shows oxyhemoglobin concentrations (µmol/L). The area of residual disease in the nonresponder and responder is identified by the black arrow. Scale bars, 1 cm.
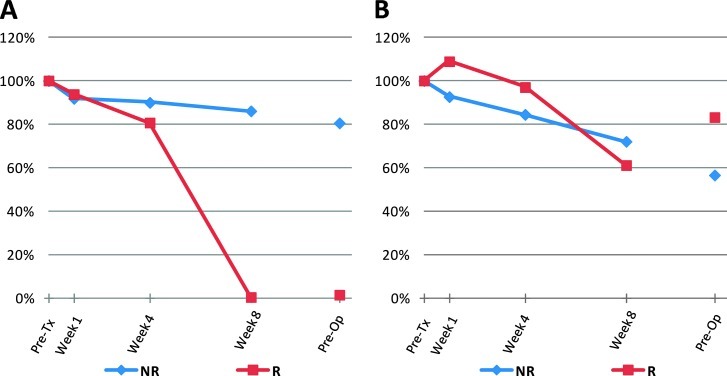
Figure 2.
Comparison of the changes in oxyhemoglobin level between the nonresponder and the responder of Figure 1. Volume-weighted method is shown in A and the whole-breast method is shown in B. The dots between week 8 and preoperative scans were not connected because of the variation in the duration between the two scans for each patient. The preoperative scan varied depending on the operation date for each patient.
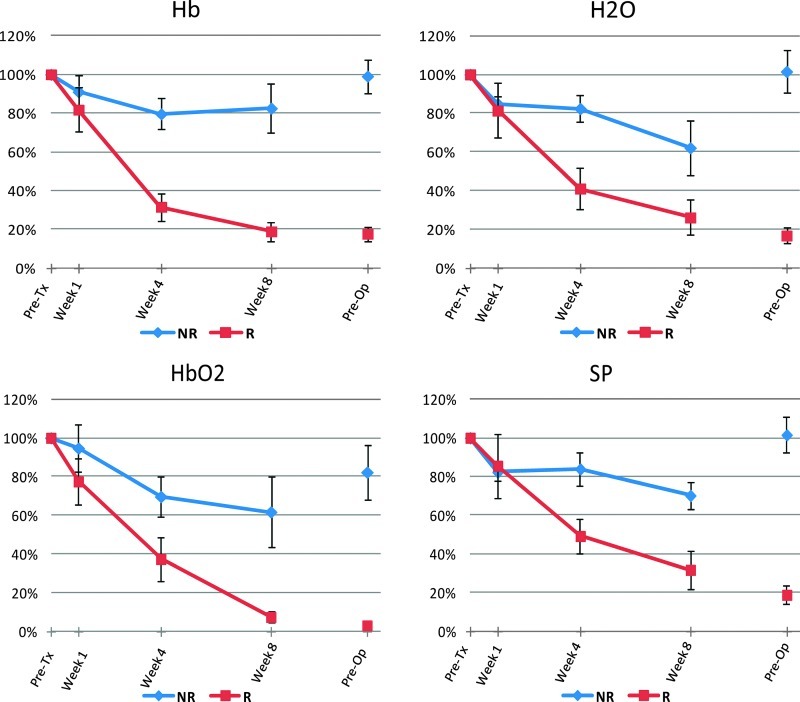
Figure 3.
Changes in deoxyhemoglobin, oxyhemoglobin, water, and scatter power measured in nonresponders and responders using the volume-weighted method. The dots between week 8 and preoperative scans were not connected because of the variation in the duration between the two scans for each patient. The preoperative scan varied depending on the operation date for each patient. Bars, SE.
Table 3.
Discriminant Analysis.
| Parameter | Responders (Mean ± SE; %) | Nonresponders (Mean ± SE; %) | P | Sensitivity (%) | Specificity (%) |
| (A) Volume-weighted method at 1 week | |||||
| Hb | 82 ± 11 | 91 ± 8 | 0.6 | 83 | 75 |
| HbO2 | 77 ± 12 | 95 ± 12 | 0.4 | 67 | 75 |
| H2O | 81 ± 14 | 85 ± 4 | 0.8 | 50 | 50 |
| SP | 86 ± 16 | 82 ± 5 | 0.9 | 50 | 50 |
| (B) Volume-weighted method at 4 weeks | |||||
| Hb | 32 ± 7 | 80 ± 8 | 0.005* | 100 | 100 |
| HbO2 | 37 ± 11 | 70 ± 10 | 0.1 | 67 | 100 |
| H2O | 41 ± 11 | 82 ± 7 | 0.04* | 83 | 100 |
| SP | 49 ± 9 | 84 ± 9 | 0.046* | 83 | 100 |
| (C) Whole-breast method at 1 week | |||||
| Hb | 117 ± 7 | 86 ± 9 | 0.04* | 100 | 83 |
| HbO2 | 108 ± 8 | 82 ± 7 | 0.04* | 75 | 83 |
| HbT | 110 ± 7 | 83 ± 7 | 0.03* | 75 | 83 |
| H2O | 111 ± 11 | 71 ± 7 | 0.01* | 75 | 100 |
| Li | 107 ± 5 | 96 ± 10 | 0.6 | ||
| StO2 | 105 ± 5 | 87 ± 8 | 0.1 | 75 | 67 |
| St | 117 ± 7 | 90 ± 12 | 0.1 | 75 | 83 |
| SA | 113 ± 6 | 96 ± 12 | 0.3 | 75 | 83 |
| SP | 103 ± 14 | 70 ± 8 | 0.06 | 75 | 83 |
| TOI | 116 ± 15 | 68 ± 9 | 0.02* | 75 | 83 |
| (D) Whole-breast method at 4 weeks | |||||
| Hb | 114 ± 6 | 72 ± 6 | 0.002* | 100 | 80 |
| HbO2 | 97 ± 13 | 66 ± 5 | 0.05 | 75 | 80 |
| HbT | 101 ± 10 | 68 ± 5 | 0.02* | 75 | 80 |
| H2O | 99 ± 13 | 71 ± 7 | 0.09 | 75 | 100 |
| Li | 113 ± 11 | 85 ± 7 | 0.07 | 75 | 80 |
| StO2 | 101 ± 14 | 79 ± 4 | 0.1 | ||
| St | 125 ± 7 | 86 ± 5 | 0.002* | 100 | 100 |
| SA | 116 ± 15 | 83 ± 7 | 0.07 | 50 | 80 |
| SP | 92 ± 12 | 72 ± 4 | 0.1 | 50 | 80 |
| TOI | 99 ± 13 | 63 ± 9 | 0.05 | 100 | 80 |
For C, normality violation was found for the Li parameter, and Mann-Whitney was used to calculate the P value. The discriminant analysis was not applicable.
For D, normality violation was found for the StO2 parameter, and Mann-Whitney was used to calculate the P value. The discriminant analysis was not applicable.
Significant result.
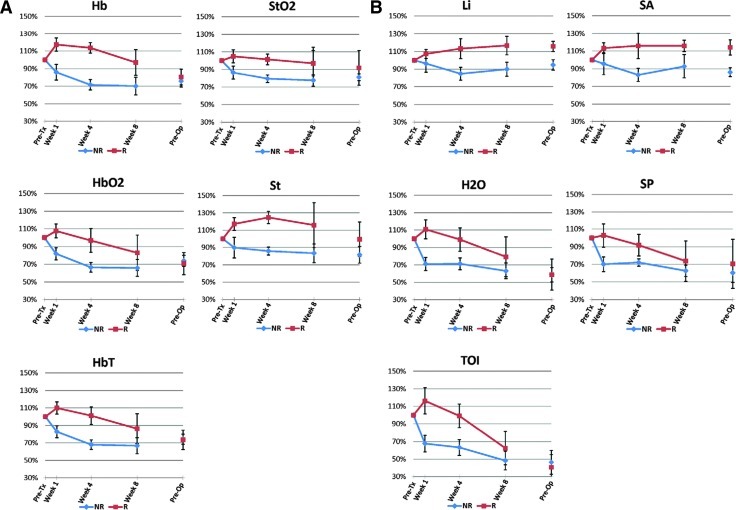
Figure 4 demonstrates the changes in 10 optical parameters between responders and nonresponders divided into blood-related and other parameters using the whole-breast method. The values as a percentage of the pretreatment scan for each of the 10 optical parameters at 1 and 4 weeks of time, respectively, are presented in Table 3, C and D. Non-responders and responders were found to be significantly different at 1 week after treatment initiation for Hb (P = .04), HbO2 (P = .04), HbT (P = .03), H2O (P = .01), and TOI (P = .02) parameters. However, only Hb (P = .002), HbT (P = .02), and St (P = .002) were found to be significantly different at 4 weeks after initiation of treatment.
Figure 4.
Changes in DOS parameters measured in nonresponders and responders using the whole-breast method. (A) Blood-related parameters: Hb, HbO2, HbT, StO2, and St. (B) Other parameters: Li, H2O, TOI, SA, and SP. The dots between week 8 and preoperative scans were not connected because of the variation in the duration between the two scans for each patient. The preoperative scan varied depending on the operation date for each patient. Bars, SE.
A linear mixed model with repeated-measures analysis was also performed to determine the statistical significance of each optical parameter. The mean percent change for Hb was 16.0% (SE = 5.5) in responders versus -21.5% (SE = 6.2) in nonresponders, where it exhibited a statistically significant difference (P = .001). The mean percent change for HbO2 was 2.8% (SE = 9.3) in responders versus -28.0% (SE = 4.6) in nonresponders. This difference was also statistically significant (P = .002). The mean percent change for H2O was 11% (SE = 9.0) in responders versus -29.3% (SE = 5.3) in non-responders and was statistically significant (P = .001). The mean percent change for St was 20.8% (SE = 2.5) in responders versus -11.7% (SE = 7.2) in nonresponders and was also statistically significant (P = .004). The mean percent change for HbT was 5.7% (SE = 7.7) in responders versus -26.3% (SE = 4.7) in nonresponders and was statistically significant (P = .002). The mean percent change for SP was 1.7% (SE = 11.1) in responders versus -29.7% (SE = 5.0) in nonresponders and was also statistically significant (P = .001). The mean percent change for TOI was 11.7% (SE = 12.5) in responders versus -33.4% (SE = 6.8) in nonresponders. This difference was statistically significant as well (P < .001). Li, StO2, and SA were found not to be statistically significant (P > .05).
Sensitivity and specificity for all parameters are also presented at 1 and 4 weeks' time in Table 3. The best predictor of therapeutic response of the volume-weighted method was Hb, particularly at 4 weeks' time, where 100% for both sensitivity and specificity were achieved. For the whole-breast method, both Hb (with 100% sensitivity, 83% specificity) and H2O (with 75% sensitivity, 100% specificity) were found to give a good prediction of the ultimate clinical-pathologic treatment response as early as 1 week after the start of treatment. At 4 weeks' time, oxygen desaturation was found to be the best predictor of the therapeutic response, with 100% for both sensitivity and specificity. Values seem to initially increase for responding patients.
Discussion
DOS is a noninvasive, nonionizing technique that uses near-infrared light to rapidly provide functional and structural tissue information. It does not require the use of exogenous markers, which makes it ideal for repetitive scans during treatment monitoring. Previous studies have demonstrated the feasibility of using DOS for monitoring treatment in patients with LABC. A study on 11 patients receiving identical ariamycin/cytoxan (AC) treatments [15] indicated significant reductions in tumor concentrations of HbT (27% ± 15%), HbO2 (33% ± 7%), and H2O (11% ± 15%) for responders when compared with pretreatment concentrations within 1 week of the start of the treatment. Nonresponders showed no significant changes for these parameters. A recent study [25] on 10 patients with greater diversity in disease extent and treatment plans demonstrated large drops from baseline of 68% ± 21%, 59% ± 20%, 51% ± 28%, and 53% ± 26% in Hb, HbO2, H2O, and SP for responders 4 weeks after treatment initiation, respectively. Small changes of only 18% ± 10%, 18% ± 21%, 15% ± 12%, and 13% ± 10% were observed in these parameters for non-responding subjects 4 weeks after initiating treatment.
In the study here, we report the results of a larger cohort (n = 15) verifying our earlier findings [25] but also investigating whole-breast DOS effects. The overall trends of DOS parameters of interest (Figure 3) were found to agree with our previous results [25]. After 4 weeks of treatment initiation, the mean Hb content was 32% ± 7% for responders versus 80% ± 8% for nonresponders. The difference was found to be statistically significant (P = .005). The mean percent in HbO2 was 37% ± 11% for responders versus 70% ± 10% for non-responders. The difference was found to only approach significance (P = .1). The mean H2O content was 41% ± 11% for responders versus 70% ± 10% for nonresponders (difference, P = .04), whereas the mean SP content was 49% ± 9% for responders versus 84% ± 9% for nonresponders (difference, P = .046). These results are encouraging and provide further support to the potential of using DOS to monitor treatment response in patients with LABC. As the tumor begins to respond to treatment, the hemoglobin decreases, which may represent a reduction in tumor oxygen consumption due to cell death. The decrease in oxyhemoglobin is potentially associated with the diminishment or the normalization of vascular supply or a reduction in pooled hypoxic blood. Water and scatter power may decrease as a result of loss of cellularity and diminished tumor edema [31].
The use of the whole-breast measurements to calculate the changes in optical parameters (whole-breast method) leads to some interesting findings. With the exception of patients 8 and 9 (discussed further in the next paragraphs), DOS parameters (Hb, HbO2, HbT, H2O, SP, and TOI) in responders exhibited a sharp increase during the first few days of the treatment and then started to drop significantly with treatment time as presented in Figure 4. In contrast, for nonresponders, most of the changes in optical parameters were marked by a small initial drop followed by a relatively slow diminishment. These whole-breast changes were found to be statistically significant for Hb (P = .04), HbO2 (P = .04), HbT (P = .03), H2O (P = .01), and TOI (P = .02) as early as 1 week from the start of the treatment. Only changes in Hb, HbT, and St were significant once the treatment reached the week 4 time point (Table 3). These findings suggest that changes in oxygen consumption, vascularity, and tissue structure in the whole breast start to occur as early as 1 week from the treatment initiation. At 4 weeks, changes in oxygen consumption are reflected by changes in Hb, HbT, and St, which provided a good separation between responders and non-responders. The fast rise in HbO2 for responders during the first week of the treatment is consistent with the recent findings reported by Roblyer et al. [28]. In their study on 24 patients with LABC receiving various treatment regimens, a significant flare in HbO2 was observed in pathologic complete responding (n = 8) and partially responding tumors (n = 11), opposed to nonresponders (n = 5), which exhibited a decrease in HbO2 levels on day 1 after treatment initiation. They suggested that such a oxyhemoglobin flare is likely due to a decrease in HbO2 conversion to Hb as a result of a decrease in cellular metabolism and/or increased tissue perfusion. As an alternative explanation, we suggest that this may be related to the active metabolism associated with cell death. This would require adenosine triphosphate (ATP) and glucose, which would be provided by an increase in blood flow, leading to an edematous and erythematous breast, often seen clinically in responders.
The trends observed in the changes of optical parameters in patients 8 and 9 were found to be similar to those of nonresponding patients. These patients had negligible invasive tumor remaining and were rated by the pathologist as good responders. However, in these patients, despite the significant reduction in the size of the invasive disease, the overall tumor size remained relatively large due an abundance of ductal carcinoma in situ cells in the breast. In addition, they were the only two patients who were both ER/PR. and Her-2-neu-positive and had carboplatinum or radiation, both of which can create DNA cross-link lesions. In these subjects, in addition to DOS measurements, patients' characteristics such as age, menopausal status, tumor composition and grade, ER/PR, Her- 2-neu, and neoadjuvant treatment may provide useful information to classify therapy response. This will be investigated in the future once more subjects with large in situ components are available.
The whole-breast method was found to be superior to the volume-weighted method because it permitted the distinction between responders and nonresponders as early as 1 week after treatment initiation when using deoxyhemoglobin (with 100% sensitivity, 83% specificity) and percent water (with 75% sensitivity, 100% specificity) as predictors of treatment response as shown in Table 3. At 4 weeks after start of treatment, 100% sensitivity and 100% specificity for deoxyhemoglobin and oxygen desaturation were achieved using the volume-weighted and whole-breast methods, respectively. These results were generally consistent and seemed to be independent of molecular drug mechanisms but rather relate to, if ultimately, cell death in the tumor was or was not induced. The different chemotherapy drugs used lead ultimately either to apoptosis or to necrosis. We hypothesize that the results of the whole-breast method may be due to concurrent changes in the optical parameters measured within the tumor in addition to the surrounding normal breast tissue over the treatment course as reported by previous investigators [24] or potentially a metabolic flare.
The whole-breast method introduced in this work offers a potential major advantage over previous methods [25,28]. The use of the whole-breast measurements to calculate the changes in optical parameters does not require any knowledge of the tumor size and location and no tumor segmentation and/or registration algorithms are needed to identify the tumor, which makes this method simple and ideal for clinical settings. This method may be dependent on tumor size but seemed to be appropriate for LABCs because changes in optical parameters occur primarily in the tumor.
In conclusion, regardless of the treatment regimens, changes in the DOS parameters corresponded well to the clinical and whole-mount pathologic response. This makes DOS potentially ideal for real-life scenarios where treatment regimens are customized based on individual clinical circumstances in women with LABC. A whole-breast method for data analysis was introduced here in which a study on 15 patients showed that five of the parameters were shown to be able to distinguish responders from nonresponders as early as 1 week after treatment initiation. This work indicates that it is possible to use this method to begin to customize treatment regimens. It can also permit the objective rational of change from ineffective therapies to effective ones early (within days to weeks) as opposed to many months later, which can be potentially too late.
Acknowledgments
The authors thank ART Canada for providing technical support.
Footnotes
This project was funded by the Canadian Breast Cancer Foundation - Ontario Region through a research grant to G.J.C. and through a fellowship to O.F. Funding for this work was also provided by the Terry Fox Foundation and Natural Sciences and Engineering Research Council of Canada. This research was also supported through a Cancer Care Ontario Research Chair in experimental therapeutics and imaging awarded to G.J.C.
References
- 1.American Cancer Society, author. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 2.Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM, Cristofanilli M. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer. 2011;117:1819–1826. doi: 10.1002/cncr.25682. [DOI] [PubMed] [Google Scholar]
- 3.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, Bruning P, Cufer T, Bonnefoi H, Tomiak E, Pritchard KI, Hamilton A, Piccart MJ, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 4.Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999;13:457–472. vii. doi: 10.1016/s0889-8588(05)70065-4. [DOI] [PubMed] [Google Scholar]
- 5.De Lena M, Varini M, Zucali R, Rovini D, Viganotti G, Valagussa P, Veronesi U, Bonadonna G. Multimodal treatment for locally advanced breast cancer. Result of chemotherapy-radiotherapy versus chemotherapy-surgery. Cancer Clin Trials. 1981;4:229–236. [PubMed] [Google Scholar]
- 6.Hortobagyi GN. Comprehensive management of locally advanced breast cancer. Cancer. 1990;66:1387–1391. doi: 10.1002/1097-0142(19900915)66:14+<1387::aid-cncr2820661414>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 8.Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, Halpern E, Moore R, Kuter I, Taghian A. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 9.Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol. 1999;17:110–119. doi: 10.1200/JCO.1999.17.1.110. [DOI] [PubMed] [Google Scholar]
- 10.Belli P, Costantini M, Malaspina C, Magistrelli A, Latorre G, Bonomo L. MRI accuracy in residual disease evaluation in breast cancer patients treated with neoadjuvant chemotherapy. Clin Radiol. 2006;61:946–953. doi: 10.1016/j.crad.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 12.Tiling R, Linke R, Untch M, Richter A, Fieber S, Brinkbaumer K, Tatsch K, Hahn K. 18F-FDG PET and 99mTc-sestamibi scintimammography for monitoring breast cancer response to neoadjuvant chemotherapy: a comparative study. Eur J Nucl Med. 2001;28:711–720. doi: 10.1007/s002590100539. [DOI] [PubMed] [Google Scholar]
- 13.Ntziachristos V, Chance B. Probing physiology and molecular function using optical imaging: applications to breast cancer. Breast Cancer Res. 2001;3:41–46. doi: 10.1186/bcr269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerussi A, Hsiang D, Shah N, Mehta R, Durkin A, Butler J, Tromberg BJ. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci USA. 2007;104:4014–4019. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N, Gibbs J, Wolverton D, Cerussi A, Hylton N, Tromberg BJ. Combined diffuse optical spectroscopy and contrast-enhanced magnetic resonance imaging for monitoring breast cancer neoadjuvant chemotherapy: a case study. J Biomed Opt. 2005;10:051503. doi: 10.1117/1.2070147. [DOI] [PubMed] [Google Scholar]
- 17.Holboke MJ, Tromberg BJ, Li X, Shah N, Fishkin J, Kidney D, Butler J, Chance B, Yodh AG. Three-dimensional diffuse optical mammography with ultrasound localization in a human subject. J Biomed Opt. 2000;5:237–247. doi: 10.1117/1.429992. [DOI] [PubMed] [Google Scholar]
- 18.Shah N, Cerussi A, Eker C, Espinoza J, Butler J, Fishkin J, Hornung R, Tromberg B. Noninvasive functional optical spectroscopy of human breast tissue. Proc Natl Acad Sci USA. 2001;98:4420–4425. doi: 10.1073/pnas.071511098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tromberg BJ, Cerussi A, Shah N, Compton M, Durkin A, Hsiang D, Butler J, Mehta R. Imaging in breast cancer: diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005;7:279–285. doi: 10.1186/bcr1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zonios G, Perelman LT, Backman V, Manoharan R, Fitzmaurice M, Van Dam J, Feld MS. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Appl Opt. 1999;38:6628–6637. doi: 10.1364/ao.38.006628. [DOI] [PubMed] [Google Scholar]
- 21.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 22.Vaupel P, Hockel M. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: characterization and therapeutic relevance. Int J Oncol. 2000;17:869–879. doi: 10.3892/ijo.17.5.869. [DOI] [PubMed] [Google Scholar]
- 23.Choe R, Corlu A, Lee K, Durduran T, Konecky SD, Grosicka-Koptyra M, Arridge SR, Czerniecki BJ, Fraker DL, DeMichele A, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Med Phys. 2005;32:1128–1139. doi: 10.1118/1.1869612. [DOI] [PubMed] [Google Scholar]
- 24.Jakubowski DB, Cerussi AE, Bevilacqua F, Shah N, Hsiang D, Butler J, Tromberg BJ. onitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: a case study. J Biomed Opt. 2004;9:230–238. doi: 10.1117/1.1629681. [DOI] [PubMed] [Google Scholar]
- 25.Soliman H, Gunasekara A, Rycroft M, Zubovits J, Dent R, Spayne J, Yaffe MJ, Czarnota GJ. Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer. Clin Cancer Res. 2010;16:2605–2614. doi: 10.1158/1078-0432.CCR-09-1510. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C, Choe R, Shah N, Durduran T, Yu G, Durkin A, Hsiang D, Mehta R, Butler J, Cerussi A, et al. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J Biomed Opt. 2007;12:051903. doi: 10.1117/1.2798595. [DOI] [PubMed] [Google Scholar]
- 27.Clarke GM, Eidt S, Sun L, Mawdsley G, Zubovits JT, Yaffe MJ. Whole-specimen histopathology: a method to produce whole-mount breast serial sections for 3-D digital histopathology imaging. Histopathology. 2007;50:232–242. doi: 10.1111/j.1365-2559.2006.02561.x. [DOI] [PubMed] [Google Scholar]
- 28.Roblyer D, Ueda S, Cerussi A, Tanamai W, Durkin A, Mehta R, Hsiang D, Butler JA, McLaren C, Chen WP, et al. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proc Natl Acad Sci USA. 2011;108:14626–14631. doi: 10.1073/pnas.1013103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khayat M, Ichalalene Z, Mincu N, Leblond F, Guilman O, Djeziri S. Optical tomography as adjunct to x-ray mammography: methods and results. In: Azar FS, editor. Multimodal Biomedical Imaging II. San Jose, CA: SPIE-The International Society for Optical Engineering; 2007. pp. 64310F-1–64310F-12. [Google Scholar]
- 30.Mincu N, Djeziri S, Ichalalene Z, Leblond F, Khayat M. Sensitivity and repeatability of diffuse optical tomography: towards breast cancer neoadjuvant treatment monitoring. In: Chance B, Alfano RR, Tromberg BJ, Tamura M, Sevick-Muraca EM, editors. Optical Tomography and Spectroscopy of Tissue VII; Proceedings of SPIE he International Society for Optical Engineering; San Jose, CA. 2007. pp. 64341D-1–64341D-9. [Google Scholar]
- 31.Tromberg BJ, Cerussi AE. Imaging breast cancer chemotherapy response with light. Commentary on Soliman et al., p. 2605. Clin Cancer Res. 2010;16:2486–2488. doi: 10.1158/1078-0432.CCR-10-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]