Abstract
Oxidative stress participates in doxorubicin (Dx)-induced cardiotoxicity. The metal complex MnDPDP and its metabolite MnPLED possess SOD-mimetic activity, DPDP and PLED have, in addition, high affinity for iron. Mice were injected intravenously with MnDPDP, DPDP, or dexrazoxane (ICRF-187). Thirty minutes later, mice were killed, the left atria were hung in organ baths and electrically stimulated, saline or Dx was added, and the contractility was measured for 60 minutes. In parallel experiments, 10 µM MnDPDP or MnPLED was added directly into the organ bath. The effect of MnDPDP on antitumor activity of Dx against two human tumor xenografts (MX-1 and A2780) was investigated. The in vitro cytotoxic activity was studied by co-incubating A2780 cells with MnDPDP, DPDP, and/or Dx. Dx caused a marked reduction in contractile force. In vivo treatment with MnDPDP and ICRF-187 attenuated the negative effect of Dx. When added directly into the bath, MnDPDP did not protect, whereas MnPLED attenuated the Dx effect by approximately 50%. MnDPDP or ICRF-187 did not interfere negatively with the anti-tumor activity of Dx, either in vivo or in vitro. Micromolar concentrations of DPDP but not MnDPDP displayed an in vitro cytotoxic activity against A2780 cells. The present results show that MnDPDP, after being metabolized to MnPLED, protects against acute Dx cardiotoxicity. Both in vivo and in vitro experiments show that cardioprotection takes place without interfering negatively with the anticancer activity of Dx. Furthermore, the results suggest that the previously described cytotoxic in vivo activity of MnDPDP is an inherent property of DPDP.
Introduction
The anthracycline doxorubicin (Adriamycin; Dx) is one of the best agents for treating human hematological malignancies and solid tumors. Its use is, however, restricted by dose-limiting cardiotoxicity [1,2]. This problem with anthracycline-induced chronic heart failure has lately been shown in retrospective studies to be even more serious than previously believed. Chronic anthracycline-induced cardiotoxicity is associated with a poor prognosis for the affected patients, and their survival seems to be worse than that of patients with ischemic cardiomyopathy. At present, there is no specific evidence-based treatment of anthracycline-induced chronic heart failure [3], and for patients with end-stage heart failure, heart transplantation remains the only option [4].
The molecular pathogenesis of anthracycline-induced cardiotoxicity remains controversial, although the oxidative stress-based hypothesis involving myocardial production of reactive oxygen species has gained the widest acceptance [2]. There is substantial evidence that superoxide anions (·O2-) [2,5] and iron [6] play key roles in the underlying deleterious effects. Many years ago, Doroshow et al. [7] suggested that cardiac tissue has weak antioxidant activity because it more or less lacks catalase. Furthermore, they showed that Dx selectively downregulates glutathione peroxidase. The relative deficiency of cardiac antioxidant defense, includes also superoxide dismutase (SOD) [7,8], which has been implicated as important in myocardial ischemia and reperfusion [9] and in xenobiotic-induced cardiac injury [5,10]. In fact, Dx has, for a long time, been considered as a prototypical model for oxygen-derived free radical-mediated cardiotoxicity [10]. The SOD mimetic AEOL 10150 was recently shown to effectively protect cardiac function from Dx-induced oxidative stress in EC-SOD knockout mice [11].
It has been known for many years that anthracycline-induced cardiotoxicity can be reduced by simultaneous administration of the intracellular iron chelator dexrazoxane (ICRF-187), presumably by reducing oxidative stress, allowing maximum cumulative doses of ICRF-187 to be doubled [12]. However, the use of ICRF-187 has been highly restricted because of suspicion that it may also protect cancer cells against Dx and hence reduce the anticancer efficacy.
MnDPDP (mangafodipir; Teslascan) is an approved magnetic resonance imaging contrast agent for use in humans that has been on the market since 1997 but was, for commercial reasons, withdrawn in 2010. During the development of mangafodipir, it was serendipitously discovered that it had profound antioxidant properties [13], presumably through its superoxide dismutase mimetic activity and high iron-binding capability [14–16]. Manganese dipyridoxyl ethylenediamine (MnPLED), a metabolite of MnDPDP, has been shown to reduce myocardial ischemia-reperfusion injury in anesthetized pigs [17,18]. Over the past decades, it has been demonstrated that increase in oxidative stress, unrelated to known drug metabolism pathways, usually occurs after exposure to a series of structurally unrelated anticancer agents, including oxaliplatin, 5-fluorouracil, and paclitaxel [19]. The mechanism(s) of initiation of reactive oxygen production during exposure by different cancer chemotherapeutic agents is, however, unclear.
Interestingly, Laurent et al. [20] and Alexandre et al. [21] have shown both in vitro and in vivo that MnDPDP protects non-cancer cells, like blood cells, against oxidative stress induced by oxaliplatin, 5-fluorouracil, and paclitaxel without interfering negatively with the antitumor activity. On the contrary, MnDPDP potentiates the antitumor effects of these chemotherapeutics and displays an anticancer effect of its own. These results are of interest because they suggest that at least some of the toxic effects of secondary reactive oxygen production after exposure to anticancer agents can be pharmacologically ameliorated, without diminishing the anticancer efficacy. This inspired James Doroshow from the National Cancer Institute to write an editorial in the Journal of the National Cancer Institute [19] where he suggested that MnDPDP should be tested in cancer patients. A first small feasibility study in patients with colon cancer on adjuvant oxaliplatin plus 5-fluorouracil (FOLFOX) chemotherapy suggests that MnDPDP protects against the dose-limiting toxicity of FOLFOX [22].
In the present study, the protective effect of MnDPDP against Dx-induced cardiotoxicity was examined and compared with that of ICRF-187. The anticancer activity of Dx in the presence of MnDPDP was also studied both under in vivo (A2780 and MX-1 xenografts) and under in vitro (A2780 and MX-1 cells) conditions.
Materials and Methods
Animals
Male CD mice, 25 ± 5 g (Charles River, Sulzfeld, Germany, and Ry, Denmark) were used in the experiment designed to study the cardioprotective effects of the test substance and female CD-1 nu/nu mice (Charles River Laboratories, Calco, Italy; aged 7 weeks at the start of the experiment). Animals received humane care in compliance with institutional guidelines.
Drugs and Cell Lines
Dx and ICRF-187 were from Pharmacia-UpJohn (Milan, Italy) or Sigma (St Louis, MO) and Teslascan (ready-to-use MnDPDP formulation). Teslascan, MnDPDP, DPDP, and MnPLED were provided by Nycomed Imaging AS (Oslo Norway; now GE Healthcare). A2780 cells (human ovarian carcinoma; European Collection of Cell Cultures, Porton Down, United Kingdom) and MX-1 cells (human breast carcinoma; CLS, Eppelheim, Germany) were cultured in RPMI-1640 media or Dulbecco modified Eagle medium/HAM F-12 media (1:1), respectively, supplemented with 10% (vol/vol) fetal bovine serum (PAA, Pasching, Austria), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin, at 37°C in humidified air with 5% CO2. Cell culture media and supplements were from Invitrogen (Paisley, United Kingdom).
In Vivo/In Vitro Cardioprotective Effects of MnDPDP and MnPLED
Male CD mice were injected intravenously (i.v.) with various doses of saline (controls), MnDPDP, DPDP, and ICRF-187. Thirty minutes later, mice were killed, and the left atria were hung in 20-ml organ baths filled with 37°C Krebs buffer and stimulated with 4-Hz, 3-millisecond supramaximal pulses (Stimulator 215/I; HSE, March, Germany). Contractions were recorded isometrically by means of conventional force transducers (F30, HSE). After equilibration (30 minutes), saline or 120 µM Dx (dose selected from pilot experiments) was added, and contractility was measured for 60 minutes.
In parallel experiments, MnDPDP or MnPLED at the indicated concentrations was added directly into organ baths containing isolated atria from untreated animals, 30 minutes before Dx addition. In subsequent experiments using a simpler experimental setup containing no reusable plastic materials, the atria displayed a much higher sensitivity against Dx; 36 µM Dx resulted in more than 70% reduction in contractile force.
Effects of MnDPDP, MnPLED, and DPDP on the Iron-Driven Fenton Reaction
Ferric iron in the form of FeCl3 (10 µM) was partially reduced to its ferrous form by cysteine (100 µM) in 150 mM acetate buffer, pH 5.0. H2O2 (100 µM) was added to initiate the production of hydroxyl radicals (·OH), as described previously [23]. Briefly, ·OH oxidizes H2DCF (nonfluorescent 2′,7′-dichlorodihydrofluorescein; 5µM) to fluorescent DCF (2′,7′-dichlorofluorescein). H2DCF was obtained by hydrolyzing its acetate ester (H2DCF-DA). Dimethyl sulfoxide (10%) and desferrioxamine (DFO) (10 µM) were used to demonstrate the formation of ·OH and the involvement of iron, respectively. MnDPDP, MnPLED, and DPDP at various concentrations were assayed for their inhibitory actions on the Fenton reaction. Fluorescence was measured in an FL600 Microplate Fluorescence reader (Bio-Tek, Winooski, VT) at an excitation of 485 nm and emission of 530 nm.
In Vivo Antitumor Activity of Dx in the Absence and Presence of MnDPDP or ICRF-187
Two human tumor lines A2780 and MX-1 were used. The ovarian carcinoma A2780 has a tumor doubling time of approximately 2 days and is sensitive to Dx (tumor volume inhibition, ∼90%). The mammary carcinoma MX-1 has a doubling time of approximately 5 days and is marginally sensitive to Dx (tumor volume inhibition, ∼50%). Tumor xenograft fragments (2–3 mm in diameter) were implanted subcutaneously in both flanks of nude mice (day 0). Therapy started when the mean diameters of the growing tumors were 5 to 8 mm. This was day 4 for both tumor lines.
Three injections of Dx at a dose of 7 mg/kg per injection were given i.v. with a weekly interval. This is the maximum tolerated dose in tumor-bearing nude mice of the strain used, taking into account late toxicity. The test substances were given i.v. 30 minutes before each injection of Dx. ICRF-187 was administered at a dose of 50 mg/kg (186 µmol/kg), MnDPDP at a dose of 10 µmol/kg, and Teslascan (ready-to-use MnDPDP formulation) at doses of 5, 10, and 20 µmol/kg. Each experimental group contained five mice.
In Vitro Cytotoxic Activity of Dx, MnDPDP, and/or DPDP
The cytotoxic activity of DPDP and MnDPDP was compared by co-incubating A2780 and MX-1 cells with MnDPDP, DPDP, and/or Dx. The viability of cells was measured using the methylthiazoletetrazolium (MTT) assay. Briefly, 8000 cells were seeded per well on a 96-well plate and grown overnight under standard conditions. Cells were then exposed for 48 hours to various concentrations of MnDPDP, DPDP, and/or Dx at 37°C. The viability of the cells was then assessed by adding 5 mg/ml MTT to a final concentration of 0.5 mg/ml and incubating cells for a further 4 hours at 37°C. The blue formazan that is formed by mitochondrial dehydrogenases of viable cells was then dissolved overnight at 37°C by adding 10% SDS and 10 mM HCl to a final concentration of 5% SDS and 5 mM HCl. Finally, the absorbance of the solution was read at 570 nm with a reference at 670 nm in a microplate reader (SpectraMax 340; Molecular Devices, Sunnyvale, CA) connected to an Apple Macintosh computer running the program Softmax Pro V1.2.0 (Molecular Devices). Viability is expressed as percent absorbance (A570nm – A670nm) relative to the untreated control cells.
Calculations and Analysis of Results
All values are given as arithmetic means ± SEM for in vivo/vitro cardioprotection and in vivo tumor growth data or ±SD for in vitro Fenton reaction and cell MTT viability data.
The contractile force during the in vitro atrium experiment is presented as the percentage (mean ± SEM) of the force generated immediately before incubation with Dx (time = 0 minute). The statistical differences between nontreated and atria pretreated with MnDPDP, ICRF-187, or MnPLED were tested by Student's t test.
In vitro responses of Dx, MnDPDP, and/or DPDP with regard to viability are presented as concentration-effect curves. The biphasic concentration-effect curve of Dx was analyzed by fitting the experimental data into a biphasic sigmoidal four- parameter logistic equation (GraphPad Prism version 5.02; GraphPad Software, La Jolla, CA). From this analysis, the low and high pD2 values (negative log of the concentration of Dx that produces half of its maximal inhibition in the two phases, -logIC50) were calculated.
The tumor diameters were measured biweekly with a Vernier caliper; volumes were calculated according to the formula (length x width2) / 2, and tumor growth curves were constructed. Antitumor activity of Dx was assessed as percentage tumor inhibition, according to the formula: 100 - (T/C x 100), where T is the mean tumor weight; and C, the mean tumor weight of control mice. Student's t test was used to test for statistically significant difference between the groups.
Results
In Vivo/In Vitro Cardioprotective Effects of MnDPDP and MnPLED
Table 1 shows that MnDPDP, when injected into the animal before it is killed and before the left atria were removed and placed in an organ bath, protects the heart muscle from the deleterious effects of Dx. Pretreating the animals with MnDPDP at 1 and 10 µmol/kg for 30 minutes produced approximately 40% and 50% protection, respectively (expressed as the relative improvement in contractility at 60 minutes compared with saline-pretreated animals). When the dose of MnDPDP was increased to 30 µmol/kg, the protective effect disappeared (not shown). Equimolar doses of DPDP alone provided much less protection (5%–15%). MnDPDP, at 10 or 30 µM, when added directly into the organ bath containing isolated atria from untreated animals did not protect against Dx (not shown). The reference substance ICRF-187 also protected against Dx-induced cardiotoxicity, although larger doses were required (93 and 186 µmol/kg, corresponding to 25 and 50 mg/kg) compared with MnDPDP (1–10 µmol/kg). The protective effect of ICRF-187 disappeared, like that of MnDPDP, when the dose was further increased to 372 mg/kg (not shown), that is, both these drugs display a bell-shaped dose-response curve.
Table 1.
Effects of In Vivo Pretreatment with MnDPDP and ICRF-187 on Preservation of Contractile Force of the Isolated Mouse Atria during In Vitro Treatment with Dx.
| In Vivo | Saline | Saline | ICRF-187 (93 µmol/kg) | ICRF-187 (186 µmol/kg) | MnDPDP (1 µmol/kg) | MnDPDP (10 µmol/kg) |
| In Vitro: | Saline n = 4 | Dx n = 9 | Dx n = 3 | Dx n = 3 | Dx n = 3 | Dx n = 3 |
| Time (min) | ||||||
| 10 | 96 ± 1 | 79 ± 2 | 96 ± 2 | 92 ± 2 | 93 ± 1 | 93 ± 4 |
| 20 | 94 ± 2 | 58 ± 3 | 88 ± 4 | 82 ± 2 | 75 ± 6 | 84 ± 5 |
| 30 | 93 ± 2 | 49 ± 3 | 73 ± 7 | 73 ± 2 | 68 ± 5 | 75 ± 6 |
| 40 | 91 ± 3 | 42 ± 3 | 68 ± 6 | 65 ± 4 | 60 ± 5 | 68 ± 6 |
| 50 | 88 ± 3 | 38 ± 3 | 62 ± 7 | 63 ± 4 | 56 ± 6 | 62 ± 6 |
| 60 | 87 ± 3 | 35 ± 2 | 58 ± 7† | 60 ± 4† | 54 ± 6‡ | 60 ± 6† |
Contractile force during the experiment is presented as the percentage (mean ± SEM) of the force generated immediately before incubation with 120 µM Dx (time = 0 minute).
P < .001, F test followed by Student's t test; substance/Dx versus saline/Dx.
P < .01, F test followed by Student's t test; substance/Dx versus saline/Dx.
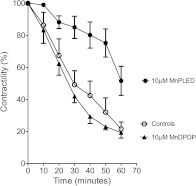
MnPLED, when added directly into the organ baths, resulted in protection in the first series of experiments, but this effect faded in later experiments. In subsequent experiments using a simpler experimental setup containing no reusable plastic materials, the atria displayed a much higher sensitivity against Dx; 36 µM Dx resulted in more than 70% reduction in contractile force. Furthermore, under these experimental conditions, 10 µM MnPLED attenuated the Dx-induced effect by approximately 50% (P < .05), whereas MnDPDP still did not result in any protection (Figure 1). The inconsistency between the results in the first series of experiments and the following is probably due to the retention of the lipophilic MnPLED to plastic holders in the organ baths. In subsequent experiments, these holders would liberate MnPLED in high-enough quantities to mask the effect of externally added MnPLED. Similar problems have been reported for nitroglycerin by Ahlner et al. [24].
Figure 1.
Effects of in vitro pretreatment with MnDPDP and MnPLED on preservation of contractile force of the isolated mouse atria during in vitro treatment with Dx (mean ± SEM; n = 5 for Controls and MnPLED and n = 3 for MnDPDP).
Effects of MnDPDP, MnPLED, and DPDP on the Iron-Driven Fenton Reaction
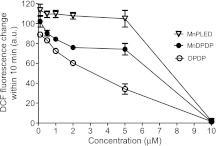
Figure 2 demonstrates that DPDP inhibited the Fenton reaction in a concentration-dependent manner; the inhibition started at 0.1 µM and was complete at 10 µM, that is, at an equimolar concentration of DPDP to that of the iron concentration in the reaction mixture. The inhibitory pattern is in accordance with the reported high-stability constant of DPDP for ferric iron (logK = 33.52) [25].
Figure 2.
The Fenton assay in presence of various concentrations of MnPLED, MnDPDP and DPDP (mean ± SD; n = 3).
The iron-chelating capacity of MnDPDP was significantly higher than that of MnPLED. In the case of MnPLED, no inhibition was evident up to and including a concentration of 5 µM, but at 10 µM, the inhibition was complete. These results are surprising and in opposition to both the reported iron- and manganese-chelating capacity of DPDP and PLED. The reported stability constants between Fe3+ and DPDP and between Fe3+ and PLED are 33.5 and 36.9 (logK), respectively, whereas the reported stability constants between Mn2+ and DPDP and Mn2+ and PLED are 15.1 and 12.6, respectively [25]. One would therefore expect MnPLED to have a higher affinity for Fe3+ and to be a much better inhibitor of the iron-driven Fenton reaction than MnDPDP.
In Vivo Antitumor Activity of Dx in the Absence and Presence of MnDPDP or ICRF-187
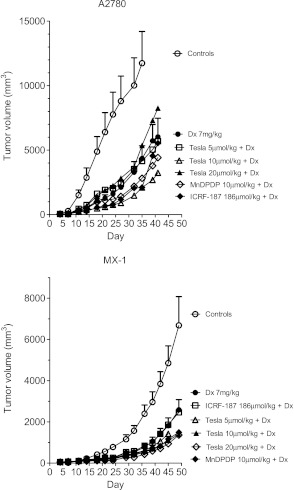
The results of A2780 and MX-1 tumor growth are given in Figure 3. Dx treatment resulted in a tumor weight inhibition (TWI) of 80% in the A2780-bearing mice. No statistically significant difference in TWI was seen in any of the A2780 groups pretreated with 5 or 20 µmol/kg Teslascan or with 10 µmol/kg MnDPDP. However, statistically significant higher TWI values (P ≤ .05) were found in the groups pretreated with 10 µmol/kg Teslascan (TWI = 91%) or 186 µmol/kg (50 mg/kg) ICRF-187 (TWI = 89%) compared to the group treated with Dx alone. The corresponding Dx treatment in MX-1-bearing mice resulted in a TWI of 68%. Closely comparable results were achieved in all other groups, except for the group pretreated with 20 µmol/kg Teslascan where TWI was increased to 83% (P ≤ .05).
Figure 3.
In vivo antitumor activity of Dx in the absence and presence of MnDPDP, Teslascan (Tesla), or dexrazoxane (ICRF-187) in mice bearing A2780 or MX-1 tumors (mean ± SEM; n = 5 in each group).
In Vitro Cytotoxic Activity of Dx, MnDPDP, and/or DPDP on A2780 and MX-1 Cells
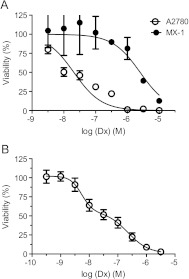
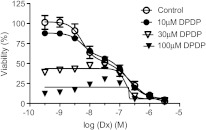
The cytotoxic activity of Dx alone on A2780 and MX-1 cancer cells is presented in Figure 4A. As expected, Dx-sensitive A2780 cells displayed an approximately 100-fold higher sensitivity to Dx than the partially Dx-sensitive MX-1 cells. It is also obvious that the concentration-response curve for A2780 displays more than one phase. When data from subsequent experiments in A2780 cells including some lower Dx concentrations were fitted into a biphasic sigmoidal four parameter logistic equation (Figure 4B), it resulted in two distinct pD2 (-logEC50) values: 8.264 (95% confidence interval = 8.001–8.528) and 6.647 (95% confidence interval = 6.273–7.020), respectively. Although results from MTT tests are not necessarily obtained at steady-state conditions, interestingly, the pD2 values correspond well to the previously described different inhibitory effects of Dx on the topoisomerase II enzyme.
Figure 4.
The cytotoxic effect of increasing concentrations of Dx in A2780 (A and B) and MX-1 cells (A) (mean ± SD; n = 3).
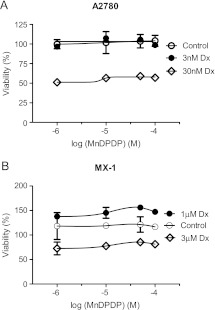
MnDPDP, alone or in combination with Dx at threshold concentrations (3 nM for A2780 and 1 µM for MX-1) and at concentrations around half-maximal effect (30 nM for A2780 and 3 µM for MX-1), did not have any obvious cytotoxic effects in either A2780 or MX-1 cells (Figure 5). Conversely, DPDP alone had cytotoxic effects on A2780 but not on MX-1 cells (Figure 6). Surprisingly, neither Dx at threshold concentrations nor at concentrations around half-maximal effect revealed any additive effect to the cytotoxic effect of DPDP alone in A2780 cells. One would expect to see a clear additive effect around the half-maximal concentrations of these two compounds.
Figure 5.
The cytotoxic effect of MnDPDP in A2780 (A) and MX-1 (B) cells in the absence and presence of various concentrations of Dx (mean ± SD; n = 3).
Figure 6.
The cytotoxic effect of DPDP in A2780 (A) and MX-1 (B) cells in the absence and presence of various concentrations of Dx (mean ± SD; n = 3).
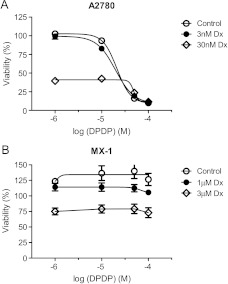
Furthermore, addition of DPDP close to the threshold concentration (10 µM) or the half-maximal concentration (30 µM) did not reveal any obvious additive effect to the cytotoxic effect of Dx alone in A2780 (Figure 7).
Figure 7.
The cytotoxic effect of Dx in the absence and presence of various concentrations of DPDP (mean ± SD; n = 3).
Discussion
The incidence of chronic anthracycline-induced cardiotoxicity and chronic heart failure ranges from 1% to 16% within weeks to months after anthracycline chemotherapy, and it further increases with the length of follow-up [26]. The study by Von Hoff et al. in 1979 [27] estimated that 7% of patients develop Dx-related chronic heart failure after a cumulative dose of 550 mg/m2, and this dose was considered for many subsequent years to be the highest recommended for Dx and daunorubicin. However, the meta-analysis by Swain et al. [28] published in 2003 estimated an incidence as high as 26% of patients at risk for Dx-related chronic heart failure for a cumulative dose of 550 mg/m2. A retrospective analysis in 2006 revealed that compared with the expected values, 30-year childhood cancer survivors had a 15-fold higher rate of heart failure, a 10-fold higher rate of other cardiovascular diseases, and a 9-fold higher rate of stroke [29].
Despite decades of research and testing of thousands of potentially protective agents, only one drug has been approved for use in clinical practice: ICRF-187 [2]. Interestingly, ICRF-187 is not an outcome of any sophisticated and rational drug design. Its cardioprotective activity was discovered accidentally during its preclinical testing as a potential anticancer drug, similar to the serendipitous discovery that the magnetic resonance imaging contrast agent MnDPDP possesses profound cytoprotective activity.
However, ICRF-187 is not recommended at the beginning of Dx chemotherapy in patients with metastatic breast cancer because of suspicion that it may reduce the anticancer effect [30,31]. This possibility has been questioned [32–36], and of 16 published clinical studies, there was only 1 study [37] showing a slightly reduced response rate but no effect on overall survival. Nevertheless, the possibility that ICRF-187 may reduce the anticancer efficacy of anthracyclines requires attention. It is furthermore known that both anthracyclines and ICRF-187 target topoisomerase II, although at different sites on the enzyme [2,34,38,39]. Taking into consideration the immense problem with anthracycline cardiotoxicity, the continuing uncertainty whether ICRF-187, the only clinically approved drug, decreases the efficacy of Dx or not is, of course, highly unsatisfactory. Nevertheless, the results from the present study add to other studies, suggesting that ICRF-187 does not decrease the efficacy of Dx.
The present results suggest that MnDPDP, like ICRF-187, protects against Dx cardiotoxicity. The finding that in vivo administration is a prerequisite for the cardioprotective effect of MnDPDP, but not for its metabolite MnPLED, demonstrates that the compound must be metabolized before becoming active, that is, MnDPDP may be acting as a prodrug. After i.v. injection, MnDPDP is rapidly dephosphorylated to MnPLED [40], which is a much more lipophilic compound than MnDPDP. MnPLED can presumably cross the plasma cellular membrane and exert pronounced cardioprotective effects.
The reason for the bell-shaped dose-response curve of both MnDPDP and ICRF-187, that is, the cardioprotective effect disappeared when the dose was increased, is far from obvious. Laurent et al. [20] suggested that MnDPDP in combination with cytostatic/cytotoxic drugs increases the intracellular level of H2O2 in normal cells from a relatively low level to one where cell survival signals are activated, whereas MnDPDP and cytostatic/cytotoxic drugs increase the intracellular level of H2O2 in cancer cells from a high level to levels where apoptosis is activated. Such a cytoprotective mechanism in normal cells may explain the bell-shaped curve. However, data from a follow-up work by Alexandre et al. [21] did not support the suggestion that MnDPDP potentiates cytostatic/cytotoxic drug.induced increases in the level of H2O2 in normal cells. Although the combination profoundly increased the H2O2 level in cancer cells, MnDPDP decreased it in normal cells.
Many mechanisms have been suggested to explain the anticancer activity of Dx and other anthracyclines [41]. However, several in vitro mechanistic experiments reported in the literature had been performed at concentrations of Dx considered to be far too high compared with peak (Cmax) and steady-state (Css) plasma concentrations observed in patients after standard bolus infusions (≈5 µM and 25–250 nM, respectively). It is therefore concluded that any study involving intact cells exposed to more than 2 µM Dx needs a cautionary reevaluation [42]. The present study clearly demonstrates that cytotoxic effects of Dx are reached at concentrations between 0.3 nM and 3 µM in Dx-sensitive A2780 cells. The concentration-response curve of Dx in A2780 cells reveals two phases, which may correlate to inhibition of the catalytic cycle of topoisomerase II at two different steps [39]. However, because of the non-steady-state conditions in the MTT test, such interpretation must be made with caution.
The DNA cleavage-enhancing Dx stabilizes the DNA-enzyme complex in its cleaved conformation and inhibits resealing [39,41,42], leading to DNA double-strand breaks and cell death. ICRF-187 is also a potent topoisomerase II catalytic inhibitor [36,38,43,44], but in contrast to the DNA cleavage-enhancing drugs, it stabilizes the DNA-enzyme complex in a “closed clamp” conformation, rendering the enzyme less sensitive to the cleavage enhancers [43]. Thus, it inhibits the enzymatic activity without the induction of DNA strand breaks.
Topoisomerase II is nowadays generally recognized to be the main cellular target of anthracyclines [2,39]. Failure to relax the super-coiled DNA blocks DNA replication and transcription. Furthermore, DNA strand breaks trigger the apoptosis of cancer cells, apparently through the p53-dependent pathway. Although some older studies have suggested that formation of reactive oxygen species and lipid peroxidation may contribute to the anticancer effects of anthracyclines, more recent studies do not support this suggestion, and there now seems to be general consensus that oxidative stress is unlikely to contribute significantly to the antitumor activity [42].
Laurent et al. [20] and Alexandre et al. [21] have shown that MnDPDP exerts antitumor effects in colon cancer (CT-26)-bearing mice and profoundly potentiates the antitumor efficacy of oxaliplatin and paclitaxel in these mice. It has in addition been demonstrated that MnDPDP potentiates the cytotoxic effects of oxaliplatin, paclitaxel, and 5-fluorouracil in CT-26 cells [21]. The present study demonstrates that neither MnDPDP nor ICRF-187 interferes negatively with the antitumor activity of Dx in A2780- and MX-1-bearing mice. Although an improved antitumor efficacy was occasionally observed, MnDPDP did not display such a profound potentiating effect on the antitumor activity of Dx as that described by Laurent et al. and Alexandre et al. for oxaliplatin and paclitaxel in CT-26-bearing mice. Furthermore, no in vitro cytotoxic activity of MnDPDP alone or in combination with Dx was seen in either A2780 or MX-1 cells. However, DPDP alone displayed cytotoxic activity in A2780 cells—30 µM DPDP kills approximately 50% of the cancer cells and 100 µM kills almost all cells (Figure 7). DPDP had little—if any—effect on the viability of MX-1 cells.
Interestingly, no obvious additive effect of DPDP was seen on the cytotoxic activity of Dx over its entire concentration-effect curve in A2780 cells. One would expect to see a rather profound additive effect around the concentrations causing half-maximal effects of these two compounds. A speculative and unproven explanation to this may be that DPDP inhibits a step in the topoisomerase II catalytic cycle that differs from the step inhibited by low (<1 µM) Dx concentrations. However, it is still difficult to understand how “one plus one seems to equal one” over the entire concentration range of Dx and DPDP. Interestingly, as described above, ICRF-187 has been revealed to inhibit a step in the topoisomerase II catalytic cycle, which differs from that inhibited by Dx; ICRF-187 inhibits ATP hydrolysis and reopening of the ATPase domain, thereby trapping topological complexes with DNA inside the enzyme [39,44]. This may render the enzyme less sensitive to DNA cleavage enhancers such as Dx. It should, however, be stressed that the present study does not reveal any antagonistic effect of DPDP on the Dx activity in A2780 cells (over a wide range of concentrations of both agents), in a similar way as has been shown with daunorubicin on the topoisomerase II enzyme activity [38]. Hofland et al. [34] have shown antagonistic effects of ICRF-187 on the efficacy of daunorubicin, both under in vitro and under in vivo conditions. However, they did not see any antagonistic effect of ICRF-187 on the Dx efficacy. They speculated that this difference might have been due to longer cellular retention of Dx compared with daunorubicin.
A recent study [36] suggests that Dx as well as ICRF-187 exert both topoisomerase II-dependent and -independent apoptopic effects. This may, in turn, explain the lack of negative interference of ICRF-187 on Dx-induced apoptosis.
The present study used an acute experimental mice model for evaluating the protective potential of MnDPDP against Dx-induced cardiotoxicity. This may be considered as a limiting factor because the main problem with Dx in the clinical setting is chronic heart failure that develops weeks and months after Dx administration. In the case of ICRF-187, however, the immediate reduction in left ventricular ejection fraction seen on Dx administration in patients is in fact considered to be a very good indicator of the risk of developing chronic heart failure [12]. Furthermore, both MnDPDP and the reference substance ICRF-187 protected against Dx in the used mice model.
In conclusion, the present results suggest that MnDPDP, presumably after being metabolized to MnPLED, protects against acute Dx cardiotoxicity, without any negative effect on the anticancer activity of Dx.
Footnotes
This work was supported by a grant from Medical Research Council of Southeast Sweden (FORSS-85191). J.O.G. Karlsson and R. Towart are founders of PledPharma AB and own shares in this company. Karlsson and Towart are inventors on two granted patent families (e.g., US6258828 and US6147094) covering the therapeutic use of mangafodipir in cancer, which are owned by GE Healthcare. Karlsson and R.G.G. Andersson are inventors on two patent applications (WO2009078794 and WO2011004323) covering the therapeutic use of PLED-derivatives in cancer, and T. Kurz is inventor on one of these (WO2009078794). Andersson is a board member of PledPharma AB and has received research funding (less than US $100,000 during the last 5 years) from PledPharma AB. Karlsson is employed by PledPharma AB, and Karlsson and Towart are former employees of GE Healthcare. D. Grant is employed by GE Healthcare.
References
- 1.Shan K, Lincoff AM, Young J. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 3.Lenihan DJ. Diagnosis and management of heart failure in cancer patients. In: Ewer MS, Yeh E, editors. Cancer and the Heart. Hamilton, Ontario, Canada: BC Decker; 2006. pp. 129–138. [Google Scholar]
- 4.Thomas X, Le QH, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81:504–507. doi: 10.1007/s00277-002-0534-8. [DOI] [PubMed] [Google Scholar]
- 5.Wallace KB. Free-radical-mediated chemical cardiomyopathies. In: Wallace KB, editor. Free Radical Toxicology. London, UK: Taylor & Francis Ltd; 1997. pp. 205–221. [Google Scholar]
- 6.Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 2005;68:261–271. doi: 10.1124/mol.105.013383. [DOI] [PubMed] [Google Scholar]
- 7.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Saari JT, Kang YJ. Weak antioxidant defences make the heart a target for damage in copper-deficient rats. Free Radical Biol Med. 1994;17:529–536. doi: 10.1016/0891-5849(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 9.Shattock MJ, Haddock PS. Oxidant stress and the heart: modulation of ion transport mechanisms during ischaemia and reperfusion. In: Black D, Winyard PG, editors. Immunopharmacology of Free Radical Species. London, UK: Academic Press Ltd; 1995. pp. 65–72. [Google Scholar]
- 10.Singal PK, Deally CMR, Weinberg LE. Subcellular effects of adriamycine in heart: a concise review. J Mol Cell Cardiol. 1987;19:817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 11.Kliment CR, Suliman HB, Tobolewski JM, Reynolds CM, Day BJ, Zhu X, McTiernan CF, McGaffin KR, Piantadosi CA, Oury TD. Extracellular superoxide dismutase regulates cardiac function and fibrosis. J Mol Cell Cardiol. 2009;47:730–742. doi: 10.1016/j.yjmcc.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, Dubin N, Ferrans V, Stecy P, Zeleniuch-Jacquotte A, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988;91:745–752. doi: 10.1056/NEJM198809223191203. [DOI] [PubMed] [Google Scholar]
- 13.Asplund A, Grant D, Karlsson JOG. Mangafodipir (MnDPDP)- and MnCl2-induced endothelium-dependent relaxation in bovine mesenteric arteries. J Pharmacol Exp Ther. 1994;271:609–614. [PubMed] [Google Scholar]
- 14.Brurok H, Ardenkjaer-Larsen JH, Hansson G, Skarra S, Berg K, Karlsson JO, Laursen I, Jynge P. Manganese dipyridoxyl diphosphate: MRI contrast agent with antioxidative and cardioprotective properties? Biochem Biophys Res Commun. 1999;254:768–772. doi: 10.1006/bbrc.1998.0131. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson JOG. Antioxidant activity of mangafodipir is not a new finding. J Hepatol. 2004;40:872–873. doi: 10.1016/j.jhep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson JO, Brurok H, Towart R, Jynge P. The magnetic resonance imaging contrast agent mangafodipir exerts antitumor activity via a previously described superoxide dismutase mimetic activity. Cancer Res. 2006;66:598. doi: 10.1158/0008-5472.CAN-05-2053. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson JOG, Brurok H, Eriksen M, Towart R, Toft KG, Moen O, Engebretsen B, Jynge P, Refsum H. Cardioprotective effects of the MR contrast agent MnDPDP and its metabolite MnPLED upon reperfusion of the ischemic porcine myocardium. Acta Radiol. 2001;42:540–547. doi: 10.1080/028418501127347340. [DOI] [PubMed] [Google Scholar]
- 18.Smith HJ. Contrast-enhanced MR imaging in the diagnosis and preservation of cardiac viability. Acta Radiol. 2001;42:539. doi: 10.1080/028418501127347232. [DOI] [PubMed] [Google Scholar]
- 19.Doroshow JH. Redox modulation of chemotherapy-induced tumor cell killing and normal tissue toxicity. J Natl Cancer Inst. 2006;98:223–225. doi: 10.1093/jnci/djj065. [DOI] [PubMed] [Google Scholar]
- 20.Laurent A, Nicco C, Chéreau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 21.Alexandre J, Nicco C, Chereau C, Laurent A, Weill B, Goldwasser F, Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98:236–244. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson JO, Adolfsson K, Thelin B, Jynge P, Andersson RG, Falkmer UG. First clinical experience with the magnetic resonance imaging contrast agent and superoxide dismutase mimetic mangafodipir as an adjunct in cancer chemotherapy—a translational study. Transl Oncol. 2012;5:32–38. doi: 10.1593/tlo.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird SK, Kurz T, Brunk UT. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem J. 2006;15:275–283. doi: 10.1042/BJ20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahlner J, Axelsson KL, Ljusegren ME, Norlander B, Andersson RG. Retention and subsequent liberation of glyceryl trinitrate in organ baths influences the relaxation of bovine mesenteric arteries contracted by various agents. Pharmacol Toxicol. 1987;61:316–319. doi: 10.1111/j.1600-0773.1987.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 25.Rocklage SM, Cacheris WP, Quay SC, Hahn FE, Raymond KN. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′-diacetate 5, 5-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg Chem. 1989;28:477–485. [Google Scholar]
- 26.Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol. 2007;7:122–128. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 27.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 28.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 29.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 30.Hensley ML, Schuchter LM, Lindley C, Meropol NJ, Cohen GI, Broder G, Gradishar WJ, Green DM, Langdon RJ, Jr, Mitchell RB, et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol. 1999;17:3333–3355. doi: 10.1200/JCO.1999.17.10.3333. [DOI] [PubMed] [Google Scholar]
- 31.Yeh ETH, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand J-B, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann K. Dexrazoxane and the ASCO guidelines for the use of chemotherapy and radiotherapy protectants: a critique. J Clin Oncol. 2000;18:2004–2006. doi: 10.1200/JCO.2000.18.9.2004. [DOI] [PubMed] [Google Scholar]
- 33.Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol. 2004;130:1–7. doi: 10.1007/s00432-003-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofland KF, Thougaard AV, Sehested M, Jensen PB. Dexrazoxane protects against myelosuppression from the DNA cleavage-enhancing drugs etoposide and daunorubicin but not doxorubicin. Clin Cancer Res. 2005;15:3915–3924. doi: 10.1158/1078-0432.CCR-04-2343. [DOI] [PubMed] [Google Scholar]
- 35.Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardio-protective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008:CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Yan T, Deng S, Metzger A, Gödtel-Armbrust U, Porter AC, Wojnowski L. Topoisomerase IIα-dependent and -independent apoptotic effects of dexrazoxane and doxorubicin. Mol Cancer Ther. 2009;8:1075–1085. doi: 10.1158/1535-7163.MCT-09-0139. [DOI] [PubMed] [Google Scholar]
- 37.Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, Jones SE, Wadler S, Desai A, Vogel C, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 38.Sehested M, Jensen PB, Sorensen BS, Holm B, Friche E, Demant EJ. Antagonistic effect of the cardioprotector (+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) on DNA breaks and cytotoxicity induced by the topoisomerase II directed drugs daunorubicin and etoposide (VP-16) Biochem Pharmacol. 1993;46:389–393. doi: 10.1016/0006-2952(93)90514-w. [DOI] [PubMed] [Google Scholar]
- 39.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;28:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toft KG, Hustvedt SO, Grant D, Martinsen I, Gordon PB, Friisk GA, Korsmo AJ, Skotland T. Metabolism and pharmacokinetics of MnDPDP in man. Acta Radiol. 1997;38:677–689. doi: 10.1080/02841859709172400. [DOI] [PubMed] [Google Scholar]
- 41.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 42.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 43.Sehested M, Jensen PB. Mapping of DNA topoisomerase II poisons (etoposide, clerocidin) and catalytic inhibitors (aclarubicin, ICRF-187) to four distinct steps in the topoisomerase II catalytic cycle. Biochem Pharmacol. 1996;12:879–886. doi: 10.1016/0006-2952(95)02241-4. [DOI] [PubMed] [Google Scholar]
- 44.Classen S, Olland S, Berger JM. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc Natl Acad Sci USA. 2003;100:10629–10634. doi: 10.1073/pnas.1832879100. [DOI] [PMC free article] [PubMed] [Google Scholar]