Absinthe is an emerald-green liqueur that achieved fantastic popularity at the close of the 19th century. It was associated with the Bohemian lifestyle and was credited with the inspiration of famous artists and poets (1, 2). Because of its widespread abuse and the associated toxicity of its content of oil of wormwood, absinthe was made illegal in most countries in the 1910s. The most likely ingredient responsible for toxicity is believed to be the terpenoid α-thujone (1–4). Oil of wormwood has convulsant activity as well as activity in killing worms and insects (5). The mechanism of action of thujone has remained speculative until now. In a recent issue of PNAS, Hold et al. (6) provided evidence that thujone acts as a γ-aminobutyric acid type A (GABAA) receptor chloride channel blocker, much like the plant convulsant picrotoxin, and related synthetic analogs.
Absinthe use is ritualistic, involving a special glass and perforated spoon and adding cold water by pouring it over sugar cubes, at which time the liqueur turns white because of precipitation of alcohol-soluble herbal ingredients. Its magical powers worshiped by the masses, absinthe became the national drink of France in the late 19th century, with workers and artists alike awaiting l'heure verte, 5–7 p.m., when they all headed for the cafes of Paris for their glass of absinthe, drinking 36–221 million liters per year around 1910 (1, 2). In 1906, Paris had 33,330 bars (and drink sellers) for 2,601,000 people, compared with 17,000 bakers. Alcohol was a major economic force. The major supplier was the Swiss factory Pernod, established in 1787, and the original French connection was probably in Algeria.
Many Parisians adopted the Bohemian lifestyle in the Belle Epoque, among them many creative artists and writers. De Musset was so often hung over from absinthe that his students found him to “absinthe himself too much from his lectures.” Beaudelaire was an habitual user of absinthe. Verlaine abused absinthe in a self-destructive overindulgence, in the midst of a homosexual affair with Rimbaud. Other big fans included Zola, Oscar Wilde, Gaugin, Toulouse-Lautrec, van Gogh, and Picasso. But the wild and destructive lifestyle led to a negative reaction and major support for prohibition in France and elsewhere. One can see the negative side of absinthe drinking in many of the poems written about it and the pictures drawn about it, as in Degas (Fig. 1). The “madness in a bottle,” i.e., absinthe, was doubly attacked for its reputation for inducing insane and criminal acts, as well as convulsions and other toxicity (2). However, statistics showed that in France in 1907, only about 1/40th of the inmates of insane asylums were absinthe drinkers, and many of those would actually drink anything alcoholic (2).
Figure 1.

L'Absinthe, E. Degas.
Finally, military and civilian leaders entering into World War I discouraged alcohol abuse and absinthe in support of the war effort. Absinthe was outlawed in most countries in 1910–1915, and all alcohol became illegal shortly after in many. Pernod, an anise-flavored, green-colored analog of absinthe lacking the oil of wormwood, has remained available. Absinthe can still be purchased in certain lands, including Czechoslovakia, and you can make your own with readily available and legal ingredients (check the web).
Absinthe was widely regarded as imparting pharmacological effects beyond those of alcohol alone, such as stimulating the imagination and aphrodisiac action, as well as producing hallucinations. Except for the toxicity, there is little research evidence supporting this view and more study is needed.
As noted, oil of wormwood had ancient herbal medicinal uses, primarily for digestion (5). It is an extract of the common European woody bush, the wormwood plant, Artemisia absinthium. Other members of the Artemisia genus include sagebrush. The natural product thujone is found in plants of the Thuja genus, which is arborvitae and cedar. They are in the conifer family Cupressacae, which also includes Juniperus (the source of gin), and nutmeg; the related Tsuga genus is hemlock (a well-known poison).
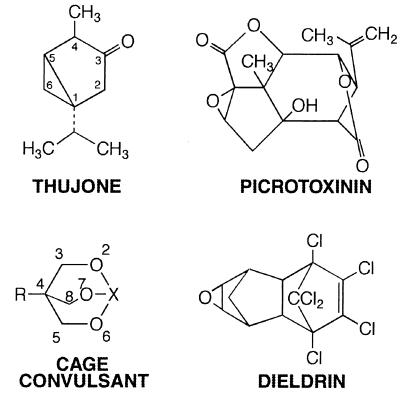
The newly noted connection with GABAA receptor channel antagonism should not be surprising in light of previous observations. The major pharmacological effect of oil of wormwood is seizures (2). The active agent (1, 7) is thujone (Fig. 2), and its only pharmacological action listed in the Merck index is “convulsant” (8). Many naturally occurring and synthetic convulsive agents are blockers of GABA-mediated inhibition (9, 10). The prototypic GABA channel blocker picrotoxinin (Fig. 2) is isolated from plants of the moonseed family, Menispermaceae, and its close relatives tutin and coriamyrtin, from the New Zealand tutu plant Coriaria arborea (11, 12), known as a “loco weed” that caused occasional poisonings in cows, and even in people.
Figure 2.
Chemical structures.
The senior author of this thujone report, Dr. John Casida, has synthesized and studied a major category of synthetic potent neurotoxic chemicals (13), the cage convulsants (Fig. 2), which were discovered to be noncompetitive GABAA receptor antagonists acting at the picrotoxinin site (13–15). One of these drugs, t-butyl bicylcophosphorothionate (TBPS), is a major research tool used to assay GABA receptors by radioligand binding (16). Synthetic butyrolactones with convulsant and anticonvulsant actions also have been described for the picrotoxinin site (17). In addition, this drug target appears to be the site of action of the experimental convulsant pentylenetetrazol (18) and numerous polychlorinated hydrocarbon insecticides, including dieldrin (Fig. 2) and α-endosulfan, mentioned in Hold et al. (6). This was demonstrated independently by both Dr. Casida (19, 20) and another author of the report, Dr. Toshio Narahashi (21), who has been a pioneer in mechanism studies on many drugs and toxins.
The report of Hold et al. (6) convincingly demonstrates that thujone acts as a GABAA receptor antagonist by four lines of evidence: (i) the symptoms of poisoning and protection by benzodiazepines and barbiturates resemble those of other GABA blockers like picrotoxinin; (ii) a strain of insects resistant to picrotoxin and GABA-blocking insecticides like dieldrin is resistant to thujone; (iii) thujone competitively inhibits the binding of a radioactive cage convulsant to the picrotoxin/convulsant site on GABAA receptors in mammalian brain membranes; and (iv) thujone reversibly blocks GABAA receptor chloride currents in mammalian neurons. These actions are shown not to be caused by ethanol. In addition, the authors demonstrate the nature and activity of the major metabolites of thujone in mammalian brain and liver. They demonstrate that α-thujone is 2.3 times more active than β-thujone in binding and that 7-hydroxy-α-thujone and dehydro-α-thujone are toxic but not as potent as α-thujone. It would have been nice to have additional biological potencies for β-thujone because it is more abundant than α-thujone in wormwood and active in the binding test. If the two stereoisomers had differed in activity it would have provided even stronger evidence that the GABAA receptor is the drug target. It is possible or likely that both isomers have some activity. Likewise, the high abundance of the metabolite 7-hydroxy thujone suggests the possibility that some of the pharmacological actions may ensue from this substance.
Now why would a drug with toxic and convulsant actions possibly be considered pleasant or at least desirable? A speculation that thujone might behave in a manner similar to tetrahydrocannabinol, the active ingredient of marijuana, was ruled out (22): thujone has a low affinity for cannabinoid receptor binding sites but none of the pharmacological actions, such as locomotor activity (open field test), immobility (ring stand test), and analgesia (hot plate test). Thus cannabinoids, but not thujone, are central nervous system depressants, like a sleeping pill. Thujone, like picrotoxin, is excitatory on the brain (analeptic). Such an agent may produce mood elevation and antidepressant effects. One may note the anxiogenic and possibly alerting effect of GABA antagonists, as opposed to the anxiolytic, sedative, but also amnestic effects of GABA-enhancing drugs like benzodiazepines and ethanol (9, 10, 23). Do not forget, however, that in absinthe one is balancing the effect of thujone with the intoxicating, disinhibitory, and depressant effects of ethanol, not to mention those of the other herbal ingredients of oil of wormwood and others added to the myriad recipes for absinthe now in existence.
I will leave with this quote of Oscar Wilde about absinthe: “After the first glass, you see things as you wish they were. After the second, you see them as they are not. Finally, you see things as they really are, which is the most horrible thing in the world” (2).
Footnotes
See companion article on page 3826 in issue 8 of volume 97.
References
- 1.Vogt D D, Montagne M. Int J Addict. 1982;17:1015–1029. doi: 10.3109/10826088209057772. [DOI] [PubMed] [Google Scholar]
- 2.Conrad B. Absinthe: History in a Bottle. San Francisco: Chronicle Books; 1988. [Google Scholar]
- 3.Arnold W N. Sci Am. 1989;260:112–117. doi: 10.1038/scientificamerican0689-112. [DOI] [PubMed] [Google Scholar]
- 4.Cushney A A. A Textbook of Pharmacology and Therapeutics. 4th Ed. Philadelphia: Lea Bros.; 1906. p. 426. [Google Scholar]
- 5.Simonetti G. Simon & Schuster's Guide to Herbs and Spices. New York: Simon & Schuster; 1990. pp. 261–262. [Google Scholar]
- 6.Hold K M, Sirisoma S I, Ikeda T, Narahashi T, Casida J E. Proc Natl Acad Sci USA. 2000;97:3826–3831. doi: 10.1073/pnas.070042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacco T, Chialva F. Planta Medica. 1988;54:93. doi: 10.1055/s-2006-962357. [DOI] [PubMed] [Google Scholar]
- 8.Windholz M, Budavari S, Stroumtsos L, Fertig M, editors. The Merck Index. 9th Ed. Rahway, NJ: Merck; 1976. p. 9139. [Google Scholar]
- 9.Macdonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 10.Olsen R W, Gordey M. In: Handbook of Experimental Pharmacology, Pharmacology of Ionic Channel Function: Activators and Inhibitors. Endo M, Kurachi Y, Mishina M, editors. Vol. 147. Heidelberg: Springer; 2000. pp. 497–515. [Google Scholar]
- 11.Porter L A. Chem Rev (Washington, DC) 1967;67:441–464. doi: 10.1021/cr60248a004. [DOI] [PubMed] [Google Scholar]
- 12.Curtis D R, Davies J, Game C J A, Johnston G A R, McCulloch R M. Brain Res. 1973;63:419–423. doi: 10.1016/0006-8993(73)90116-9. [DOI] [PubMed] [Google Scholar]
- 13.Casida J E, Palmer C J. Adv Biochem Psychopharmacol. 1988;45:109–123. [PubMed] [Google Scholar]
- 14.Bowery N G, Collins J F, Hill R G. Nature (London) 1976;261:601–603. doi: 10.1038/261601a0. [DOI] [PubMed] [Google Scholar]
- 15.Ticku M K, Olsen R W. Neuropharmacology. 1979;18:315–318. doi: 10.1016/0028-3908(79)90132-1. [DOI] [PubMed] [Google Scholar]
- 16.Squires R F, Casida J E, Richardson M, Saederup E. Mol Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- 17.Klunk W E, Covey D F, Ferrendelli J A. Mol Pharmacol. 1982;22:444–450. [PubMed] [Google Scholar]
- 18.Ramanjaneyulu R, Ticku M K. Eur J Pharmacol. 1984;98:337–345. doi: 10.1016/0014-2999(84)90282-6. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence L J, Casida J E. Life Sci. 1984;35:171–178. doi: 10.1016/0024-3205(84)90136-x. [DOI] [PubMed] [Google Scholar]
- 20.Cole L M, Casida J E. Life Sci. 1986;39:1855–1862. doi: 10.1016/0024-3205(86)90295-x. [DOI] [PubMed] [Google Scholar]
- 21.Narahashi T. In: Insecticide Action: From Molecule to Organism. Narahashi T, Chambers J E, editors. New York: Plenum; 1989. pp. 55–84. [Google Scholar]
- 22.Meschler J P, Howlett A C. Pharmacol Biochem Behav. 1999;62:473–480. doi: 10.1016/s0091-3057(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 23.Paul S M. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 87–94. [Google Scholar]